Identification of Breed Differences in Known and New Fescue Toxicosis Associated Phenotypes in Charolais-and Hereford-Sired Crossbred Beef Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Phenotyping Techniques
2.3. Statistical Analysis
3. Results
3.1. Analysis of Known Phenotypes in Response to Fescue Exposure
3.1.1. Average Daily Gain and Body Conditioning
3.1.2. Physiological Measures of Heat Stress/Fescue Toxicosis
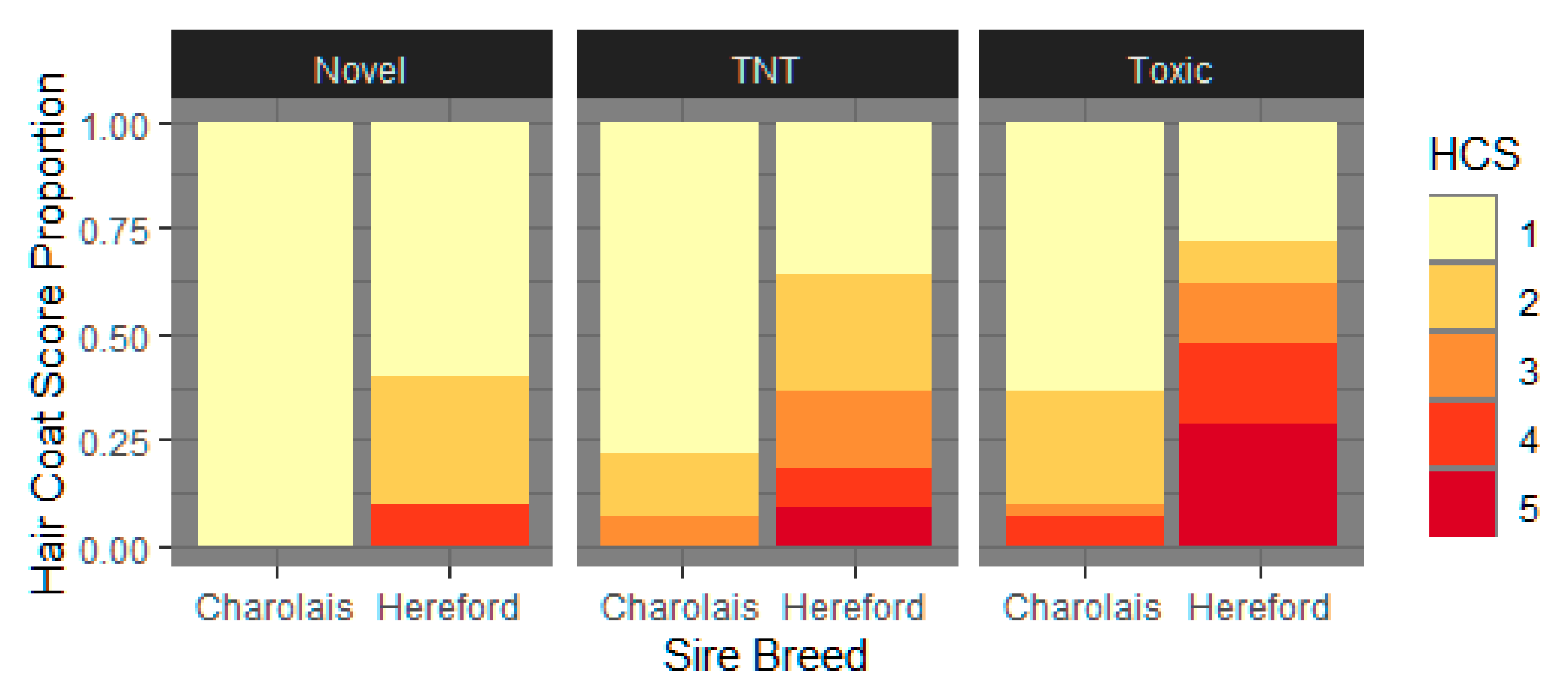
3.1.3. Hair Coat Score
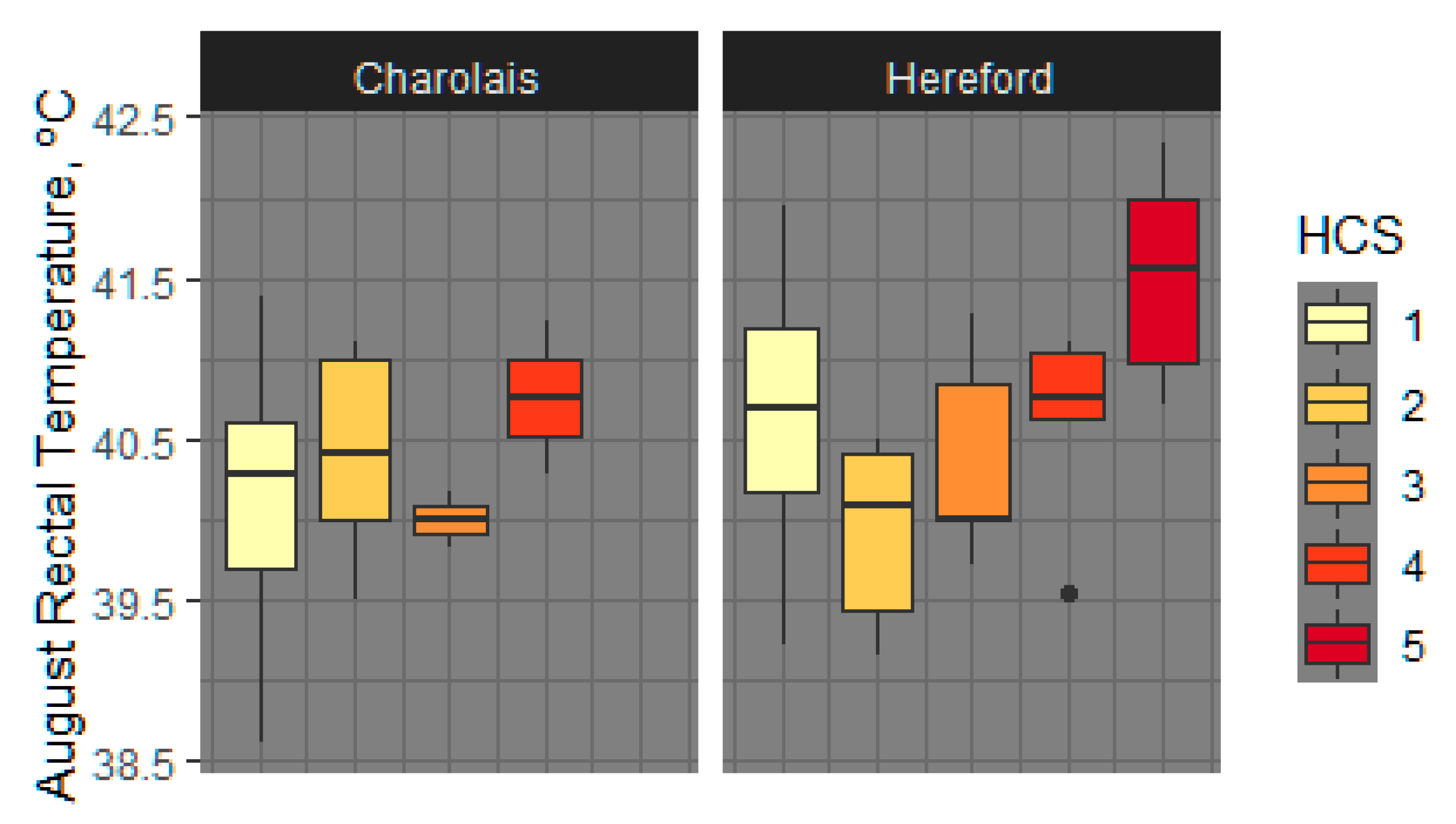
3.1.4. Rectal Temperature
3.1.5. August HCS as Covariate for Rectal Temperature
3.2. Analysis of New Phenotypes in Response to Fescue Exposure
3.2.1. Blood Serum Mineral Concentration
3.2.2. Hair Reduction Rate
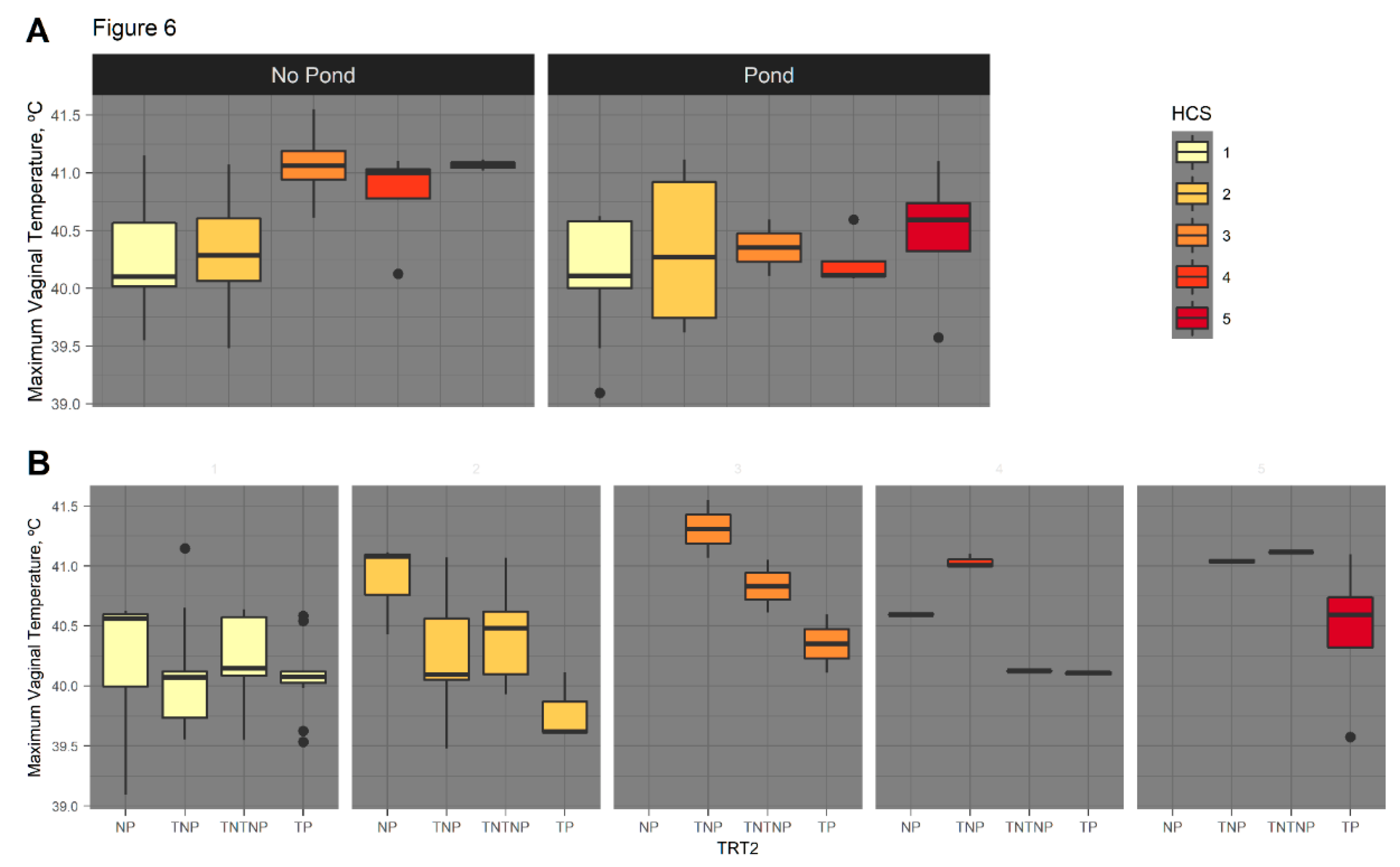
3.2.3. Serial Vaginal Temperature
3.2.4. August HCS as Covariate of Serial Vaginal Temperature
3.3. Comparison of Trait Correlations between Toxic, Alternating (TNT), and Novel Fescue Pastures
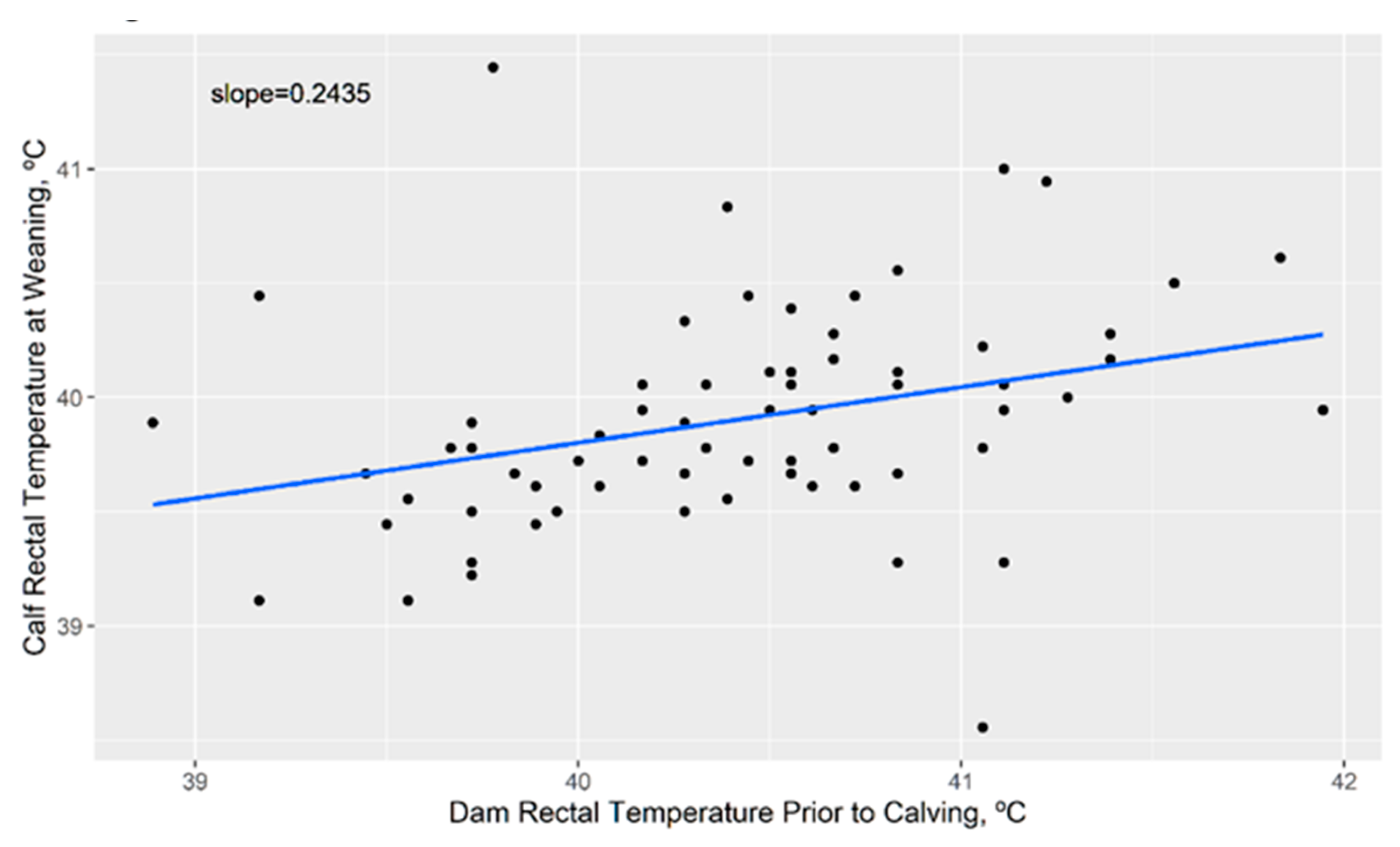
3.4. Impact of Cow Body Temperature on Calf Body Temperature Later in Life
4. Discussion
4.1. Weight Gain and Body Condition Traits Differed by Toxic Fescue Exposure and Sire Breed
4.2. Respiration Rates Were Impacted by Fescue Type and Sire Breed and Blood Pressure Were Impacted by Toxic Fescue
4.3. Blood Serum Phosphorus and Magnesium Are Impacted by Sire Breed and Toxic Fescue Exposure
4.4. Hair shedding and HCS Are Impacted by Toxic Fescue Exposure and Exhibit within And across Sire Breed Variability
4.5. Rectal and Vaginal Temperatures Differ by Sire Breed, Hair Coat Thickness, and Tend to Differ by Fescue Pasture Type
4.6. Calf Body Temperature at Weaning Is Impacted by Maternal Heat Stress
4.7. Evidence of Genetic Differences in Hereford-and Charolais-Sired Cows in Response to Toxic Fescue
