Ultrasound-Guided Radiofrequency Ablation of Chemodectomas in Five Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walter, J.H.; Rudolph, R. Systemic, metastatic, eu- and heterotope tumours of the heart in necropsied dogs. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 1996, 43, 31–45. [Google Scholar] [CrossRef]
- Girard, C.; Hélie, P.; Odin, M. Intrapericardial neoplasia in dogs. J. Vet. Diagnostic Investig. 1999, 11, 73–78. [Google Scholar] [CrossRef]
- Ware, W.A.; Hopper, D.L. Cardiac tumors in dogs: 1982–1995. J. Vet. Intern. Med. 1999, 13, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Surakiatchanukul, S.; Goodsitt, E.; Storer, J. Chemodectoma of the aortic body. Chest 1971, 60, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Yates, W.D.; Lester, S.J.; Mills, J.H. Chemoreceptor tumors diagnosed at the Western College of Veterinary Medicine 1967–1979. Can. Vet. J. 1980, 21, 124–129. [Google Scholar]
- Hayes, H.M.; Sass, B. Chemoreceptor Neoplasia: A Study of the Epidemiological Features of 357 Canine Cases. J. Vet. Med. Ser. A 1988, 35, 401–408. [Google Scholar] [CrossRef]
- Guglielmini, C.; Baron Toaldo, M.; Quinci, M.; Romito, G.; Luciani, A.; Cipone, M.; Drigo, M.; Diana, A. Sensitivity, specificity, and interobserver variability of survey thoracic radiography for the detection of heart base masses in dogs. J. Am. Vet. Med. Assoc. 2016, 248, 1391–1398. [Google Scholar] [CrossRef]
- Magestro, L.M.; Gieger, T.L.; Nolan, M.W. Stereotactic body radiation therapy for heart-base tumors in six dogs. J. Vet. Cardiol. 2018, 20, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Arias-Stella, J.; Bustos, F. Chronic hypoxia and chemodectomas in bovines at high altitudes. Arch. Pathol. Lab. Med. 1976, 100, 636–639. [Google Scholar]
- Lam, S.Y.; Tipoe, G.L.; Liong, E.C.; Fung, M.L. Chronic hypoxia upregulates the expression and function of proinflammatory cytokines in the rat carotid body. Histochem. Cell Biol. 2008, 130, 549–559. [Google Scholar] [CrossRef]
- Saldana, M.J.; Salem, L.E.; Travezan, R. High altitude hypoxia and chemodectomas. Hum. Pathol. 1973, 4, 251–263. [Google Scholar] [CrossRef]
- Aupperle, H.; März, I.; Ellenberger, C.; Buschatz, S.; Reischauer, A.; Schoon, H.A. Primary and Secondary Heart Tumours in Dogs and Cats. J. Comp. Pathol. 2007, 136, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, N.; Ehrhart, E.J.; Willis, J.; Sisson, D.; Constable, P.; Greenfield, C.; Manfra-Maretta, S.; Hintermeister, J. Analysis of factors affecting survival in dogs with aortic body tumors. Vet. Surg. 2002, 31, 44–48. [Google Scholar] [CrossRef]
