Biofilm and Equine Limb Wounds
Abstract
Simple Summary
Abstract
1. Introduction
2. Biofilm in General
“A structured community of microbes with genetic diversity and variable gene expression (phenotype) that creates behaviours and defences used to produce unique infections (chronic infection). Biofilms are characterised by significant tolerance to antibiotics and biocides while remaining protected from host immunity”.
3. Similarities between Equine and Human Wounds with Delayed Healing and the Potential to Use the Horse as a Model
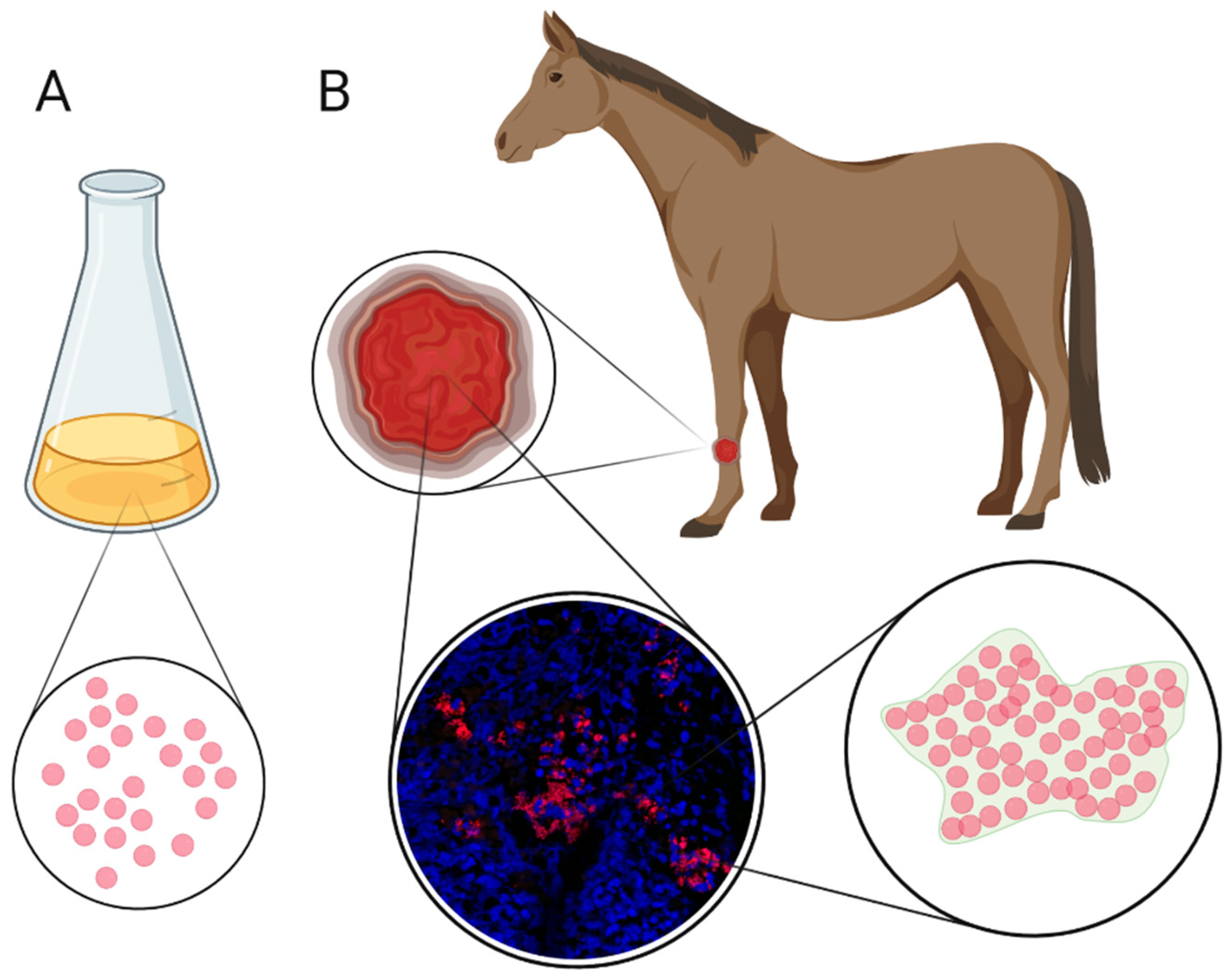
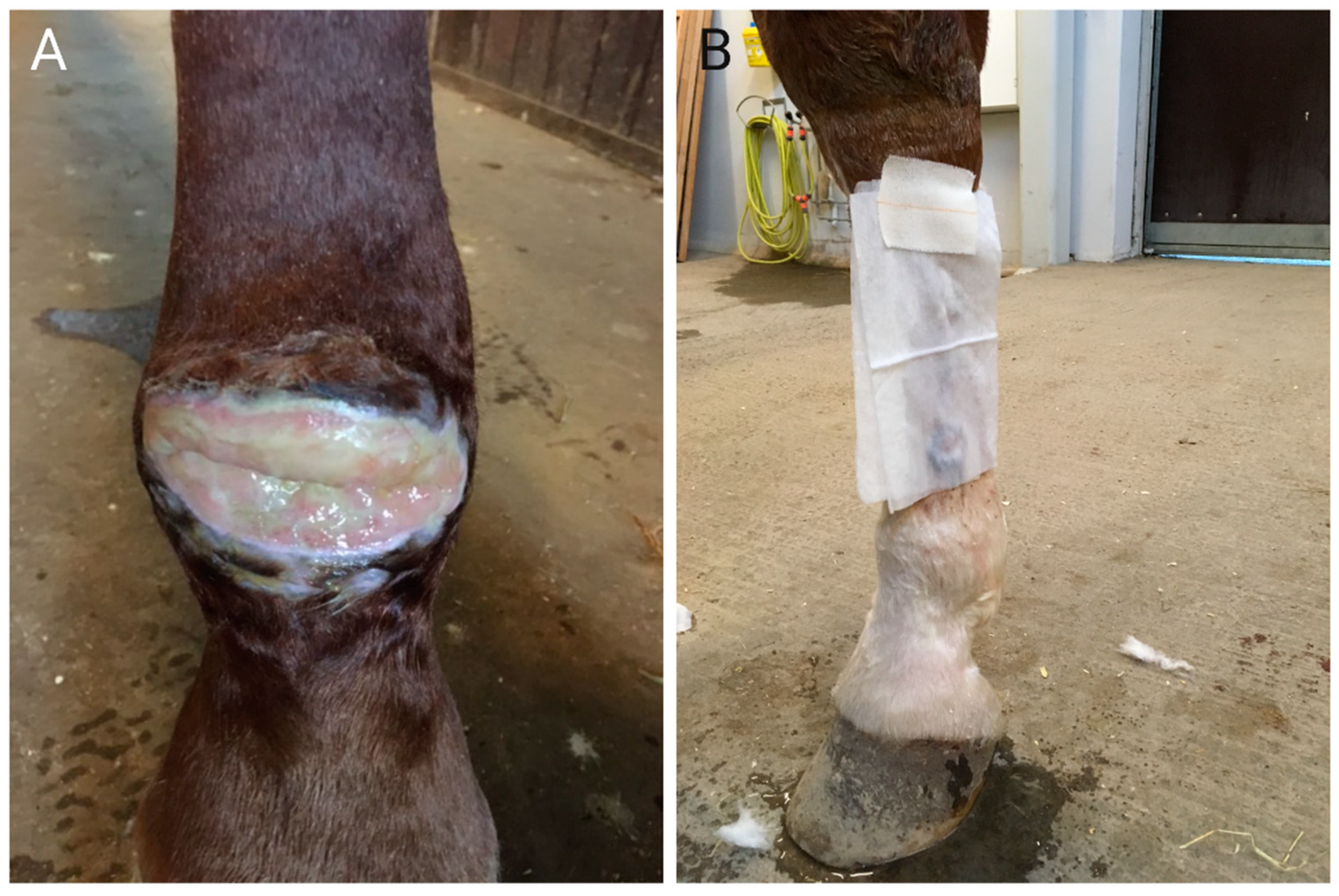
4. Biofilm and Equine Limb Wounds
5. Handling Biofilms in Wounds
5.1. Detection of Biofilm in Wounds
5.2. Treatment of Biofilm in Wounds
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bundgaard, L.; Bendixen, E.; Sørensen, M.A.; Harman, V.M.; Beynon, R.J.; Petersen, L.J.; Jacobsen, S. A selected reaction monitoring based analysis of acute phase proteins in interstitial fluids from experimental equine wounds healing by secondary intention. Wound Repair Regen. 2016, 24, 525–532. [Google Scholar] [CrossRef]
- Celeste, C.J.; Deschene, K.; Riley, C.B.; Theoret, C.L. Regional differences in wound oxygenation during normal healing in an equine model of cutaneous fibroproliferative disorder. Wound Repair Regen. 2010, 19, 89–97. [Google Scholar] [CrossRef]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef]
- Sørensen, M.A.; Pedersen, L.J.; Bundgaard, L.; Toft, N.; Jacobsen, S. Regional disturbances in metabolism and blood flow in equine limb wounds healing with formation of exuberant granulation tissue. Wound Repair Regen. 2014, 22, 647–653. [Google Scholar] [CrossRef]
- Uccioli, L.; Izzo, V.; Meloni, M.; Vainieri, E.; Ruotolo, V.; Giurato, L. Non-healing foot ulcers in diabetic patients: General and local interfering conditions and management options with advanced wound dressings. J. Wound Care 2015, 24, 35–42. [Google Scholar] [CrossRef]
- Wilmink, J.M.; Stolk, P.W.T.; Van Weeren, P.R.; Barneveld, A. Differences in second-intention wound healing between horses and ponies: Macroscopic aspects. Equine Veter.-J. 1999, 31, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.; Madsen, K.G.; Phipps, R.K.; Krogfelt, K.A.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D.; et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef]
- Cochrane, C.A.; Freeman, K.; Woods, E.; Welsby, S.; Percival, S.L. Biofilm evidence and the microbial diversity of horse wounds. Can. J. Microbiol. 2009, 55, 197–202. [Google Scholar] [CrossRef]
- Van Hecke, L.; Hermans, K.; Haspeslagh, M.; Chiers, K.; Pint, E.; Boyen, F.; Martens, A. A quantitative swab is a good non-invasive alternative to a quantitative biopsy for quantifying bacterial load in wounds healing by second intention in horses. Veter.-J. 2017, 225, 63–68. [Google Scholar] [CrossRef]
- Westgate, S.J.; Percival, S.L.; Knottenbelt, D.C.; Clegg, P.D.; Cochrane, C.A. Microbiology of equine wounds and evidence of bacterial biofilms. Veter.-Microbiol. 2011, 150, 152–159. [Google Scholar] [CrossRef]