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoveland, C.S. Importance and economic significance of the Acremonium endophytes to performance of animals and grass plant. Agric. Ecosyst. Environ. 1993, 44, 3–12. [Google Scholar] [CrossRef]
- Hill, N.S.; Belesky, D.P.; Stringer, W.C. Competitiveness of tall fescue as influenced by acremonium coenophialum. Crop Sci. 1991, 31, 185–190. [Google Scholar] [CrossRef]
- Foote, A.P.; Kristensen, N.B.; Klotz, J.L.; Kim, D.H.; Koontz, A.F.; McLeod, K.R.; Bush, L.P.; Schrick, F.N.; Harmon, D.L. Ergot alkaloids from endophyte-infected tall fescue decrease reticuloruminal epithelial blood flow and volatile fatty acid absorption from the washed reticulorumen. J. Anim. Sci. 2013, 91, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.L.; Brown, K.R.; Xue, Y.; Matthews, J.C.; Boling, J.A.; Burris, W.R.; Bush, L.P.; Strickland, J.R. Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 2012, 90, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.R.; Aiken, G.E.; Spiers, D.E.; Fletcher, L.R.; Oliver, J.W. Physiological Basis of Fescue Toxicosis. In Tall Fescue Twenty-First Century, Agronomy Monographs; Wiley: Hoboken, NJ, USA, 2009; pp. 203–227. [Google Scholar]
- Strickland, J.R.; Looper, M.L.; Matthews, J.C.; Rosenkrans, C.F.; Flythe, M.D.; Brown, K.R. Board-invited review: St. Anthony’s Fire in livestock: Causes, mechanisms, and potential solutions. J. Anim. Sci. 2011, 89, 1603–1626. [Google Scholar] [CrossRef]
- Aldrich, C.G.; Paterson, J.A.; Tate, J.L.; Kerley, M.S. The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. J. Anim. Sci. 1993, 71, 164–170. [Google Scholar] [CrossRef]
- Al-Haidary, A.; Spiers, D.E.; Rottinghaus, G.E.; Garner, G.B.; Ellersieck, M.R. Thermoregulatory ability of beef heifers following intake of endophyte-infected tall fescue during controlled heat challenge. J. Anim. Sci. 2001, 79, 1780–1788. [Google Scholar] [CrossRef]