- Noszczyk-Nowak, A.; Nowak, M.; Paslawska, U.; Atamaniuk, W.; Nicpon, J. Cases with manifestation of chemodectoma diagnosed in dogs in Department of Internal Diseases with Horses, Dogs and Cats Clinic, Veterinary Medicine Faculty, University of Environmental and Life Sciences, Wroclaw, Poland. Acta Vet. Scand. 2010, 52, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rancilio, N.J.; Higuchi, T.; Gagnon, J.; McNiel, E.A. Use of three-dimensional conformal radiation therapy for treatment of a heart base chemodectoma in a dog. J. Am. Vet. Med. Assoc. 2012, 241, 472–476. [Google Scholar] [CrossRef]
- Vicari, E.D.; Brown, D.C.; Holt, D.E.; Brockman, D.J. Survival times of and prognostic indicators for dogs with heart base masses: 25 cases (1986–1999). J. Am. Vet. Med. Assoc. 2001, 219, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Domenech, O.; Romito, G. Surgical excision of a heart base chemodectoma in a dog. Veterinaria 2013, 27, 23–29. [Google Scholar]
- Obradovich, J.E.; Withrow, S.J.; Powers, B.E.; Walshaw, R. Carotid Body Tumors in the Dog Eleven Cases (1978–1988). J. Vet. Intern. Med. 1992, 6, 96–101. [Google Scholar] [CrossRef]
- Lew, F.H.; McQuown, B.; Borrego, J.; Cunningham, S.; Burgess, K.E. Retrospective evaluation of canine heart base tumours treated with toceranib phosphate (Palladia): 2011–2018. Vet. Comp. Oncol. 2019, 17, 465–471. [Google Scholar] [CrossRef]
- Coto, G.M.; Musser, M.L.; Tropf, M.A.; Ward, J.L.; Seo, Y.J.; Mochel, J.P.; Johannes, C.M. A Multi-Institutional Retrospective Analysis of Toceranib Phosphate for Presumed or Confirmed Canine Aortic Body Chemodectomas. Front. Vet. Sci. 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kruckman-Gatesy, C.R.; Ames, M.K.; Griffin, L.R.; Boss, M.K.; Rao, S.; Leary, D.; LaRue, S.M. A retrospective analysis of stereotactic body radiation therapy for canine heart base tumors: 26 cases. J. Vet. Cardiol. 2020, 27, 62–77. [Google Scholar] [CrossRef]
- Maeda, A.; Murakami, M.; Iwasaki, R.; Goto, S.; Kitagawa, K.; Sakai, H.; Mori, T. Three-dimensional conformal radiation therapy for canine aortic body tumour: 6 cases (2014–2019). J. Small Anim. Pract. 2021, 62, 385–390. [Google Scholar] [CrossRef]