- König, L.; Klopfleisch, R.; Kershaw, O.; Gruber, A.D. Prevalence of biofilms on surgical suture segments in wounds of dogs, cats, and horses. Veter.-Pathol. 2014, 52, 295–297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- König, L.M.; Klopfleisch, R.; Höper, D.; Gruber, A.D. Next generation sequencing analysis of biofilms from three dogs with postoperative surgical site infection. Int. Sch. Res. Not. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.A.; Freeman, L.J.; Seleem, M.N.; Snyder, P.W. Biofilm-infected wounds in a dog. J. Am. Veter.-Med. Assoc. 2014, 244, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, E.; Bay, L.; Bjarnsholt, T.; Bundgaard, L.; Sørensen, M.; Jacobsen, S. The occurrence of biofilm in an equine experimental wound model of healing by secondary intention. Veter. Microbiol. 2017, 204, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, E.; Bay, L.; Skovgaard, L.T.; Bjarnsholt, T.; Jacobsen, S. An equine wound model to study effects of bacterial aggregates on wound healing. Adv. Wound Care 2019, 8, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, C.A. Models in vivo of wound healing in the horse and the role of growth factors. Veter.-Dermatol. 1997, 8, 259–272. [Google Scholar] [CrossRef]
- Knottenbelt, D.C. Equine wound management: Are there significant differences in healing at different sites on the body? Veter. Dermatol. 1997, 8, 273–290. [Google Scholar] [CrossRef]
- Lefebvre-Lavoie, J.; Lussier, J.G.; Theoret, C.L. Profiling of differentially expressed genes in wound margin biopsies of horses using suppression subtractive hybridization. Physiol. Genom. 2005, 22, 157–170. [Google Scholar] [CrossRef][Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Genet. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Cornforth, D.M.; Dees, J.L.; Ibberson, C.B.; Huse, H.K.; Mathiesen, I.H.; Kirketerp-Møller, K.; Wolcott, R.D.; Rumbaugh, K.P.; Bjarnsholt, T.; Whiteley, M. Pseudomonas aeruginosa transcriptome during human infection. Proc. Natl. Acad. Sci. USA 2018, 115, E5125–E5134. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.; Høiby, N. The in vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef]
- Roberts, A.E.; Kragh, K.N.; Bjarnsholt, T.; Diggle, S.P. The limitations of in vitro experimentation in understanding biofilms and chronic infection. J. Mol. Biol. 2015, 427, 3646–3661. [Google Scholar] [CrossRef]
- Haesler, E.; Swanson, T.; Ousey, K.; Carville, K. Clinical indicators of wound infection and biofilm: Reaching international consensus. J. Wound Care 2019, 28, s4–s12. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Biophysics of biofilm infection. Pathog. Dis. 2014, 70, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Genet. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Givskov, M. The role of quorum sensing in the pathogenicity of the cunning aggressor Pseudomonas aeruginosa. Anal. Bioanal. Chem. 2006, 387, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Bjarnsholt, T.; Jensen, P.O.; Givskov, M.; Høiby, N. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: Current and emerging inhibitors. Future Microbiol. 2013, 8, 901–921. [Google Scholar] [CrossRef]
- Singh, R.; Ray, P. Quorum sensing-mediated regulation of staphylococcal virulence and antibiotic resistance. Future Microbiol. 2014, 9, 669–681. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Genet. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- Stewart, P.S. Antimicrobial tolerance in biofilms. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The calgary biofilm device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Cerca, N.; Jefferson, K.; Oliveira, R.; Pier, G.B.; Azeredo, J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect. Immun. 2006, 74, 4849–4855. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef]