- Drewnoski, M.E.; Oliphant, E.J.; Poore, M.H.; Green, J.T.; Hockett, M.E. Growth and reproductive performance of beef heifers grazing endophyte-free, endophyte-infected and novel endophyte-infected tall fescue. Livest. Sci. 2009, 125, 254–260. [Google Scholar] [CrossRef]
- Jackson, J.J.; Lindemann, M.D.; Boling, J.A.; Matthews, J.C. Summer-long grazing of high vs. low endophyte (Neotyphodium coenophialum)-infected tall fescue by growing beef steers results in distinct temporal blood Analyte Response Patterns, with Poor Correlation to Serum Prolactin Levels. Front. Vet. Sci. 2015, 2, 77. [Google Scholar] [CrossRef]
- Brown, M.A.; Brown, A.H.; Jackson, W.G.; Miesner, J.R. Genotype X environment interactions in Angus, Brahman, and reciprocal-cross cows and their calves grazing common bermudagrass, endophyte-infected tall fescue pastures, or both forages. J. Anim. Sci. 1993, 78, 546–551. [Google Scholar] [CrossRef]
- Browning, R. Physiological responses of Brahman and Hereford steers to an acute ergotamine challenge. J. Anim. Sci. 2000, 78, 124–130. [Google Scholar] [CrossRef]
- Cole, N.A.; Brown, M.A.; Phillips, W.A. Genetic x environment interactions on blood constituents of Angus, Brahman, and reciprcal-cross cows and calves grazing common bermudagrass or endophyte-infected tall fescue. J. Anim. Sci. 2001, 79, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Lees, J.; Lisle, A.T.; Sullivan, M.L.; Gaughan, J. Effect of heat stress on rumen temperature of three breeds of cattle. Int. J. Biometeorol. 2018, 62, 207–215. [Google Scholar] [CrossRef]
- Rolf, M.M. Genetic Basis for Heat Tolerance in Cattle. Cab 2015, 9, 948. [Google Scholar]
- Smith, T.; Cassady, J.P. Genetic resistance to the effects of grazing endophyte-infected tall fescue. J. Anim. Sci. 2015, 93, 5506–5511. [Google Scholar] [CrossRef]