- Bussadori, C.M.; Claretti, M.; Borgonovo, S.; Boz, E.; Papa, M.; Rossi, C.; Martelli, F.; Aimi, M.; Signorelli, S.; Marinelli, R. Branch pulmonary artery stent placement in a dog with heart base neoplasia. J. Vet. Cardiol. 2020, 30, 17–22. [Google Scholar] [CrossRef]
- Taylor, S.; Rozanski, E.; Sato, A.F.; Rush, J.E. Vascular stent placement for palliation of mass-associated chylothorax in two dogs. J. Am. Vet. Med. Assoc. 2017, 251, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Melnik, R. Thermal ablation of biological tissues in disease treatment: A review of computational models and future directions. Electromagn. Biol. Med. 2020, 39, 49–88. [Google Scholar] [CrossRef]
- Ellis, L.M.; Curley, S.A.; Tanabe, K.K. Radiofrequency Ablation: Current Indications, Techniques and Outcomes; Ellis, L.M., Curley, S.A., Tanabe, K.K., Eds.; Springer: New York, NY, USA, 2004; Volume 242, ISBN 978-0-387-21598-3. [Google Scholar]
- Wood, B.J.; Abraham, J.; Hvizda, J.L.; Alexander, H.R.; Fojo, T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer 2003, 97, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Chen, K.Y.; Chen, A.; Chen, C.N. Differences in the ultrasonographic appearance of thyroid nodules after radiofrequency ablation. Clin. Endocrinol. 2021, 95, 489–497. [Google Scholar] [CrossRef]
- Shibamoto, K.; Mimura, H.; Fukuhara, Y.; Nishino, K.; Kawamoto, H.; Kato, K. Feasibility, safety, and efficacy of artificial carbon dioxide pneumothorax for computed tomography fluoroscopy-guided percutaneous radiofrequency ablation of hepatocellular carcinoma. Jpn. J. Radiol. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Alyusuf, E.Y.; Ekhzaimy, A.A.; Rivera, J.A. Radiofrequency Ablation as a Primary Therapy for Benign Functioning Insulinoma. AACE Clin. Case Rep. 2021, 7, 153–157. [Google Scholar] [CrossRef]
- Dai, Y.; Covarrubias, D.; Uppot, R.; Arellano, R.S. Image-Guided Percutaneous Radiofrequency Ablation of Central Renal Cell Carcinoma: Assessment of Clinical Efficacy and Safety in 31 Tumors. J. Vasc. Interv. Radiol. 2017, 28, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.F.; Szejnfeld, D.; Xavier, A.C.W.; Kater, C.E.; Freire, F.; Ribeiro, C.A.; Goldman, S.M. Percutaneous ablation of functioning adrenal adenoma: A report on 11 cases and a review of the literature. Abdom. Imaging 2013, 38, 1130–1135. [Google Scholar] [CrossRef]
