Simple Summary
Fat content and the degree of fatty acid unsaturation in meat are two major concerns for consumers. Fat concentration and its molecular structure (fatty acid positional distribution) are related to the nutritional fat value and tissue rheological properties. Changes in fat concentration and/or fatty acid profile related to modifications of dietary treatments are well described in the literature. Nevertheless, studies aimed to control fatty acid positional distribution by dietary intervention in pigs are scarce, and studies have shown that the internal sn-2 position is highly regulated and resistant to dietary manipulation. However, this study demonstrated that heavy pigs fed on free-range with high levels of oleic acid can alter the fatty acid composition of the internal position of the triglyceride, thus affecting the nutritional value of their fat as well as their physicochemical properties.
Abstract
The nutritional value of fat consumption depends on both the fatty acid composition and the positional distribution of fatty acids within the triglyceride molecule. This research studies the effect of feeding with three different diets (4% lard-enriched; 11.5% high-oleic sunflower-enriched; and extensive feeding mainly with acorns) on the composition of fatty acids in the sn-2 position (and sn-1,3) of triglycerides and the textural properties of subcutaneous fat in heavy Iberian pigs (n = 210 castrated males). A moderate dietary enrichment with oleic acid in mixed diets did not alter the regulation of the sn-2 position of triglyceride (69.9% and 13.9% of palmitic and oleic acids, respectively), but the extremely high intake of oleic acid in pigs fed mainly on acorns changed the proportions of palmitic and oleic acids at the sn-2 position in the subcutaneous fat of pigs (55.0% and 27.2%, respectively). Hardness, adhesiveness, cohesiveness, gumminess, and chewiness showed the least values in EXT pigs, and the greatest values in LARD-fed barrows. SUN cohesiveness and gumminess did not differ from those fed LARD. In addition, Iberian pigs raised in free-range conditions had a more favorable nutritional lipid profile for human health compared to pigs fed conventional diets.
1. Introduction
Dietary fats are the main energy supply in pig diets and other monogastric animals, but their utilization for metabolic purposes depends on its absorption in the digestive system [1]. Different factors may affect their absorption and the fatty acid composition of the triglyceride structure [2]. Studies show that the external position in sn-1 and sn-3 in the triglyceride may impair fat absorption when compared to sn-2, which may affect its energy value [3]. Pork fat, compared to other animal and vegetable fats and oils, present saturated fatty acids (SFA) preferentially located in the internal sn-2 position of the triacylglycerol molecule (TAG), reaching a concentration over 75 g SFA/100 g of total fatty acids [4]. This is considered a drawback for the nutritional value of pig meat due to the association between human diets with high SFA concentration in this position and obesity and cardiovascular diseases [5,6]. Moreover, the technological properties of pig fat are affected by the fatty acid (FA) distribution within the sn-2 position [7,8]. Therefore, greater fat consistency has been associated with an increase in the sn-1,3 position of SFA, which could be an interesting approach in order to control an excess of soft fat in specific pig genotypes.
Studies aimed to control the FA distribution by dietary intervention in pigs are scarce and evidence that the FA composition in the internal sn-2 position is highly regulated and resistant to dietary manipulation [7,9], thus maintaining a narrow range of variation. Accordingly, previous studies in pigs evaluated the effects, such as dietary glycerol or saturated diets, that resulted in limited modifications at the sn-2 position in the TAG [10]. Other dietary supplementations, such as the use of compounds that reduce the Δ9-desaturase enzyme activity, have been related to an increase in the proportion of saturated FA at the sn-1,3 position [9]. There is no further information on the effects of the feeding with other kinds of fats or natural resources on the TAG structure. However, since a relationship between the high monounsaturated supply [11] and outdoors feeding system [12], and the increase in certain desaturases was found, their possible effect on the triglyceride structure deserves to be further explored.
Traditional feeding of Iberian pigs involves the intake of natural resources, mainly acorns and pasture. Acorns provide high-levels of monounsaturated fatty acids (MUFA), particularly oleic acid. Therefore, leading to an extremely high concentration of MUFA in pig tissues (over 55 g oleic acid/100 g total FA) [13,14]. Iberian pigs are slaughtered at high weights and studies have shown a particular lipid metabolism with increased activities of both lipogenic and desaturase enzymes [15,16]. However, there is a lack of information on how the metabolic pathways of heavy Iberian pigs fed different fat sources may affect the TAG structure. Concurrently with this line and within the industry interest of mimicking meat quality from the outdoor feeding system, monounsaturated fats have been used in the indoor dry feed. Iberian lard has been used in a traditional way as a good source of MUFA for a long time until the appearance of modified sunflower oils, that not only provide a high content of oleic acid, but also provide a low SFA in the same way as the resources that the pig consumes in free-range [14].
It was hypothesized that feeding heavy pigs with high levels of MUFA can alter the FA composition of the internal position of the TAG, thus affecting the nutritional value of their fat as well as their physicochemical properties. The objectives were to (1) study the effect of a wide range of dietary FA (saturated or monounsaturated-mixed diets indoors and monounsaturated outdoors from natural resources) given to Iberian pigs on the triglyceride structure; and (2) study the effect of the FA positional distribution changes on the rheological properties of the subcutaneous fat.