- Jensen, P.; Givskov, M.; Bjarnsholt, T.; Moser, C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol. Med Microbiol. 2010, 59, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Koch, C. Cystic fibrosis. 1. Pseudomonas aeruginosa infection in cystic fibrosis and its management. Thorax 1990, 45, 881–884. [Google Scholar] [CrossRef]
- James, G.A.; Zhao, A.G.; Usui, M.; Underwood, R.A.; Nguyen, H.; Beyenal, H.; deLancey Pulcini, E.; Agostinho, D.; Hunt, A.A.; Bernstein, H.C.; et al. Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen. 2016, 24, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Micro. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Turner, R.J.; Marques, L.L.R.; Ceri, H. Biofilms. Am. Sci. 2005, 93, 508–515. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.-M. Management of biofilm-associated infections: What can we expect from recent research on biofilm lifestyles? Med. Sci 2012, 28, 727–739. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr. Infect. Dis. Rep. 2008, 10, 394–403. [Google Scholar] [CrossRef]
- Wolcott, R.; Rhoads, D.; Bennett, M.; Wolcott, B.; Gogokhia, L.; Costerton, J.; Dowd, S. Chronic wounds and the medical biofilm paradigm. J. Wound Care 2010, 19, 45–53. [Google Scholar] [CrossRef]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Høiby, N.; Jensen, P. Biofilms and host response—helpful or harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef]
- Olson, M.; Ceri, H.; Morck, D.W.; Buret, A.G.; Read, R.R. Biofilm bacteria: Formation and comparative susceptibility to antibiotics. Can. J. Veter.-Res. 2002, 66, 86–92. [Google Scholar]
- Clutterbuck, A.; Woods, E.; Knottenbelt, D.; Clegg, P.; Cochrane, C.; Percival, S. Biofilms and their relevance to veterinary medicine. Veter.-Microbiol. 2007, 121, 1–17. [Google Scholar] [CrossRef]
- Pedersen, R.R.; Krömker, V.; Bjarnsholt, T.; Dahl-Pedersen, K.; Buhl, R.; Jørgensen, E. Biofilm research in bovine mastitis. Front. Veter.-Sci. 2021, 8. [Google Scholar] [CrossRef]
- Wilmink, J.M.; Van Herten, J.; Van Weeren, P.R.; Barneveld, A. Retrospective study of primary intention healing and sequestrum formation in horses compared to ponies under clinical circumstances. Equine Veter.-J. 2010, 34, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.H.; Broeckx, S.; Van de Walle, G.; Polettini, M. The effects of equine peripheral blood stem cells on cutaneous wound healing: A clinical evaluation in four horses. Clin. Exp. Dermatol. 2013, 38, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.R.; Singer, E.R.; Clegg, P.D.; Ireland, J.L.; Pinchbeck, G.L. Identification of risk factors for traumatic injury in the general horse population of north-west England, Midlands and north Wales. Equine Veter.-J. 2011, 44, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Agina, O.A.; Ihedioha, J.I. Occurrence of wounds in Nigerian horses. J. Appl. Anim. Welf. Sci. 2017, 20, 372–380. [Google Scholar] [CrossRef]