- Nunes, T.F.; Szejnfeld, D.; Xavier, A.C.W.; Goldman, S.M. Percutaneous ablation of functioning adenoma in a patient with a single adrenal gland. BMJ Case Rep. 2013, 2013, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Chen, T.; Yang, B.; Liu, N.; Qian, X.; Xia, B.; Feng, D.; Chen, S. Magnetic resonance imaging-guided microwave ablation for lung tumor: A case report. Quant. Imaging Med. Surg. 2021, 11, 2780–2784. [Google Scholar] [CrossRef] [PubMed]
- Rimbaș, M.; Rizzatti, G.; Larghi, A. EUS-guided ablation of pancreatic neoplasms. Minerva Gastroenterol. 2021. [Google Scholar] [CrossRef]
- Qu, C.; Li, X.-Q.; Li, C.; Xia, F.; Feng, K.; Ma, K. The Short-Term Efficacy of Novel No-Touch Combined Directional Perfusion Radiofrequency Ablation in the Treatment of Small Hepatocellular Carcinoma with Cirrhosis. J. Investig. Surg. Off. J. Acad. Surg. Res. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Koo, J.S.; Chung, S.H. The Efficacy of Radiofrequency Ablation for Bone Tumors Unsuitable for Radical Excision. Clin. Orthop. Surg. 2021, 13, 278–285. [Google Scholar] [CrossRef]
- Inoue, T.; Yoneda, M. Updated evidence on the clinical impact of endoscopic radiofrequency ablation in the treatment of malignant biliary obstruction. Dig. Endosc. Off. J. Japan Gastroenterol. Endosc. Soc. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kuroda, H.; Sakakura, N.; Sato, Y.; Chatani, S.; Murata, S.; Yamaura, H.; Nakada, T.; Oya, Y.; Inaba, Y. Novel strategy to treat lung metastases: Hybrid therapy involving surgery and radiofrequency ablation. Thorac. Cancer 2021, 12, 2085–2092. [Google Scholar] [CrossRef]
- Galletti, B.; Gazia, F.; Galletti, C.; Freni, F.; Galletti, C.; Bruno, R.; Sireci, F.; Galletti, F. Radiofrequency vs cold surgery to treat oral papillomatous lesions. Iran. J. Otorhinolaryngol. 2021, 33, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-M.; Cui, M.; Yang, W.; Wang, H.; Wang, S.; Zhang, Z.-Y.; Wu, W.; Chen, M.-H.; Yan, K.; Goldberg, S.N. The 10-year Survival Analysis of Radiofrequency Ablation for Solitary Hepatocellular Carcinoma 5 cm or Smaller: Primary versus Recurrent HCC. Radiology 2021, 300, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Pollard, R.E.; Long, C.D.; Nelson, R.W.; Hornof, W.J.; Feldman, E.C. Percutaneous ultrasonographically guided radiofrequency heat ablation for treatment of primary hyperparathyroidism in dogs. J. Am. Vet. Med. Assoc. 2001, 218, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Rasor, L.; Pollard, R.; Feldman, E.C. Retrospective evaluation of three treatment methods for primary hyperparathyroidism in dogs. J. Am. Anim. Hosp. Assoc. 2007, 43, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bucy, D.; Pollard, R.; Nelson, R. Analysis of Factors Affecting Outcome of Ultrasound-Guided Radiofrequency Heat Ablation for Treatment of Primary Hyperparathyroidism in Dogs. Vet. Radiol. Ultrasound 2017, 58, 83–89. [Google Scholar] [CrossRef]