2. Materials and Methods
2.1. Experimental Design
Castrated males (n = 210) from the mating of purebred Iberian dams mated to Iberian × Duroc sires were selected at a live weight of 87.5 ± 5 kg and allocated at random to one of the three dietary regimens: (1) Indoors feeding with a 4%-lard-enriched diet (LARD) (8 pens; 10 pigs/pen); (2) Indoors feeding with a 11.5%-high-oleic-sunflower-enriched diet (SUN) (8 pens; 10 pigs/pen); (3) Extensive feeding in free-range conditions with acorns and grass (EXT) (n = 50). The indoor pigs were restricted until 87.5 kg (8 months old) and then fed ad libitum in the final fattening phase with the specific diet, according to the normal productive practices in Iberian pig production [17] (Table 1 and Table 2). Outdoor pigs were fed according to the traditional production practices in extensive conditions. This group is considered a reference in terms of maximum quality standards aimed at obtaining high-quality meat products. In this case, following the indications of the quality standard [17], the pigs received a longer period of restriction, in order to reach a weight of 90 kg at 12 months. The last fattening period was carried out in the Mediterranean forest with acorns and grass [13,14,18]. Food intake was not measured in free-range pigs since it is difficult to measure in outdoor conditions. However, the weight of the pigs was taken at different intervals in order to know the amount of feed to provide to indoor groups and to achieve a similar growth rate to the pigs fed under free-range conditions [12,14,18]. Water was provided ad libitum. At a live weight of approximately 150 ± 7 kg, 8 animals (castrated males) from each dietary treatment were slaughtered by electrical stunning and exsanguinated at a local abattoir (Mataderos Salamanca S.L., Mozárbez, Salamanca, Spain; certified under the Spanish Quality Standard for the Iberian pig R.D. 4/2014) [17]. At slaughter, back fat samples were taken at the level of the last rib and frozen under liquid N2. Samples were transported and kept at −80 °C until analysis (within 1 month).

Table 1.
Diet composition.

Table 2.
Triacylglyceride (TAG), position 2 (sn-2), and position 1 and/or 3 (sn-1,3) fatty acid composition (g/100 g quantified fatty acids) of fat sources (lard, high-oleic sunflower oil and acorns) used in the experiment.
2.2. Fat Extraction and Triacylglyceride Purification
The total lipids of the subcutaneous fat were extracted from 1 g of the outer layer [19]. TAG were purified by thin-layer chromatography (TLC) on 0.25 cm-thick silica gel plates that were developed with hexane:ethyl ether:acetic acid (75:25:1 by volume) using 30 µL of total lipids. To detect the position of the TAG, the TLC plates were sprayed with a 0.05% solution of primuline in acetone:water (8:2 by volume). Then, TAG fractions were scraped off the plates and eluted from silica with hexane:diethyl ether (95:5 by volume). In each case, the samples of purified TAG were analyzed by both gas chromatography (GC) to quantify the fatty acid profile and by lipase hydrolysis to determine the TAG structure [4].
2.3. Fatty Acid Profile of Subcutaneous Fat
Fatty acid methyl esters (FAME) were obtained from isolated lipids by heating the samples at 80 °C for 1 h in 3 mL of methanol:toluene:H2SO4 (88:10:2 by volume), according to the procedures outlined by Garcés and Mancha [20]. After cooling, 1 mL of hexane was added and the samples were mixed. The upper phase was recovered and the FAME were separated and quantified using a gas chromatograph (HP 6890 Series GC System, Agilent, Avondale, PA) equipped with a flame ionization detector. Separation was performed with a J&W GC Column, Innowax Polyethylene Glycol (30 m × 0.316 mm × 0.25 μm, Hewlett Packard). After injection of 5 μL, the oven temperature was raised from 170 to 210 °C at a rate of 3.5 °C/min, then to 250 °C at a rate of 7 °C/min and held constant for 1 min. The flame ionization, injector, and detector were held at 250 °C. N2 was used as the carrier gas, no split ratio was used, and FAME peaks were identified by comparing retention times with those of authentic standards (Sigma-Aldrich, Alcobendas, Spain).
2.4. Triacylglyceride Structure Analysis
For the positional analysis of TAG sn-2 FA, 10 mg of the purified TAG (see above) were hydrolyzed with 2 mg of pancreatic lipase in a 1 mL Tris–HCl buffer (1 M, pH 8), 0.1 mL of 22% CaCl2, and 0.25 mL of 0.1% deoxycholate. The reaction was stopped when approximately 60% of the TAG were hydrolyzed (1 to 2 min) by adding 0.5 mL HCl 6 N. The lipids were extracted 3 times with 1.5-mL aliquots of ethyl ether, and the reaction products were separated by TLC, as described previously. The free fatty acids (FFA) band, representing the positions 1 and 3 (sn-1,3), and the sn-2-monoacylglycerol band, representing the position 2 (sn-2) of TAG, were scraped off the plate and transmethylated [4]. The validity of the procedure was confirmed by comparing the FA composition of the original TAG with those remaining after the partial hydrolysis.