- Theoret, C.L.; Bolwell, C.F.; Riley, C. A cross-sectional survey on wounds in horses in New Zealand. N. Z. Veter.-J. 2015, 64, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Theoret, C.L.; Wilmink, J.M. Aberrant wound healing in the horse: Naturally occurring conditions reminiscent of those observed in man. Wound Repair Regen. 2013, 21, 365–371. [Google Scholar] [CrossRef]
- Textor, J.A.; Clark, K.C.; Walker, N.J.; Aristizobal, F.A.; Kol, A.; Lejeune, S.S.; Bledsoe, A.; Davidyan, A.; Gray, S.N.; Bohannon-Worsley, L.K.; et al. Allogeneic stem cells alter gene expression and improve healing of distal limb wounds in horses. Stem Cells Transl. Med. 2017, 7, 98–108. [Google Scholar] [CrossRef]
- Gordillo, G.M.; Bernatchez, S.F.; Diegelmann, R.; Di Pietro, L.A.; Eriksson, E.; Hinz, B.; Hopf, H.; Kirsner, R.; Liu, P.; Parnell, L.K.; et al. Preclinical models of wound healing: Is man the model? Proceedings of the wound healing society symposium. Adv. Wound Care 2013, 2, 1–4. [Google Scholar] [CrossRef]
- Harman, R.M.; Theoret, C.L.; Van De Walle, G.R. The horse as a model for the study of cutaneous wound healing. Adv. Wound Care 2019, 10, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.A.; Leach, D.H.; Fretz, P.B.; Townsend, H.G.G. Comparative aspects of the healing of excisional wounds on the leg and body of horses. Veter.-Surg. 2008, 13, 83–90. [Google Scholar] [CrossRef]
- Theoret, C. Physiology of Wound Healing. In Equine Wound Management, 3rd ed.; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2017; pp. 1–13. [Google Scholar]
- Cochrane, C.A.; Pain, R.; Knottenbelt, D.C. In-Vitro wound contraction in the horse: Differences between body and limb wounds. Wounds 2003, 15, 175–181. [Google Scholar]
- Sardari, K.; Kazemi, H.; Emami, M.R.; Movasaghi, A.R.; Goli, A.A. Role of collagen cross-linking on equine wound contraction and healing. Comp. Haematol. Int. 2008, 18, 239–247. [Google Scholar] [CrossRef]
- Davidson, J.M.; Yu, F.; Opalenik, S.R. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv. Wound Care 2013, 2, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Mogford, J.E.; Mustoe, T.A. Experimental models of wound healing. In Cutaneous Wound Healing, 1st ed.; Falanga, V., Ed.; Martin Dunitz Ltd.: Hampshire, UK, 2001; pp. 109–122. [Google Scholar]
- Wilmink, J.M.; Weeren, P.R.; Stolk, P.W.T.; Mil, F.N.; Barneveld, A. Differences in second-intention wound healing between horses and ponies: Histological aspects. Equine Veter.-J. 1999, 31, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bodaan, C.J.; Wise, L.M.; Wakelin, K.; Stuart, G.S.; Real, N.C.; Mercer, A.; Riley, C.; Theoret, C. Short-term treatment of equine wounds with orf virus IL-10 and VEGF-E dampens inflammation and promotes repair processes without accelerating closure. Wound Repair Regen. 2016, 24, 966–980. [Google Scholar] [CrossRef]
- Beidler, S.K.; Douillet, C.D.; Berndt, D.F.; Keagy, B.A.; Rich, P.B.; Marston, W.A. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J. Vasc. Surg. 2009, 49, 1013–1020. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Jørgensen, E.; Hjerpe, F.B.; Hougen, H.P.; Bjarnsholt, T.; Berg, L.C.; Jacobsen, S. Histologic changes and gene expression patterns in biopsy specimens from bacteria-inoculated and noninoculated excisional body and limb wounds in horses healing by second intention. Am. J. Veter.-Res. 2020, 81, 276–284. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Jull, A.B.; Arroll, B.; Parag, V.; Waters, J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst. Rev. 2012, 12, CD001733. [Google Scholar] [CrossRef] [PubMed]