- Leal, R.O.; Pascual, L.F.; Hernandez, J. The use of percutaneous ultrasound-guided radiofrequency heat ablation for treatment of primary hyperparathyroidism in eight dogs: Outcome and complications. Vet. Sci. 2018, 5, 91. [Google Scholar] [CrossRef] [Green Version]
- Mallery, K.F.; Pollard, R.E.; Nelson, R.W.; Hornof, W.J.; Feldman, E.C. Percutaneous ultrasound-guided radiofrequency heat ablation for treatment of hyperthyroidism in cats. J. Am. Vet. Med. Assoc. 2003, 223, 1602–1607. [Google Scholar] [CrossRef]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Barr, F. Percutaneous biopsy of abdominal organs under ultrasound guidance. J. Small Anim. Pract. 1995, 36, 105–113. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Gazelle, G.S.; Compton, C.C.; Mueller, P.R.; Tanabe, K.K. Treatment of intrahepatic malignancy with radiofrequency ablation: Radiologic-pathologic correlation. Cancer 2000, 88, 2452–2463. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, K.; Yan, K.; Wang, S.; Meng, Y.; Liu, B.; Wu, H.; Wang, H. Percutaneous radiofrequency ablation near large vessels in beagle livers: The impact of time and distance on the ablation zone. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Gr. 2021, 38, 1263–1270. [Google Scholar] [CrossRef]
- Boysen, S.R.; Lisciandro, G.R. The use of ultrasound for dogs and cats in the emergency room AFAST and TFAST. Vet. Clin. N. Am.-Small Anim. Pract. 2013, 43, 773–797. [Google Scholar] [CrossRef]
- Faraoni, D.; Willems, A.; Melot, C.; de Hert, S.; van der Linden, P. Efficacy of tranexamic acid in paediatric cardiac surgery: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2012, 42, 781–786. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, D.J.; Blackstock, K.J.; Epstein, K.; Brainard, B.M. Evaluation of tranexamic acid and ε-aminocaproic acid concentrations required to inhibit fibrinolysis in plasma of dogs and humans. Am. J. Vet. Res. 2014, 75, 731–738. [Google Scholar] [CrossRef]