2.5. Melting Point of Subcutaneous Fat
The melting point, as determined by the slip point temperature, was performed in triplicate. Briefly, the lipids were drawn 1 cm into capillary tubes while still warm, and the tubes were stored at 4 °C overnight. Then, the tubes were placed vertically in a chilled water bath, the water temperature was increased gradually (2 °C/min), and the temperature at which the lipid began to move up the capillary tube was recorded (ISO 6321:2002) [21].
2.6. Texture Profile Analysis
The texture profile analysis (TPA) was carried out using a TA.XT2i SMS Stable Micro Systems Texture Analyzer (Stable Microsystems Ltd., Surrey, England) with the Texture Expert programs. Textural tests were carried out at about 22 °C. Briefly, 4 cylinders (1 cm height and 1.5 cm diameter) were prepared from each subcutaneous sample. A double compression cycle test was performed up to 50% compression of the original portion height with a 2 cm diameter aluminum cylinder probe (5 s were allowed to elapse between the two compression cycles). Force-time deformation curves were obtained with a 30 kg load cell applied at a crosshead speed of 2 mm/s. The following parameters were quantified [22]: Hardness (N) = maximum force required to compress the sample; springiness (m) = ability of the sample to recover its original form after the deforming force was removed; adhesiveness (N × s) = area under the abscissa after the first compression; cohesiveness = extent to which the sample could be deformed prior to rupture; gumminess (N) = force to disintegrate a semisolid meat sample for swallowing (hardness × cohesiveness); and chewiness (J) = work required to masticate the sample before swallowing (hardness × cohesiveness × springiness).
2.7. Statistical Analysis
The chemical and TPA analyses were carried out by triplicate. Response data were analyzed as a completely randomized design, two-way ANOVA, in PROC GLM of SAS v. 9.4 (SAS Institute, Inc., Cary, NC, USA, 2014) [23], with the dietary treatment as the main effect in the model. The least squares means were computed, and Duncan’s test was used to separate the means at a p < 0.05.
3. Results and Discussion
The dietary FA composition and positional distribution are important determinants in FA digestion and absorption [2]. Marked differences in FA positional distribution were found between the different fat sources of the diets (lard and high-oleic sunflower oil or acorns) (Table 2). Lard was characterized by having approximately 47.1% of oleic acid (C18:1n-9) mostly located in sn-1,3; 23.6% of palmitic acid (C16:0) almost fully occupying the sn-2 position (68.9%), and similar concentrations (12-14%) of stearic and linoleic acids (C18:0 and C18:2n-6, respectively) located in a 2:1 ratio external vs. internal position. High-oleic sunflower oil and acorns had a similar concentration of C18:1n-9 (59.7%), which was located in sn-1,3 (sunflower oil) and in sn-2 (acorns), whereas C16:0 was fully located in sn-1,3 of acorns and randomly distributed in high-oleic sunflower oil. In addition, C18:0 and C18:2n-6 were also located in a randomized way in high-oleic sunflower oil, and acorns had the lowest concentration of C18:0 in the sn-1,3 position and the highest concentration of C18:2n-6, mostly found at the sn-2 location. Previous studies reported high proportions of sn-2 C16:0 in lard [8,24] and high sn-2 C18:1n-9 in high-oleic sunflower oil [25]. However, there is scarce information on the triglyceride structure of fat from acorns. Mattson and Volpenhein [26] found that acorns had high proportions of sn-2 C18:1n-9 and C18:2n-6, which agrees with the results observed in the present study, but there is no further evidence to our knowledge.
The effect of dietary treatment on the FA of TAG is presented in Table 3. Proportions of C14:0, C16:0, C18:0, as well as the total SFA and SFA/PUFA index, were less (p < 0.05) in subcutaneous fat of EXT pigs than those of the LARD- and SUN-fed groups (Table 3). Subcutaneous fat from pigs fed the LARD and SUN diets had greater (p < 0.05) proportions of palmitoleic acid (C16:1n-7), but lower proportions of eicosenoic acid (C20:1n-9) (p = 0.015), than the EXT pigs. Fat samples from EXT pigs had the greatest (p < 0.05) proportions of MUFA and UI, especially C18:1n-9, whereas the SUN-fed group had intermediate values. Similar high proportions of monounsaturated fatty acids mainly C18:1n-9 have been reported by other authors when heavy pigs were fed in extensive conditions with acorns in comparison to those fed a diet enriched with lard [27] or a fat mixture rich in monounsaturated fatty acids (lard + olive oil oleine) [18]. In addition, proportions of all PUFA, and specifically linoleic (C18:2n-6) and linolenic (C18:3n-3) acids, were greater (p < 0.05) in the fat from EXT pigs than fat from SUN- and LARD-fed ones. Other authors [18] reported similar results when free-range pigs were compared to the group receiving a monounsaturated-enriched diet. However, Ventanas et al. [17] reported lower PUFA in those pigs fed extensively when compared to others fed lard or a MUFA-enriched diet. Changes in the PUFA accumulation in tissues between studies may be attributed to the duration of the free-range feeding, since lower periods outdoors have resulted in higher PUFA proportions in subcutaneous fat [14]. Moreover, the PUFA proportion may be affected not only by diet, but also by other factors such as the different metabolic use by the pig, since a preferential use of this kind of fatty acids for energy supply has been reported [28].