- Beidler, S.K.; Douillet, C.D.; Berndt, D.F.; Keagy, B.A.; Rich, P.B.; Marston, W.A. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008, 16, 642–648. [Google Scholar] [CrossRef]
- Hanson, R.R. Complications of equine wound management and dermatologic surgery. Veter.-Clin. N. Am. Equine Pract. 2008, 24, 663–696. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-infective Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Kirketerp-Møller, K.; Jensen, P.; Fazli, M.M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef]
- Seth, A.K.; Geringer, M.R.; Hong, S.J.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. In vivo modeling of biofilm-infected wounds: A review. J. Surg. Res. 2012, 178, 330–338. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Trøstrup, H.; Thomsen, K.; Calum, H.; Hoiby, N.; Moser, C. Animal models of chronic wound care: The application of biofilms in clinical research. Chronic Wound Care Manag. Res. 2016, 3, 123–132. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Schwarz, R.; Neurand, K. The skin of domestic mammals as a model for human skin, with speical reference to the domestic pig. Curr. Probl. Dermatol. 1978, 7, 39–52. [Google Scholar] [PubMed]
- Klein, P.; Sojka, M.; Kucera, J.; Matonohova, J.; Pavlik, V.; Nemec, J.; Kubickova, G.; Slavkovsky, R.; Szuszkiewicz, K.; Danek, P.; et al. A porcine model of skin wound infected with a polybacterial biofilm. Biofouling 2018, 34, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Sinha, M.; Mathew-Steiner, S.S.; Das, A.K.; Roy, S.; Sen, C.K. Chronic wound biofilm model. Adv. Wound Care 2015, 4, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J.; Bohn, G.; Hanft, J.; Harkless, L.; Kim, P.; Lavery, L.; Schultz, G.; Wolcott, R. Wound biofilm: Current perspectives and strategies on biofilm disruption and treatments. Wounds 2017, 29, S1–S17. [Google Scholar] [PubMed]
- Westgate, S.J.; Percival, S.L.; Knottenbelt, D.C.; Clegg, P.D.; Cochrane, C.A. Chronic equine wounds: What is the role of infection and biofilms. Wounds 2010, 22, 138–145. [Google Scholar]
- Bischofberger, A.S.; Dart, C.M.; Perkins, N.R.; Kelly, A.; Jeffcott, L.; Dart, A.J. The effect of short- and long-term treatment with manuka honey on second intention healing of contaminated and noncontaminated wounds on the distal aspect of the forelimbs in horses. Veter.-Surg. 2012, 42, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Hopf, H.W.; Rollins, M.D. Wounds: An overview of the role of oxygen. Antioxid. Redox Signal. 2007, 9, 1183–1192. [Google Scholar] [CrossRef]
- Alhede, M.; Bjarnsholt, T.; Jensen, P.; Phipps, R.K.; Moser, C.; Christophersen, L.; Christensen, L.D.; Van Gennip, M.; Parsek, M.; Høiby, N.; et al. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 2009, 155, 3500–3508. [Google Scholar] [CrossRef]
- Nadzir, N.A.A.; Zakaria, Z.; Adzahan, N.M.; Mayaki, A.M. Antibiotic susceptibilities of biofilm producing bacteria isolated from horse wounds. Explor. Anim. Med. Res. 2020, 10, 42–49. [Google Scholar]
- Johani, K.; Malone, M.; Jensen, S.; Gosbell, I.; Dickson, H.; Hu, H.; Vickery, K. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. Int. Wound J. 2017, 14, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.C.; Khansa, I.; Gordillo, G.M. A formidable foe is sabotaging your results: What you should know about biofilms and wound healing. Plast. Reconstr. Surg. 2017, 139, 1184e–1194e. [Google Scholar] [CrossRef]