- Marín, L.M.; Iazbik, M.C.; Zaldivar-Lopez, S.; Guillaumin, J.; Mcloughlin, M.A.; Couto, C.G. Epsilon Aminocaproic Acid for the Prevention of Delayed Postoperative Bleeding in Retired Racing Greyhounds Undergoing Gonadectomy. Vet. Surg. 2012, 41, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, E.; Pedro, B.; Dukes-McEwan, J.; Gelzer, A.R.; Blackwood, L. A descriptive review of cardiac tumours in dogs and cats. Vet. Comp. Oncol. 2017, 15, 273–288. [Google Scholar] [CrossRef] [Green Version]
- Hoffmeier, A.; Sindermann, J.R.; Scheld, H.H.; Martens, S. Cardiac Tumors-Diagnosis and Surgical Treatment. Dtsch. Arztebl. Int. 2014, 111, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Huo, J.L.; Choi, J.C.; DeLuna, A.; Lee, D.; Fleischmann, D.; Berry, G.J.; Deuse, T.; Haddad, F. Cardiac paraganglioma: Diagnostic and surgical challenges. J. Card. Surg. 2012, 27, 178–182. [Google Scholar] [CrossRef]
- D’Avila, A.; Houghtaling, C.; Gutierrez, P.; Vragovic, O.; Ruskin, J.N.; Josephson, M.E.; Reddy, V.Y. Catheter ablation of ventricular epicardial tissue: A comparison of standard and cooled-tip radiofrequency energy. Circulation 2004, 109, 2363–2369. [Google Scholar] [CrossRef] [Green Version]
- Lawrenz, T.; Borchert, B.; Leuner, C.; Bartelsmeier, M.; Reinhardt, J.; Strunk-Mueller, C.; Meyer Zu Vilsendorf, D.; Schloesser, M.; Beer, G.; Lieder, F.; et al. Endocardial radiofrequency ablation for hypertrophic obstructive cardiomyopathy: Acute results and 6 months’ follow-up in 19 patients. J. Am. Coll. Cardiol. 2011, 57, 572–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.S.; Chon, M.K.; Jun, E.J.; Park, Y.H.; Lee, S.H.; Kim, J.S.; Shin, D.H.; Lee, S.Y.; Cho, M.S.; Lee, S.W.; et al. Septal Reduction Using Transvenous Intramyocardial Cerclage Radiofrequency Ablation: Preclinical Feasibility. JACC Basic Transl. Sci. 2020, 5, 988–998. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Biondetti, P.; Ferrante, G.; Carugo, S.; Carrafiello, G. Immediate Clinical Success after Percutaneous Ablation of Extra-adrenal Paraganglioma. Cardiovasc. Intervent. Radiol. 2018, 41, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.F.; Atwell, T.D.; Charboneau, W.J.; Young, W.F.; Wass, T.C.; Callstrom, M.R. Minimally invasive treatment of metastatic pheochromocytoma and paraganglioma: Efficacy and safety of radiofrequency ablation and cryoablation therapy. J. Vasc. Interv. Radiol. 2011, 22, 1263–1270. [Google Scholar] [CrossRef]