Table 3.
Effect of dietary treatments on the fatty acid (g/100 g quantified fatty acids) profile of triacylglycerides of subcutaneous fat.
The positional distribution of FA within the TAG molecule of pig subcutaneous fat is shown in Table 4. In contrast to other species, the sn-2 in the TAG of pig adipose tissue was occupied mainly by C16:0, C14:0, C16:1n-7, and the SFA and SFA/PUFA index (p = 0.0001), as similarly described by other authors [29,30]. C18:0 was mainly esterified at the sn-1,3 of the TAG (p = 0.0001), as well as C18:1n-9 (p = 0.0001), C18:2n-6 (p = 0.0001), C18:3n-3 (p = 0.0001), C20:1n-9, and the UI index (p = 0.0001). In the case of C20:0, C20:4n-6, and Σn-6/Σn-3, higher amounts were also observed in sn-1,3 than in sn-2 (p < 0.05), but no differences were detected in the total proportion of triglycerides among dietary treatments (Table 3). A similar distribution has been reported earlier in a variety of pig tissues [8,31], human milk substitutes [32,33], plasma, and milk of rats and rabbits [34], thereby indicating that FA are not randomly esterified to the glycerol hydroxyl groups in animal fats.

Table 4.
Triacylglyceride sn-2 and sn-1,3 fatty acid composition (g/100 g quantified fatty acids) profile of subcutaneous fat. Effect of dietary treatment (D), positional distribution (P), and the interaction (D × P).
The increase of C18:1n-9 proportion in the SUN treatment implied a slight decrease of C16:0 (p = 0.0045) and SFA (p = 0.0001) in the sn-1,3 position of subcutaneous fat that did not result in changes in C16:0 or SFA sn-2 when compared to the fat samples from pigs fed lard-enriched diet. Whereas, the SUN group showed lower C18:0 accumulation in the sn-2 position (p = 0.0001) when compared to the subcutaneous fat from the lard-enriched group. The decrease in the C18:0 sn-2 proportion in fat was also observed in pigs from the EXT group when compared to fat from the LARD group in the present study. Although the change implied only around 1% variation, it has been described that the mentioned obesity and/or cardiovascular diseases related to SFA are truly related to the digested/absorbed C18:0, therefore dependent on the C18:0 concentration in the sn-2 position [5,6]. A previous dietary intervention has also been proven to alter FA in the external sn-1,3 position of the TAG rather than the sn-2 location. Smith et al. [7] observed that the depressing desaturase enzyme activity increased the concentration of C18:0 located in the external sn-1,3 position in bovine adipose tissue, but had no appreciable effect on sn-2. In pigs, Segura et al. [10] observed that the substitution of lard by palm oil as a dietary fat only produced slight changes in sn-1,3.
The main changes by dietary manipulations on the triglyceride structure in the present study were detected in the group fed in extensive conditions mainly based on the acorns intake. Therefore, pigs fed EXT had lower C14:0, C16:0, C18:0, C20:0, and SFA (p < 0.05) in the sn-2 position compared to those fed LARD or SUN diets. A decrease was also observed in sn-1,3 of SFA in the EXT group when compared to the others but changes were of lower magnitude that those observed for the sn-2 position. Moreover, the EXT group had an increase in sn-2 MUFA (mainly C18:1n-9) (p = 0.0001) and in less magnitude in sn-1,3 when compared to LARD or SUN. The different changes in the response to the positional distribution according to diets were confirmed by the statistically significant interaction effect (Table 4, Figure 1). Therefore, fat from EXT pigs showed a higher decrease in C14:0 and C16:0 sn-2 and more substantial increase in C18:1n-9 sn-2 than the other groups (p < 0.05). Other fatty acids (C18:2n-6, C18:3n-3, C20:0, C20:3n-6, and C20:4n-6) did not show any interaction effect depending on the dietary treatment and positional distribution.
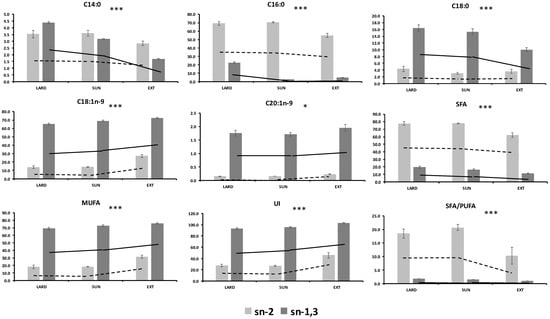
Figure 1.