- Percival, S.; Vuotto, C.; Donelli, G.; Lipsky, B.A. Biofilms and wounds: An identification algorithm and potential treatment options. Adv. Wound Care 2015, 4, 389–397. [Google Scholar] [CrossRef]
- Thomsen, T.R.; Aasholm, M.S.; Rudkjøbing, V.B.; Saunders, A.M.; Bjarnsholt, T.; Givskov, M.; Kirketerp-Møller, K.; Nielsen, P.H. The bacteriology of chronic venous leg ulcer examined by culture-independent molecular methods. Wound Repair Regen. 2010, 18, 38–49. [Google Scholar] [CrossRef]
- Price, L.B.; Liu, C.M.; Frankel, Y.M.; Melendez, J.H.; Aziz, M.; Buchhagen, J.; Contente-Cuomo, T.; Engelthaler, D.M.; Keim, P.S.; Ravel, J.; et al. Macroscale spatial variation in chronic wound microbiota: A cross-sectional study. Wound Repair Regen. 2010, 19, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Moser, C.; Abu Al-Soud, W.; Sørensen, S.; Høiby, N.; Nielsen, P.H.; Thomsen, T.R. Culture-dependent and -independent investigations of microbial diversity on urinary catheters. J. Clin. Microbiol. 2012, 50, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef]
- Hill, K.; Davies, C.; Wilson, M.; Stephens, P.; Harding, K.; Thomas, D. Molecular analysis of the microflora in chronic venous leg ulceration. J. Med. Microbiol. 2003, 52, 365–369. [Google Scholar] [CrossRef]
- Penterman, J.; Nguyen, D.; Anderson, E.; Staudinger, B.J.; Greenberg, E.P.; Lam, J.S.; Singh, P.K. Rapid evolution of culture-impaired bacteria during adaptation to biofilm growth. Cell Rep. 2014, 6, 293–300. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, S.; Nelson, E.A.; Golder, S.; Dalton, J.E.; Craig, D.; Iglesias, C.; on behalf of the DASIDU Steering Group. Systematic review of methods to diagnose infection in foot ulcers in diabetes. Diabet. Med. 2006, 23, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.E.; Frantz, R.A.; Saltzman, C.L.; Hillis, S.; Park, H.; Scherubel, M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen. 2006, 14, 548–557. [Google Scholar] [CrossRef]
- Rondas, A.A.L.M.; Schols, J.M.G.A.; Halfens, R.J.G.; Stobberingh, E.E. Swab versus biopsy for the diagnosis of chronic infected wounds. Adv. Ski. Wound Care 2013, 26, 211–219. [Google Scholar] [CrossRef]
- Levine, N.S.; Lindberg, R.B.; Mason, A.D.; Pruitt, B.A. The quantitative swab culture and smear: A quick, simple method for determining the number of viable aerobic bacteria on open wounds. J. Trauma Inj. Infect. Crit. Care 1976, 16, 89–94. [Google Scholar] [CrossRef]
- Bonham, P.A. Swab cultures for diagnosing wound infections: A literature review and clinical guideline. J. Wound Ostomy Cont. Nurs. 2009, 36, 389–395. [Google Scholar] [CrossRef]
- Saegeman, V.; Flamaing, J.; Muller, J.; Peetermans, W.E.; Stuyck, J.; Verhaegen, J. Clinical evaluation of the Copan ESwab for methicillin-resistant Staphylococcus aureus detection and culture of wounds. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 943–949. [Google Scholar] [CrossRef]