- Kohlenberg, J.; Welch, B.; Hamidi, O.; Callstrom, M.; Morris, J.; Sprung, J.; Bancos, I.; Young, W. Efficacy and safety of ablative therapy in the treatment of patients with metastatic pheochromocytoma and paraganglioma. Cancers 2019, 11, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, F.M.; Antoch, G.; Veit, P.; Freudenberg, L.S.; Blechschmid, N.; Diersch, O.; Bockisch, A.; Barkhausen, J.; Kuehl, H. Morphologic and Functional Changes in Nontumorous Liver Tissue after Radiofrequency Ablation in an In Vivo Model: Comparison of18F-FDG PET/CT, MRI, ultrasound, and CT. J. Nucl. Med. 2007, 48, 1836–1844. [Google Scholar] [CrossRef] [Green Version]
- Lesser, T.G.; Ritter, F.; Schlosser, H.; Boltze, C.; Hackenbroich, C. Effects of radiofrequency ablation on normal lung tissue in a swine model. Acad. Radiol. 2011, 18, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Yamakado, K.; Takaki, H.; Yamada, T.; Yamanaka, T.; Uraki, J.; Kashima, M.; Nakatsuka, A.; Takeda, K. Incidence and cause of hypertension during adrenal radiofrequency ablation. Cardiovasc. Intervent. Radiol. 2012, 35, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Y.; Song, X.D.; Liu, L.; Wang, X.B.; Liu, P.; Zhang, X.L.; Zhou, Y.J.; Que, D.D.; Yu, W.J.; Li, Y.Q.; et al. Intramural hematoma versus thrombus: Radiation-induced heart disease results in mass formation after radiofrequency ablation. Chin. Med. J. 2016, 129, 2762–2764. [Google Scholar] [CrossRef]
- Ancona, R.; Pinto, S.C.; Caso, P.; Di Palma, V.; Pisacane, F.; Martiniello, A.R.; Quarto, C.; de Rosa, N.; Pisacane, C.; Calabrò, R. Right atrial mass following transcatheter radiofrequency ablation for recurrent atrial fibrillation: Thrombus, endocarditis or mixoma? Monaldi Arch. Chest Dis.-Card. Ser. 2009, 72, 40–42. [Google Scholar] [CrossRef] [Green Version]
- Pires, L.A.; Wagshal, A.B.; Huang, S.K. Development of an aortic valve mass after radiofrequency catheter ablation. Pacing Clin. Electrophysiol. 1994, 17, 398–399. [Google Scholar] [CrossRef]
- Rubio Alvarez, J.; Martinez de Alegria, A.; Sierra Quiroga, J.; Adrio Nazar, B.; Rubio Taboada, C.; Martinez Comendador, J.M. Rapid growth of left atrial myxoma after radiofrequency ablation. Texas Heart Inst. J. 2013, 40, 459–461. [Google Scholar]
- Sirin, G.; Ozker, E.; Fotbolcu, H.; Ozden, K.; Demirsoy, E. Myxoma developing after open heart surgery: Is radiofrequency ablation responsible for the formation of the tumor? Acta Chir. Belg. 2012, 112, 154–156. [Google Scholar] [CrossRef]
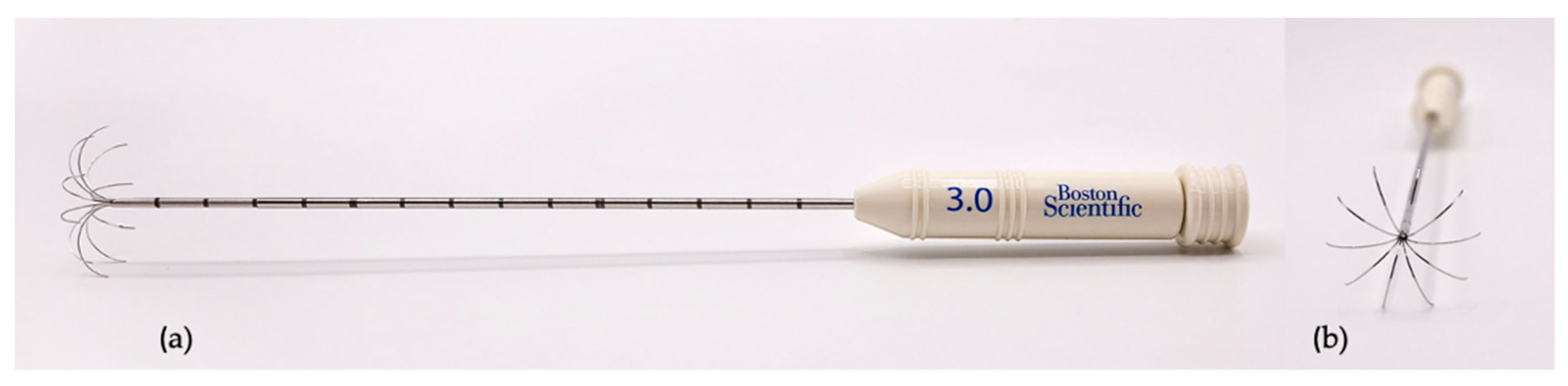
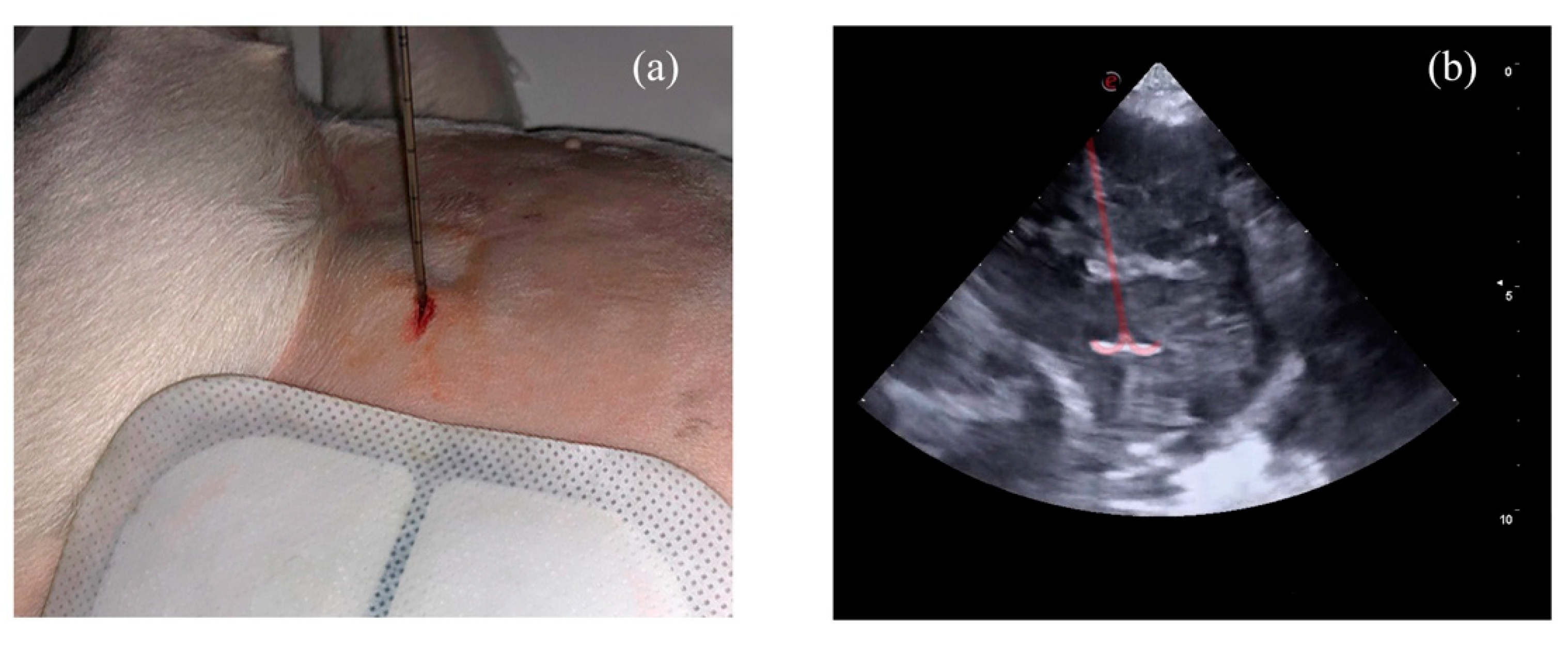

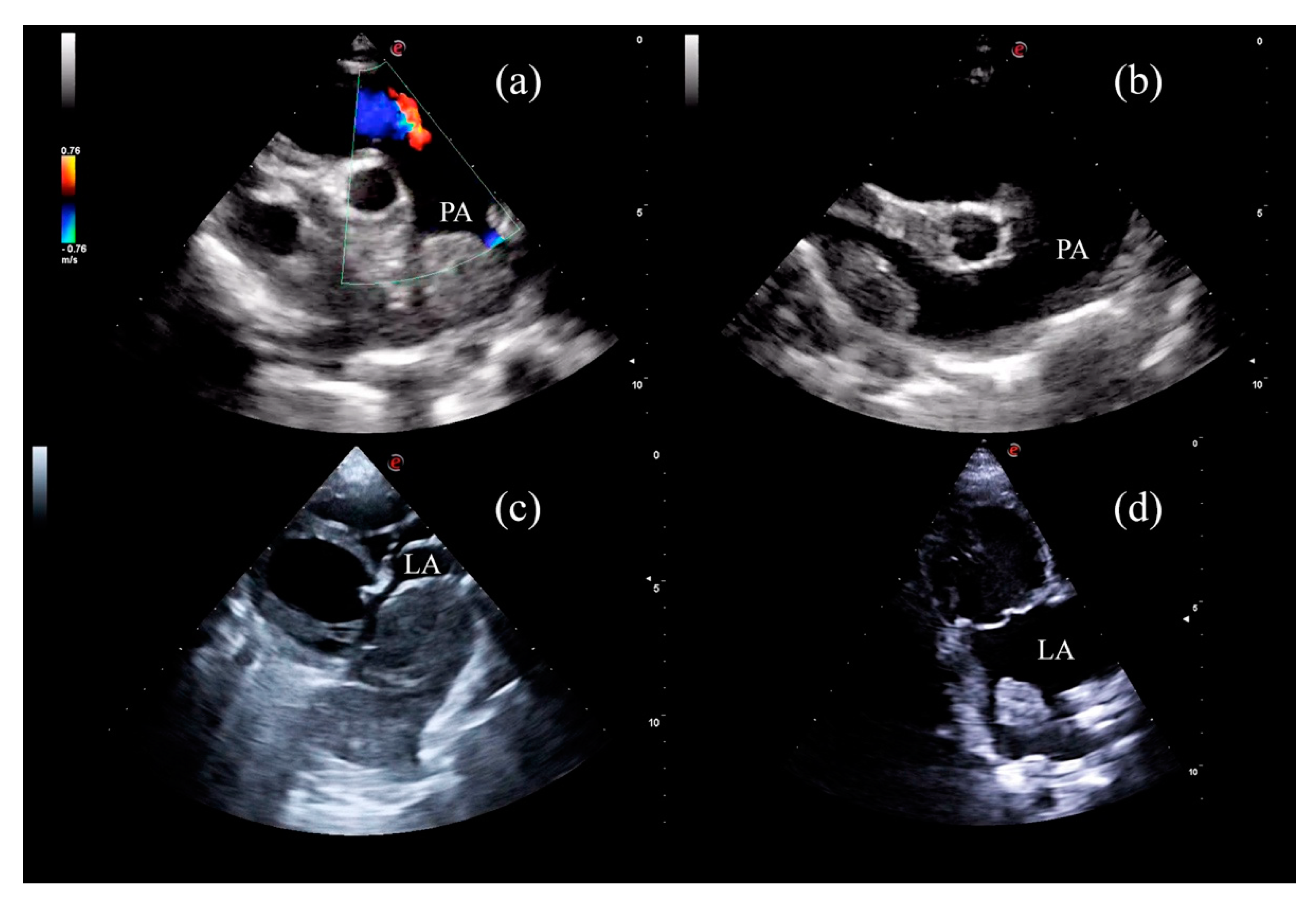
| Dog | Breed | Sex and Reproductive Status | BW (kg) | Age (years) |
|---|---|---|---|---|