- Settivari, R.S.; Evans, T.J.; Yarru, L.P.; Eichen, P.A.; Sutovsky, P.; Rottinghaus, G.E.; Antoniou, E.; Spiers, D.E. Effects of short-term heat stress on endophytic ergot alkaloid-induced alterations in rat hepatic gene expression. J. Anim. Sci. 2009, 87, 3142–3155. [Google Scholar] [CrossRef]
- Larson, B.; Samford, M.; Turner, J.; Kerley, M.; Paterson, J. Effects of endophyte-infected tall fescue, environmental temperature and prazosin injection on the rat. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 114, 39–44. [Google Scholar] [CrossRef]
- Foote, A.P.; Harmon, D.L.; Brown, K.R.; Strickland, J.R.; McLeod, K.R.; Bush, L.P.; Klotz, J.L. Constriction of bovine vasculature caused by endophyte-infected tall fescue seed extract is similar to pure ergovaline. J. Anim. Sci. 2012, 90, 1603–1609. [Google Scholar] [CrossRef]
- Stowe, H.M.; Miller, M.; Burns, M.G.; Calcatera, S.M.; Andrae, J.G.; Aiken, G.E.; Schrick, F.N.; Cushing, T.; Bridges, W.C.; Pratt, S.L. Effects of fescue toxicosis on bull growth, semen characteristics, and breeding soundness evaluation. J. Anim. Sci. 2013, 91, 3686–3692. [Google Scholar] [CrossRef]
- Eisemann, J.H.; Huntington, G.B.; Williamson, M.; Hanna, M.; Poore, M. Physiological responses to known intake of ergot alkaloids by steers at environmental temperatures within or greater than their thermoneutral zone. Front. Chem. 2014, 2, 96. [Google Scholar] [CrossRef]
- Shoup, L.; Miller, L.; Srinivasan, M.; Ireland, F.; Shike, D. Effects of cows grazing toxic endophyte-infected tall fescue or novel endophyte-infected tall fescue in late gestation on cow performance, reproduction, and progeny growth performance and carcass characteristics. J. Anim Sci. 2016, 94, 5105–5113. [Google Scholar] [CrossRef] [PubMed]
- Aiken, G.E.; Flythe, M.D. Vasconstrictive responses by the carotid and auricular arteries in goats to ergot alkaloid exposure. Front. Chem. 2014, 2, 101. [Google Scholar] [CrossRef] [PubMed]