Interaction Treatment × Position of main fatty acid positional distribution. * (p < 0.05); *** (p < 0.0001) of the interaction Treatment × Position. LARD: Iberian × Duroc (IB × DR) pigs reared indoors and fed with lard as a fat source. SUN: IB × DR pigs reared indoors and fed on a diet containing high-oleic sunflower oil (115 g/kg of diet). EXT: IB × DR breed pigs free-range reared and exclusively fed on acorns and grass according to the traditional feeding system. sn-2 (in 2-position of triglyceride), sn-1,3 (average of 1- and 3-position of triglyceride). SFA (saturated fatty acids) = C14:0 + C15:0 + C16:0 + C17:0 + C18:0 + C20:0, MUFA (monounsaturated fatty acids) = C16:1n-7 + C17:1 + C18:1n-9 + C10:1n-9, UI (unsaturation index) = [(C16:1n-7 + C17:1 + C19:1n-9 + C20:1n-9) + 2 × (C18:2n-6) + 3 × (C18:3n-3 + C20:3n-6) + 4 × C20:4n-6].
The predominant TAG structure accumulated in pig subcutaneous fat was partly related to the TAG found in the diets. Hunter [35], Mu and Porsgaard [36], and Innis [33] reported that the FA located at the sn-2 position suffer little alteration during digestion, with approximately 70% of the FA located in this position conserved in chylomicrons. Innis and Dyer [37] provided diets differing in the total FA composition and distribution within the TAG, and reported a limited effect of dietary treatment on liver lipid in piglets, suggesting the metabolic regulation of FA composition at the sn-2 position. Moreover, the presence of C16:0 in sn-2 of the dietary TAG in human infants and piglets during lactation seems to be of importance for the adequate development of the organism [33,38]. Gastric and pancreatic lipases hydrolyze FA from the external positions of the TAG. With the positional distribution of dietary fat sources used in the present experiment, the formation of FFA and 2-monoglycerides during the digestion of fat is markedly different depending on the dietary fat [30], thus the reassembly of the TAG could not be convergent.
The increase of TAG C18:2n-6 in fat from the EXT group when compared to the others, was reflected equally in both positions in the present study, thus implying a high regulation to keep a constant ratio between the external and internal position amounts of this FA. Couëdelo et al. [39] reported that the amount of linoleic acid moved from the sn-2 position of structured TAG to the sn-1,3 increased during absorption. Further research is needed to clarify whether this finding on the C18:2n-6 positional structure was completely due to special feeding conditions or to any other aspect of importance in outdoor production.
The effect of dietary treatments on subcutaneous fat moisture, melting point, and textural characteristics is presented in Table 5. The moisture content was higher in fat from the LARD group followed by the SUN and EXT groups. The decrease of fat moisture from pigs fed diets enriched with MUFA was also observed by other authors [40], who noted that the more saturated the fat was, the greater the moisture level resulted. The melting point, hardness, adhesiveness, cohesiveness, gumminess, and chewiness were least (p < 0.05) in subcutaneous fat of EXT pigs, and greatest (p < 0.05) in fat samples from LARD-fed barrows. Cohesiveness and gumminess of fat from pigs fed SUN-enriched diets did not differ from those fed LARD, whereas fat from LARD-fed pigs had greater (p < 0.05) chewiness values than the other groups.

Table 5.
Effect of dietary treatments on moisture, melting point, and textural parameters of pig subcutaneous fat 1.
The different responses of the melting point in subcutaneous fat from heavy pigs according to dietary fats has been reported previously [41]. In fact, the C18:0 content [42] and the relationship between MUFA and SFA [43] have been considered the best predictors of the melting point. In a more detailed study [8], in which the effect of positional distribution within the TAG molecule on selected physical properties of subcutaneous fat of dry-cured hams was analyzed, the melting point oscillations were related to the concentration and positional distribution of FA. Therefore, when C16:0 is preponderant in sn-2, the melting point depends largely on the FA present in sn-1,3. In the present study, an increase of slip point was observed when C16:0 or C18:0 were located in sn-1,3 (LARD-fed pigs) and a decrease when C18:1n-9 was increased in this position. However, when the C18:1n-9 concentration at sn-2 increased, the increase of C18:1n-9 and C18:2n-6 (and no C18:0) in sn-1,3 caused the slip point to decrease in fat from the SUN-fed and EXT pigs.
Concerning fat hardness, other authors reported that higher proportions of C18:0 and lower proportions of C18:2n-6 fatty acids led to a harder fat [43]. Segura et al. [8] also observed that hardness was correlated with the FA of external positions of the TAG molecule. These authors reported positive correlations of hardness with C16:0, C18:0, and SFA in the sn-1,3 position and negative correlations when C18:2n-6 and total PUFA occupied sn-1,3. In the present study, the lowest hardness values in fat from EXT pigs was coincident with the lowest level of C18:0 and the highest levels of C18:1n-9 and C18:2n-6 in sn-1,3, whereas the opposite was observed in the fat of LARD-fed pigs. Between the fat from LARD and SUN-fed pigs, the only differences in sn-1,3 were found for C18:1n-9, total MUFA, and total SFA proportions, which could explain the changes observed on hardness between these groups.