| 1 | Shar pei | F, N | 26 | 12 |
| 2 | Boxer | M, E | 32 | 10 |
| 3 | French Bulldog | F, N | 18 | 10 |
| 4 | French Bulldog | M, N | 14 | 9 |
| 5 | Spanish Water dog | M, E | 22 | 12 |
| Dog | Compression Site | Apparent CD Size at T0 (cm) | Main Clinical Signs at T0 |
|---|---|---|---|
| 1 | Pulmonary artery | 6.8 | Ascites |
| 2 | Caudal vena cava | 4.0 | Syncope |
| 3 | Right atrium with left atrium invasion | 8.5 | Ascites and syncope |
| 4 | Pulmonary artery | 7.6 | Ascites |
| 5 | Pulmonary artery | 5.8 | Ascites |
| Apparent CD Size (cm) | Pulmonary Artery Velocity (m/s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dog | Compression Site | Ablation Time (min) | T0 | T1 | T2 | T3 | T0 | T1 | T2 | T3 |
| 1 | Pulmonary artery | 18 | 6.8 | 1.2 | 0.8 | 0.8 | 3.2 | 1.2 | 1.1 | 1.2 |
| 2 | Caudal vena cava | 11 | 4.0 | 2.2 | 1.6 | 1.3 | 0.8 | 0.7 | 0.7 | 0.8 |
| 3 | Right atrium with left atrium invasion | 28 | 8.5 | 6.5 | 5.8 | 5.6 | 1.1 | 0.9 | 0.9 | 0.9 |
| 4 | Pulmonary artery | 22 | 7.6 | 2.4 | 2.3 | 2.4 | 2.8 | 0.8 | 0.9 | 1.0 |
| 5 | Pulmonary artery | 14 | 5.8 | 1.6 | 1.3 | 1.2 | 2.2 | 0.6 | 0.7 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Ochoa, P.; Alférez, M.D.; de Blas, I.; Fernendes, T.; Sánchez Salguero, X.; Balañá, B.; Meléndez Lazo, A.; Barbero Fernandez, A.; Caivano, D.; Corda, F.; et al. Ultrasound-Guided Radiofrequency Ablation of Chemodectomas in Five Dogs. Animals 2021, 11, 2790. https://doi.org/10.3390/ani11102790
Gómez Ochoa P, Alférez MD, de Blas I, Fernendes T, Sánchez Salguero X, Balañá B, Meléndez Lazo A, Barbero Fernandez A, Caivano D, Corda F, et al. Ultrasound-Guided Radiofrequency Ablation of Chemodectomas in Five Dogs. Animals. 2021; 11(10):2790. https://doi.org/10.3390/ani11102790
Chicago/Turabian StyleGómez Ochoa, Pablo, María Dolores Alférez, Ignacio de Blas, Telmo Fernendes, Xavier Sánchez Salguero, Beatriz Balañá, Antonio Meléndez Lazo, Alicia Barbero Fernandez, Domenico Caivano, Francesca Corda, and et al. 2021. "Ultrasound-Guided Radiofrequency Ablation of Chemodectomas in Five Dogs" Animals 11, no. 10: 2790. https://doi.org/10.3390/ani11102790
APA StyleGómez Ochoa, P., Alférez, M. D., de Blas, I., Fernendes, T., Sánchez Salguero, X., Balañá, B., Meléndez Lazo, A., Barbero Fernandez, A., Caivano, D., Corda, F., & Corda, A. (2021). Ultrasound-Guided Radiofrequency Ablation of Chemodectomas in Five Dogs. Animals, 11(10), 2790. https://doi.org/10.3390/ani11102790