- LaPorta, J.; Fabris, T.; Skibiel, A.; Powell, J.; Hayen, M.; Horvath, K.; Miller-Cushon, E.; Dahl, G. In utero exposure to heat stress during late gestation has prolonged effects on the activity patterns and growth of dairy calves. J. Dairy Sci. 2017, 100, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.T.; Dahl, G.E. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 2016, 99, 8443–8450. [Google Scholar] [CrossRef]
- Armstrong, D.V. Heat Stress Interaction with Shade and Cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Polsky, L.; Von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- Kurtz, T.W.; Griffin, K.A.; Bidani, A.K.; Davisson, R.L.; Hall, J.E. Recommendations for blood pressure measurement in animals: Summary of an AHA scientific statement from the council on high blood pressure research, professional and public education subcommittee. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 478–479. [Google Scholar] [CrossRef]
- Richards, M.W.; Spitzer, J.C.; Warner, M.B. Effect of Varying Levels of Postpartum Nutrition and Body Condition at Calving on Subsequent Reproductive Performance in Beef Cattle23. J. Anim. Sci. 1986, 62, 300–306. [Google Scholar] [CrossRef]
- Gray, K.A.; Smith, T.; Maltecca, C.; Overton, P.; Parish, J.A.; Cassady, J.P. Differences in hair coat shedding, and effects on calf weaning weight and BCS among Angus dams. Livest. Sci. 2011, 140, 68–71. [Google Scholar] [CrossRef]
- Dikmen, S.; Khan, F.A.; Huson, H.J.; Sonstegard, T.S.; Moss, J.I.; Dahl, G.E.; Hansen, P.J. The SLICK hair locus derived from Senepol cattle confers thermotolerance to intensively managed lactating Holstein cows. J. Dairy Sci. 2014, 97, 5508–5520. [Google Scholar] [CrossRef]
- Casler, M.; Albrecht, K.; Lehmkuhler, J.; Brink, G.; Combs, D. Forage Fescues in the Northern USA. 2008. Available online: https://cias.wisc.edu/wp-content/uploads/sites/194/2008/10/fescuefinalweb1.pdf (accessed on 17 September 2021).