Other textural parameters have also been related with the fatty acid profile and specific triglyceride structure. In fat of bovine kidney, Casutt et al. [44] and Nishioka and Irie [45] found that greater adhesiveness was associated with higher proportions of SFA or C18:2n-6. Furthermore, Segura et al. [8] observed that adhesiveness was dependent on the FA proportion in sn-2. A positive correlation was found specifically with C18:0 and C18:2n-6 sn-2, and was inversely proportional to the proportion of C18:1n-9 at sn-2. Therefore, the decreased adhesiveness of fat from EXT pigs found in the present study may be explained by the increase of C18:2n-6 and the decrease of SFA in sn-2.
A direct dependence of springiness and cohesiveness, and FA or triglyceride structure has not been clarified in our results. In fact, Sumena et al. [46] concluded that the contribution to the texture features, mainly cohesiveness, was the network of predominantly collagen and small quantities of elastic and reticular fibers, and not the adipocyte composition itself.
4. Conclusions
The sn-2 position of the triacylglyceride in pig subcutaneous fat is highly regulated and concentrates C16:0 around 70 g/100 g fatty acids. A moderate dietary enrichment with C18:1n-9 in mixed diet produce slight changes. However, the extremely high intake of C18:1n-9 in extensively reared pigs fed mainly on acorns surpasses this regulation, thereby modifying fatty acids in the sn-2 position. Therefore, extensive feeding increased C18:1n-9 sn-2 and decreased C16:0 sn-2 proportions when compared to the other dietary fats. Remarkably, the C18:0 proportion in sn-2 decreased with dietary treatments, but the C18:2n-6 proportion and positional distribution showed a strong regulation to invariability. Consequently, Iberian pigs raised extensively would have a more favorable lipid profile from the human health perspective and distinctive fat rheological properties compared to pigs fed mixed diets containing lard or high-oleic sunflower oil. The high differences in the triglyceride structure between groups fed high-oleic diets and extensive feeding would indicate that this analysis could be an interesting tool for free-range feeding authentication.
Author Contributions
Data curation, J.S., A.I.R., R.E. and M.D.R.d.Á.; formal analysis, J.S., A.I.R., Á.O., M.I.C., R.E., M.D.R.d.Á. and C.L.-B.; funding acquisition, C.L.-B.; investigation, J.S., A.I.R., Á.O., M.I.C. and C.L.-B.; methodology, J.S.; project administration, C.L.-B.; resources, A.P.; supervision, J.S. and C.L.-B.; validation, J.S. and A.P.; writing—original draft, J.S.; writing—review and editing, J.S., A.I.R., Á.O., M.I.C. and C.L.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This project was partially supported by MEDGAN (S2013/ABI-2913). Comunidad de Madrid and CEI Campus Moncloa UCM-UPM, 28040 Madrid, Spain.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (Research Committee) of University Complutense of Madrid.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to the Mataderos Salamanca S.L. technical staff for animal management, animal slaughter, and carcass fabrication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noblet, J.; van Milgen, J. Energy value of pig feeds: Effect of pig body weight and energy evaluation system. J. Anim. Sci. 2004, 82, E229–E238. [Google Scholar] [CrossRef]
- Mu, H.L.; Hoy, C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Bracco, U. Effect of triglyceride structure on fat absorption. Am. J. Clin. Nutr. 1994, 60, 1002S–1009S. [Google Scholar] [CrossRef]
- Segura, J.; Ruiz-López, N.; Menoyo, D.; Cambero, M.I.; López-Bote, C.J. Comparison of analytical techniques for the determination of the positional distribution of fatty acids in triacylglycerols. Relationship with pig fat melting point and hardness. Grasas Aceites 2015, 66, e076. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Lewandowski, P.; Nesaratnam, K.; Dunshea, F.R.; Gill, H. Differential effects of natural palm oil, chemically-and enzymatically-modified palm oil on weight gain, blood lipid metabolites and fat deposition in a pediatric pig model. Nutr. J. 2011, 10, 1–7. [Google Scholar] [CrossRef][Green Version]