- Kalan, L.R.; Meisel, J.S.; Loesche, M.; Horwinski, J.; Soaita, I.; Chen, X.; Uberoi, A.; Gardner, S.E.; Grice, E.A. Strain- and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe 2019, 25, 641–655.e5. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2015, 24, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Burmølle, M.; Thomsen, T.R.; Fazli, M.M.; Dige, I.; Christensen, L.; Homøe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef]
- Malic, S.; Hill, K.E.; Hayes, A.; Percival, S.; Thomas, D.; Williams, D. Detection and identification of specific bacteria in wound biofilms using peptide nucleic acid fluorescent in situ hybridization (PNA FISH). Microbiology 2009, 155, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S. Topical Wound Treatments and Wound-Care Products. In Equine Wound Management; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2017; pp. 75–103. [Google Scholar]
- Coenye, T.; Kjellerup, B.; Stoodley, P.; Bjarnsholt, T.; Biofilm Bash, P. The future of biofilm research—Report on the ‘2019 Biofilm Bash’. Biofilm 2019, 2, 100012. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, G.; Schultz, G.; Kitamura, A.; Minematsu, T.; Akamata, K.; Suga, H.; Kurita, M.; Hayashi, C.; Sanada, H. Rapid detection of biofilm by wound blotting following sharp debridement of chronic pressure ulcers predicts wound healing: A preliminary study. Int. Wound J. 2019, 17, 191–196. [Google Scholar] [CrossRef]
- Ashrafi, M.; Novak-Frazer, L.; Bates, M.; Baguneid, M.; Alonso-Rasgado, T.; Xia, G.; Rautemaa-Richardson, R.; Bayat, A. Validation of biofilm formation on human skin wound models and demonstration of clinically translatable bacteria-specific volatile signatures. Sci. Rep. 2018, 8, 9431. [Google Scholar] [CrossRef]
- Dowd, S.; Wolcott, R.; Kennedy, J.; Jones, C.; Cox, S. Molecular diagnostics and personalised medicine in wound care: Assessment of outcomes. J. Wound Care 2011, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Howell-Jones, R.S.; Wilson, M.J.; Hill, K.E.; Howard, A.J.; Price, P.E.; Thomas, D. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J. Antimicrob. Chemother. 2005, 55, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. Executive summary: 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infectionsa. Clin. Infect. Dis. 2012, 54, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Edstrom, L.E.; Krizek, T.J.; Groskin, M.G. The efficacy of systemic antibiotics in the treatment of granulating wounds. J. Surg. Res. 1974, 16, 299–306. [Google Scholar] [CrossRef]
- McCullough, C.J.; Wheeler, M.H.; Neale, M.L.; Heard, G.E. The influence of penicillin on experimental wound contamination with staphylococci: Studies with chromic catgut and monofilament nylon closure. BJS 1977, 64, 120–124. [Google Scholar] [CrossRef]
- Wolcott, R.; Rumbaugh, K.; James, G.; Schultz, G.; Phillips, P.; Yang, Q.; Watters, C.; Stewart, P.; Dowd, S. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J. Wound Care 2010, 19, 320–328. [Google Scholar] [CrossRef]
- Malone, M.; Swanson, T. Biofilm-based wound care: The importance of debridement in biofilm treatment strategies. Br. J. Community Nurs. 2017, 22, S20–S25. [Google Scholar] [CrossRef]
- Edwards, J.; Stapley, S. Debridement of diabetic foot ulcers. Cochrane Database Syst. Rev. 2010, 2010, CD003556. [Google Scholar] [CrossRef]
- Elraiyah, T.; Domecq, J.P.; Prutsky, G.; Tsapas, A.; Nabhan, M.; Frykberg, R.G.; Hasan, R.; Firwana, B.; Prokop, L.J.; Murad, M.H. A systematic review and meta-analysis of débridement methods for chronic diabetic foot ulcers. J. Vasc. Surg. 2016, 63, 37S–45S.e2. [Google Scholar] [CrossRef]