- Schmidt, S.P.; Osborn, T.G. Effects of endophyte-infected tall fescue on animal performance. Agric. Ecosyst. Environ. 1993, 44, 233–262. [Google Scholar] [CrossRef]
- Browning, R., Jr. Effects of endophyte-infected tall fescue on indicators of thermal status and growth in Hereford and Senepol steers. J. Anim. Sci. 2004, 82, 634–643. [Google Scholar] [CrossRef][Green Version]
- Finch, V. Body temperature in beef cattle: Its control and relevance to production in the tropics. J. Anim. Sci. 1986, 62, 531–542. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef]
- Neary, J.M.; Garry, F.B.; Holt, T.N.; Thomas, M.G.; Enns, R.M. Mean pulmonary arterial pressures in Angus steers increase from cow-calf to feedlot-finishing phases. J. Anim. Sci. 2015, 93, 3854–3861. [Google Scholar] [CrossRef]
- Goff, J.P. Treatment of calcium, phosphorus, and magnesium balance disorders. Vet. Clin. North Am. Food Anim. Pract. 1999, 15, 619–639. [Google Scholar] [CrossRef]
- Grünberg, W.; Constable, P.; Schröder, U.; Staufenbiel, R.; Morin, D.; Rohn, M. Phosphorus homeostasis in dairy cows with abomasal displacement or abomasal volvulus. J. Vet. Intern. Med. 2005, 19, 894–898. [Google Scholar] [CrossRef]
- Beede, D.K.; Collier, R.J. Potential Nutritional Strategies for Intensively Managed Cattle during Thermal Stress. J. Anim. Sci. 1986, 62, 543–554. [Google Scholar] [CrossRef]
- Joo, S.; Lee, S.; Park, D.; Kim, D.; Gu, B.-H.; Park, Y.; Rim, C.; Kim, M.; Kim, E. Changes in Blood Metabolites and Immune Cells in Holstein and Jersey Dairy Cows by Heat Stress. Animals 2021, 11, 974. [Google Scholar] [CrossRef]
- Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Meyer, M.B.; Pike, J.W.; Christakos, S. Evidence for a Role of Prolactin in Calcium Homeostasis: Regulation of Intestinal Transient Receptor Potential Vanilloid Type 6, Intestinal Calcium Absorption, and the 25-Hydroxyvitamin D3 1α Hydroxylase Gene by Prolactin. Endocvrinology 2010, 151, 2974–2984. [Google Scholar] [CrossRef]
- Bazydlo, L.A.L.; Needham, M.; Harris, N.S. Calcium, Magnesium, and Phosphate. Lab. Med. 2014, 45, e44–e50. [Google Scholar] [CrossRef]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Mote, R.S.; Hill, N.S.; Uppal, K.; Tran, V.L.T.; Jones, D.P.; Filipov, N.M. Metabolomics of fescue toxicosis in grazing beef steers. Food Chem. Toxicol. 2017, 105, 285–299. [Google Scholar] [CrossRef]
- Care, A.D. The absorption of phosphate from the digestive tract of ruminant animals. Br. Vet. J. 1994, 150, 197–205. [Google Scholar] [CrossRef]
- Tomas, F.M.; Potter, B.J. The site of magnesium absorption from the ruminant stomach. Br. J. Nutr. 1976, 36, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Aiken, G.E.; Klotz, J.L.; Looper, M.L.; Tabler, S.F.; Schrick, F.N. Disrupted hair follicle activity in cattle grazing endophyte-infected tall fescue in the summer insulates core body temperatures. Prof. Anim. Sci. 2011, 27, 336–343. [Google Scholar] [CrossRef]
- Durbin, H.J.; Lu, D.; Yampara-iquise, H.; Miller, S.P.; Decker, J.E. Development of a genetic evaluation for hair shedding in American Angus cattle to improve thermotolerance. Genet. Sel. Evol. 2020, 52, 63. [Google Scholar] [CrossRef] [PubMed]
- Walsberg, G.E. Quantifying radiative heat gain in animals. Integr. Comp. Biol. 1992, 32, 217–223. [Google Scholar] [CrossRef]
- Finch, V.A.; Bennett, I.L.; Holmes, C.R. Coat colour in cattle: Effect on thermal balance, behaviour and growth, and relationship with coat type. J. Agric. Sci. 1984, 102, 141–147. [Google Scholar] [CrossRef]