- Gouk, S.W.; Cheng, S.F.; Mok, J.S.L.; Ong, A.S.H.; Chuah, C.H. Long-chain SFA at the sn-1, 3 positions of TAG reduce body fat deposition in C57BL/6 mice. Br. J. Nutr. 2013, 110, 1987–1995. [Google Scholar] [CrossRef]
- Smith, S.B.; Yang, A.J.; Larsen, T.W.; Tume, R.K. Positional analysis of triacylglycerols from bovine adipose tissue lipids varying in degree of unsaturation. Lipids 1998, 33, 197–207. [Google Scholar] [CrossRef]
- Segura, J.; Escudero, R.; Romero de Ávila, M.D.; Cambero, M.I.; López-Bote, C.J. Effect of fatty acid composition and positional distribution within the triglyceride on selected physical properties of dry-cured ham subcutaneous fat. Meat Sci. 2015, 103, 90–95. [Google Scholar] [CrossRef]
- King, D.A.; Behrends, J.M.; Jenschke, B.E.; Rhoades, R.D.; Smith, S.B. Positional distribution of fatty acids in triacylglycerols from subcutaneous adipose tissue of pigs fed diets enriched with conjugated linoleic acid, corn oil, or beef tallow. Meat Sci. 2004, 67, 675–681. [Google Scholar] [CrossRef]
- Segura, J.; Cambero, M.I.; Cámara, L.; Loriente, C.; Mateos, G.G.; López-Bote, C.J. Effect of sex, dietary glycerol or dietary fat during late fattening, on fatty acid composition and positional distribution of fatty acids within the triglyceride in pigs. Animal 2015, 9, 1904–1911. [Google Scholar] [CrossRef][Green Version]
- Rey, A.I.; López-Bote, C.J.; Kerry, J.P.; Lynch, P.B.; Buckley, D.J.; Morrissey, P.A. Modification of lipid composition and oxidation in porcine muscle and muscle microsomes as affected by dietary supplementation of n-3 with either n-9 or n-6 fatty acids and α-tocopheryl acetate. Anim. Feed Sci. Technol. 2004, 113, 223–238. [Google Scholar] [CrossRef]
- Daza, A.; Rey, A.I.; Olivares, A.; Cordero, G.; Toldrá, F.; López-Bote, C.J. Physical activity-induced alterations on tissue lipid composition and lipid metabolism in fattening pigs. Meat Sci. 2009, 81, 641–646. [Google Scholar] [CrossRef]
- Rey, A.I.; Daza, A.; López-Carrasco, C.; López-Bote, C.J. Quantitative study of the α- and γ-tocopherols accumulation in muscle and backfat from Iberian pigs kept free-range as affected by time of free-range feeding or weight gain. Anim. Sci. 2006, 82, 901–908. [Google Scholar] [CrossRef]
- Daza, A.; Mateos, A.; Rey, A.I.; Ovejero, I.; López-Bote, C.J. Effect of duration of feeding under free-range conditions on production results and carcass and fat quality in Iberian pigs. Meat Sci. 2007, 76, 411–416. [Google Scholar] [CrossRef]
- Daza, A.; Rey, A.I.; Menoyo, D.; Bautista, J.M.; Olivares, A.; López-Bote, C.J. Effect of level of feed restriction during growth and/or fattening on fatty acid composition and lipogenic enzyme activity in heavy pigs. Anim. Feed Sci. Technol. 2007, 138, 61–74. [Google Scholar] [CrossRef]
- Ayuso, M.; Fernández, A.; Núñez, Y.; Benítez, R.; Isabel, B.; Barragán, C.; Fernández, A.I.; Rey, A.I.; Medrano, J.F.; Cánovas, A.; et al. Comparative Analysis of Muscle Transcriptome between Pig Genotypes Identifies Genes and Regulatory Mechanisms Associated to Growth, Fatness and Metabolism. PLoS ONE 2015, 10, e0145162. [Google Scholar] [CrossRef]
- BOE. Real Decreto 4/2014, de 10 de Enero, por el que se Aprueba la Norma de Calidad para la Carne, el Jamón, la Paleta y la Caña de Lomo Ibérico; Boletín Oficial del Estado, Num.10, de 11 de Enero de 2014; Government of Spain: Madrid, Spain, 2014; pp. 1569–1585. [Google Scholar]
- Daza, A.; Rey, A.I.; Ruiz, J.; López-Bote, C.J. Effects of feeding in free-range conditions or in confinement with different dietary MUFA/PUFA ratios and α-tocopheryl acetate, on antioxidants accumulation and oxidative stability in Iberian pigs. Meat Sci. 2005, 69, 151–163. [Google Scholar] [CrossRef]
- Segura, J.; Calvo, L.; Óvilo, C.; González-Bulnes, A.; Olivares, A.; Cambero, M.I.; López-Bote, C.J. Alternative method for intramuscular fat analysis using common laboratory equipment. Meat Sci. 2015, 103, 24–27. [Google Scholar] [CrossRef]
- Garcés, R.; Mancha, M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993, 211, 139–143. [Google Scholar] [CrossRef]
- ISO 6321:2002. Animal and Vegetable Fats and Oils—Determination of Melting Point in Open Capillary Tubes (Slip Point), Second Edition, 2–15. 2002. Available online: www.iso.org/obp/ui/#iso:std:iso:6321:ed-2:v1:en (accessed on 30 May 2021).