- Skärlina, E.M.; Wilmink, J.M.; Fall, N.; Gorvy, D.A. Effectiveness of conventional and hydrosurgical debridement methods in reducing Staphylococcus aureus inoculation of equine musclein vitro. Equine Veter.-J. 2014, 47, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, S.; James, G.; Goeres, D.; Bjarnsholt, T.; Vickery, K.; Percival, S.; Stoodley, P.; Schultz, G.; Jensen, S.; Malone, M. The efficacy of topical agents used in wounds for managing chronic biofilm infections: A systematic review. J. Infect. 2019, 80, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.L.; Ashton, N.M.; Elce, Y.A.; Hammond, A.; Hollis, A.R.; Quinn, G. BEVA primary care clinical guidelines: Wound management in the horse. Equine Veter.-J. 2020, 53, 18–29. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Jensen, P.; Nielsen, A.K.; Johansen, H.K.; Homøe, P.; Høiby, N.; Givskov, M.; Kirketerp-Møller, K. Antibiofilm properties of acetic acid. Adv. Wound Care 2015, 4, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.H.; Cunningham, M. Biofilms in chronic wounds and the potential role of negative pressure wound therapy: An integrative review. J. Wound Ostomy Cont. Nurs. 2013, 40, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, L.; Han, L.; Wang, G.; Yin, P.; Li, Z.; Zhang, L.; Guoqi, W.; Liu, D.; Tang, P. Early application of negative pressure wound therapy to acute wounds contaminated with Staphylococcus aureus: An effective approach to preventing biofilm formation. Exp. Ther. Med. 2016, 11, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M. Interference of Pseudomonas aeruginosa signalling and biofilm formation for infection control. Expert Rev. Mol. Med. 2010, 12, e11. [Google Scholar] [CrossRef]
- Burian, E.A.; Sabah, L.; Kirketerp-Møller, K.; Ibstedt, E.; Fazli, M.M.; Gundersen, G. The safety and antimicrobial properties of stabilized hypochlorous acid in acetic acid buffer for the treatment of acute wounds—A human pilot study and in vitro data. Int. J. Low. Extrem. Wounds 2021. [Google Scholar] [CrossRef]
- Bourély, C.; Cazeau, G.; Jarrige, N.; Leblond, A.; Madec, J.; Haenni, M.; Gay, E. Antimicrobial resistance patterns of bacteria isolated from dogs with otitis. Epidemiol. Infect. 2019, 147, e121. [Google Scholar] [CrossRef]
- Woo, K.Y.; Sibbald, R.G. A cross-sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manag. 2009, 55, 40. [Google Scholar]
- Haalboom, M.; Blokhuis-Arkes, M.H.; Beuk, R.J.; Klont, R.; Guebitz, G.; Heinzle, A.; Van Der Palen, J. Wound swab and wound biopsy yield similar culture results. Wound Repair Regen. 2018, 26, 192–199. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jørgensen, E.; Bjarnsholt, T.; Jacobsen, S. Biofilm and Equine Limb Wounds. Animals 2021, 11, 2825. https://doi.org/10.3390/ani11102825
Jørgensen E, Bjarnsholt T, Jacobsen S. Biofilm and Equine Limb Wounds. Animals. 2021; 11(10):2825. https://doi.org/10.3390/ani11102825
Chicago/Turabian StyleJørgensen, Elin, Thomas Bjarnsholt, and Stine Jacobsen. 2021. "Biofilm and Equine Limb Wounds" Animals 11, no. 10: 2825. https://doi.org/10.3390/ani11102825
APA StyleJørgensen, E., Bjarnsholt, T., & Jacobsen, S. (2021). Biofilm and Equine Limb Wounds. Animals, 11(10), 2825. https://doi.org/10.3390/ani11102825