- Hamblen, H.; Hansen, P.J.; Zolini, A.M.; Oltenacu, P.A.; Mateescu, R.G. Thermoregulatory response of Brangus heifers to naturally occurring heat exposure on pasture. J. Anim. Sci. 2018, 96, 3131–3137. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Gebremedhin, K.G. Thermal biology of domestic animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.; Ricconi, K.; Avila, S.; Klotz, J.L.; Harmon, D.L. Ruminal motility, reticuloruminal fill, and eating patterns in steers exposed to ergovaline. J. Anim. Sci. 2020, 98, skz374. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The impact of heat load on cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Heat stress on reproductive function and fertility in mammals. Reprod. Med. Biol. 2012, 11, 37–47. [Google Scholar] [CrossRef]
- Porter, J.K.; Thompson, F.N. Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 1992, 70, 1594–1603. [Google Scholar] [CrossRef]
- Zakari, A.Y.; Molokwu, E.C.I.; Osori, D.I.K. Effects of rectal and ambient temperatures and humidity on conception rates. Theriogenology 1981, 16, 331–336. [Google Scholar] [CrossRef]
- Caldwell, J.D.; Coffey, K.P.; Jennings, J.A.; Philipp, D.; Young, A.N.; Tucker, J.D.; Hubbell, D.S.; Hess, T.; Looper, M.L.; West, C.P.; et al. Performance by spring and fall-calving cows grazing with full, limited, or no access to toxic Neotyphodium coenophialum-infected tall fescue. J. Anim. Sci. 2013, 91, 465–476. [Google Scholar] [CrossRef]
- Gould, L.S.; Hohenboken, W.D. Differences between progeny of beef sires in susceptibility to fescue toxicosis. J. Anim. Sci. 1993, 71, 3025–3032. [Google Scholar] [CrossRef]



| Novel | TNT 1 | Toxic | p-Value * | |
|---|---|---|---|---|
| N | 25 | 25 | 50 | |
| August single time point phenotypes | ||||
| Systolic Blood Pressure, mmHg | 107.1 ± 9.412 a | 119.6 ± 8.036 ab | 130.7 ± 6.253 b | 0.0383 |
| Rectal Temperature, °C | 40.64 ± 0.159 a | 39.73 ± 0.130 b | 40.62 ± 0.109 a | <0.0001 |
| Respiration Rate, breaths/min | 70.56 ± 5.203 a | 55.60 ± 4.253 b | 81.13 ± 3.573 a | <0.0001 |
| Serum Phosphorus Concentration, mg/L 2 | 6.775 ± 0.249 a | 7.744 ± 0.203 b | 6.005 ± 0.171 c | <0.0001 |
| Serum Magnesium Concentration, mg/L 2 | 2.233 ± 0.048 a | 2.422 ± 0.039 b | 2.203 ± 0.033a | <0.0001 |
| Serial Phenotypes over time | ||||
| Average Daily Gain, kg/day | 0.692 ± 0.050 a | 0.720 ± 0.041 a | 0.452 ± 0.034 b | <0.0001 |
| Hair Coat Score 3 | 1.356 ± 0.286 a | 1.748 ± 0.231 ab | 2.352 ± 0.199 b | 0.0011 |
| Hair Reduction Rate, ∆HCS/day | −0.028 ± 0.002 a | −0.024 ± 0.002 a | −0.016 ± 0.001 b | <0.0001 |
| Body Condition Score 4 | 6.371 ± 0.167a | 6.418 ± 0.136 a | 5.699 ± 0.114 b | <0.0001 |
| Sire Breed | |||||
|---|---|---|---|---|---|
| Charolais | Hereford | Diff 1 | SE 2 | p-Value 3 | |
| n | 58 | 42 | |||
| August Phenotypes | |||||
| Average Daily Gain, kg/day | 0.701 ± 0.033 | 0.542 ± 0.0392 | 0.1586 | 0.0428 | 0.0004 |
| Rectal Temperature, °C | 40.04 ± 0.107 | 40.62 ± 0.126 | −0.5771 | 0.1375 | <0.0001 |
| Respiration Rate, breaths/min | 62.03 ± 3.502 | 76.16 ± 4.106 | −14.125 | 4.493 | 0.0023 |
| Vaginal Temperature, °C | |||||
| Mean | 39.02 ± 0.072 | 39.30 ± 0.082 | −0.2793 | 0.0873 | 0.0020 |
| Maximum | 40.02 ± 0.083 | 40.43 ± 0.093 | −0.4148 | 0.0997 | <0.0001 |
| Range | 1.978 ± 0.071 | 2.286 ± 0.080 | −0.3076 | 0.0852 | 0.0005 |
| Variance | 0.183 ± 0.013 | 0.233 ± 0.015 | −0.050 | 0.0158 | 0.0022 |
| Mean daily AUC, °C × h 4 | 23.54 ± 1.052 | 27.67 ± 1.186 | −4.128 | 1.2669 | 0.0016 |
| A. | Fescue Type | |||
|---|---|---|---|---|
| Novel | TNT 2 | Toxic | p-Value | |
| n | 25 | 25 | 50 | |
| Trial Day | ||||
| 0 | 4.908 ± 0.063 | 4.893 ± 0.062 | 4.817 ± 0.044 | 0.4045 |
| 28 | 4.400 ± 0.102 | 4.431 ± 0.101 | 4.391 ± 0.072 | 0.9495 |
| 57 | 3.433 ± 0.145 | 3.621 ± 0.143 | 3.810 ± 0.102 | 0.1016 |
| 83 | 2.389 ± 0.168 | 2.780 ± 0.166 | 3.361 ± 0.118 | <0.0002 |
| 112 | 1.756 ± 0.161 | 2.264 ± 0.167 | 3.087 ± 0.119 | <0.0001 |
| 140 | 1.511 ± 0.180 | 2.024 ± 0.178 | 2.775 ± 0.126 | <0.0001 |
| 149 | 1.394 ± 0.194 | 1.863 ± 0.192 | 2.443 ± 0.136 | <0.0001 |
| 156 | 1.505 ± 0.219 | 1.815 ± 0.223 | 2.259 ± 0.161 | 0.0197 |