- Romero de Ávila, M.D.; Cambero, M.I.; Ordóñez, J.A.; de la Hoz, L.; Herrero, A.M. Rheological behaviour of commercial cooked meat propducts evaluated by tensile test and texture profile analysis (TPA). Meat Sci. 2014, 98, 310–315. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS 9.4 for Windows; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Kritchevsky, D.; Tepper, S.A.; Kuksis, A.; Eghtedary, K.; Klurfeld, D.M. Cholesterol vehicle in experimental atherosclerosis. 21. native and randomized lard and tallow. J. Nutr. Biochem. 1998, 9, 582–585. [Google Scholar] [CrossRef]
- Ruiz-Gutiérrez, V.; Morgado, N.; Prada, J.L.; Pérez-Jiménez, F.; Muriana, F.J. Composition of human VLDL triacylglycerols after ingestion of olive oil and high oleic sunflower oil. J. Nutr. 1998, 128, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Mattson, F.H.; Volpenhein, R.A. The specific distribution of unsaturated fatty acids in the triglycerides of plants. J. Lipid Res. 1963, 4, 392–396. [Google Scholar] [CrossRef]
- Ventanas, S.; Tejeda, J.F.; Estévez, M. Chemical composition and oxidative status of tissues from Iberian pigs as affected by diets: Extensive feeding v. oleic acid- and tocopherol-enriched mixed diets. Animal 2008, 2, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; Menoyo, D.; Segura, J.; López-Bote, C.J.; Calvo, L. Combination of dietary glycaemic index and fasting time prior to slaughter as strategy to modify quality of pork. Meat Sci. 2020, 161. [Google Scholar] [CrossRef]
- Christie, W.W.; Moore, J.H. A comparison of structure of triglycerides from various pig tissues. Biochim. Biophy. Acta 1970, 210, 46–56. [Google Scholar] [CrossRef]
- Innis, S.M.; Nelson, C.M. Dietary triacylglycerols rich in sn-2 palmitate alter post-prandial lipoprotein and unesterified fatty acids in term infants. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W.; Moore, J.H. The variation of triglyceride structure with fatty acid composition in pig adipose tissue. Lipids 1970, 5, 921–928. [Google Scholar] [CrossRef]
- Christie, W.W.; Clapperton, J.L. Structures of the triglycerides of cows’ milk, fortified milks (including infant formulae), and human milk. Int. J. Dairy Technol. 1982, 35, 22–24. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary Triacylglycerol Structure and Its Role in Infant Nutrition. Adv. Nutr. 2011, 2, 275–283. [Google Scholar] [CrossRef]
- Christie, W.W. Structure of the triacyl-sn-glycerols in the plasma and milk of the rat and rabbit. J. Dairy Res. 1985, 52, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 2001, 36, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.L.; Porsgaard, T. The metabolism of structured triacylglycerols. Prog. Lipid Res. 2005, 44, 430–448. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Dyer, R. Dietary triacylglycerols with palmitic acid (16:0) in the 2-position increase 16:0 in the 2-position of plasma and chylomicron triacylglycerols, but reduce phospholipid arachidonic and docosahexaenoic acids, and alter cholesteryl ester metabolism in formula-fed piglets. J. Nutr. 1997, 127, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Dyer, R.; Quinlan, P.T.; Diersen-Schade, D. Dietary triacylglycerol structure and saturated fat alter plasma and tissue fatty acids in piglets. Lipids 1996, 31, 497–505. [Google Scholar] [CrossRef]
- Couëdelo, L.; Vaysse, C.; Vaique, E.; Guy, A.; Gosse, I.; Durand, T.; Pinet, S.; Cansell, M.; Combe, N. The fraction of α-linolenic acid present in the sn-2 position of structured triacylglycerols decreases in lymph chylomicrons and plasma triacylglycerols during the course of lipid absorption in rats. J. Nutr. 2012, 142, 70–75. [Google Scholar] [CrossRef]
- Ventanas, S.; Estevez, M.; Tejeda, J.F.; Ruiz, J. Protein and lipid oxidation in Longissimus dorsi and dry cured loin from Iberian pigs as affected by crossbreeding and diet. Meat Sci. 2006, 72, 647–655. [Google Scholar] [CrossRef]
- López-Bote, C.J.; Isabel, B.; Daza, A. Partial replacement of poly- with monounsaturated fatty acids and vitamin E supplementation in pig diets: Effect on fatty acid composition of subcutaneous and intramuscular fat and on fat and lean firmness. Anim. Sci. 2002, 75, 349–358. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Lea, C.H.; Swoboda, P.A.T.; Gatherum, D.P. A chemical study if sift fat ub criss-bred pigs. J. Agric. Sci. 1970, 74, 279–284. [Google Scholar] [CrossRef]
- Casutt, M.M.; Scheeder, M.R.L.; Escher, F.; Dufey, P.A.; Kreuzer, M. Relating texture properties and composition of bovine fat tissue. Fett-Lipid 1999, 101, 283–290. [Google Scholar] [CrossRef]
- Nishioka, T.; Irie, M. Evaluation method for firmness and stickiness of porcine perirenal fat. Meat Sci. 2005, 70, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Sumena, K.B.; Lucy, K.M.; Chungath, J.J.; Ashok, N.; Harshan, K.R. Regional histology of the subcutaneous tissue and the Sweat glands of large white Yorkshire pigs. Tamilnadu J. Vet. Anim. Sci. 2010, 6, 128–135. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).