| B. | Sire Breed | |||
| Charolais | Hereford | p-Value | ||
| n | 58 | 42 | ||
| Trial Day | ||||
| 0 | 4.843 ± 0.043 | 4.902 ± 0.050 | 0.369 | |
| 28 | 4.269 ± 0.069 | 4.565 ± 0.082 | 0.0116 | |
| 57 | 3.340 ± 0.098 | 3.903 ± 0.116 | 0.0004 | |
| 83 | 2.505 ± 0.114 | 3.182 ± 0.134 | 0.0002 | |
| 112 | 2.037 ± 0.115 | 2.701 ± 0.135 | 0.0003 | |
| 140 | 1.756 ± 0.122 | 2.451 ± 0.144 | 0.0004 | |
| 149 | 1.456 ± 0.132 | 2.344 ± 0.155 | <0.0001 | |
| 156 | 1.345 ± 0.153 | 2.394 ± 0.177 | <0.0001 | |
| Toxic | TNT | Novel | ||
|---|---|---|---|---|
| n = 50 | n = 25 | n = 25 | LEGEND: | |
| HCS x VTmean | 0.38 * | 0.66 * | 0.36 | Positive correlation ≥ 0.50 1 |
| HCS × ADG | −0.41 * | −0.61 * | NS | Positive correlation < 0.50 1 |
| HCS × RR | 0.50 * | NS | NS | Negative correlation ≥ 0.50 1 |
| HRR × RR | 0.52 * | NS | NS | Negative correlation < 0.50 1 |
| HRR × RT | 0.50 * | 0.52 * | NS | NS (non-significant correlation) |
| HRR × VTmean | 0.38 * | 0.66 * | 0.38 | HCS (hair coat score, 1 = 100% shed to 5 = 0% shed [30]) |
| RR × AUC | NS | NS | 0.55 * | VTmean (serial vaginal temperature mean, °C) |
| RR × P | −0.59 * | NS | NS | ADG (average daily grain, kg/day) |
| RT × Mg | NS | −0.53 * | NS | RR (respiration rate, breaths/minute) |
| RT × AUC | NS | 0.64 * | 0.39 | HRR (hair reduction rate, ∆HCS/day) |
| RT × RR | 0.53 * | NS | 0.49* | RT (rectal temperature, °C) |
| RT × VTmax | 0.26 | 0.58 * | NS | AUC (average daily accumulation of degree area under the curve × hours, °C × h) |
| RT × VTmean | 0.25 | 0.57 * | NS | P (blood serum phosphorus, mg/L) |
| RT × VTrange | NS | 0.52 * | NS | Mg (blood serum magnesium, mg/L) |
| RT × VTvar | NS | 0.55 ** | NS | VTmax (serial vaginal temperature maximum, °C) |
| VTmax × Cu | −0.53 * | NS | NS | VTrange (serial vaginal temperature range, °C) |
| VTmax × Mg | −0.26 | −0.53 * | NS | VTvar (serial vaginal temperature variance, °C2) |
| VTmax × Zn | NS | −0.53 * | NS | Cu (blood serum copper, mg/L) |
| VTmean × Zn | −0.26 | −0.59 * | NS | Zn (blood serum zinc, mg/L) |
| VTmin × Zn | −0.29* | −0.64 * | NS | VTmin (serial vaginal temperature minimum, °C) |
| VTrange × Mg | NS | −0.50 * | −0.37 | Na (blood serum sodium, mg/L) |
| Na × K | −0.60 * | NS | −0.41 * | K (blood serum potassium, mg/L) |
| Ca × Fe | 0.38 * | 0.65 * | 0.52 * | Ca (blood serum calcium, mg/L) |
| Ca × Zn | 0.26 | 0.52 * | NS | Fe (blood serum iron, mg/L) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, K.M.; Koltes, D.A.; Meyer, L.R.; Tucker, J.D.; Hubbell, D.S., III; Powell, J.G.; Apple, J.K.; Koltes, J.E. Identification of Breed Differences in Known and New Fescue Toxicosis Associated Phenotypes in Charolais-and Hereford-Sired Crossbred Beef Cows. Animals 2021, 11, 2830. https://doi.org/10.3390/ani11102830
Lucas KM, Koltes DA, Meyer LR, Tucker JD, Hubbell DS III, Powell JG, Apple JK, Koltes JE. Identification of Breed Differences in Known and New Fescue Toxicosis Associated Phenotypes in Charolais-and Hereford-Sired Crossbred Beef Cows. Animals. 2021; 11(10):2830. https://doi.org/10.3390/ani11102830
Chicago/Turabian StyleLucas, Kayla M., Dawn A. Koltes, Laura R. Meyer, John D. Tucker, Donald S. Hubbell, III, Jeremy G. Powell, Jason K. Apple, and James E. Koltes. 2021. "Identification of Breed Differences in Known and New Fescue Toxicosis Associated Phenotypes in Charolais-and Hereford-Sired Crossbred Beef Cows" Animals 11, no. 10: 2830. https://doi.org/10.3390/ani11102830
APA StyleLucas, K. M., Koltes, D. A., Meyer, L. R., Tucker, J. D., Hubbell, D. S., III, Powell, J. G., Apple, J. K., & Koltes, J. E. (2021). Identification of Breed Differences in Known and New Fescue Toxicosis Associated Phenotypes in Charolais-and Hereford-Sired Crossbred Beef Cows. Animals, 11(10), 2830. https://doi.org/10.3390/ani11102830






