Accuracy of Imputation of Microsatellite Markers from a 50K SNP Chip in Spanish Assaf Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Genotypes and Quality Control
2.2. Imputation Procedure
2.3. Imputation Performance Metrics
2.4. Population Structure, Effective Population Size, and Parental Relationships
3. Results
3.1. Genotype Quality Control
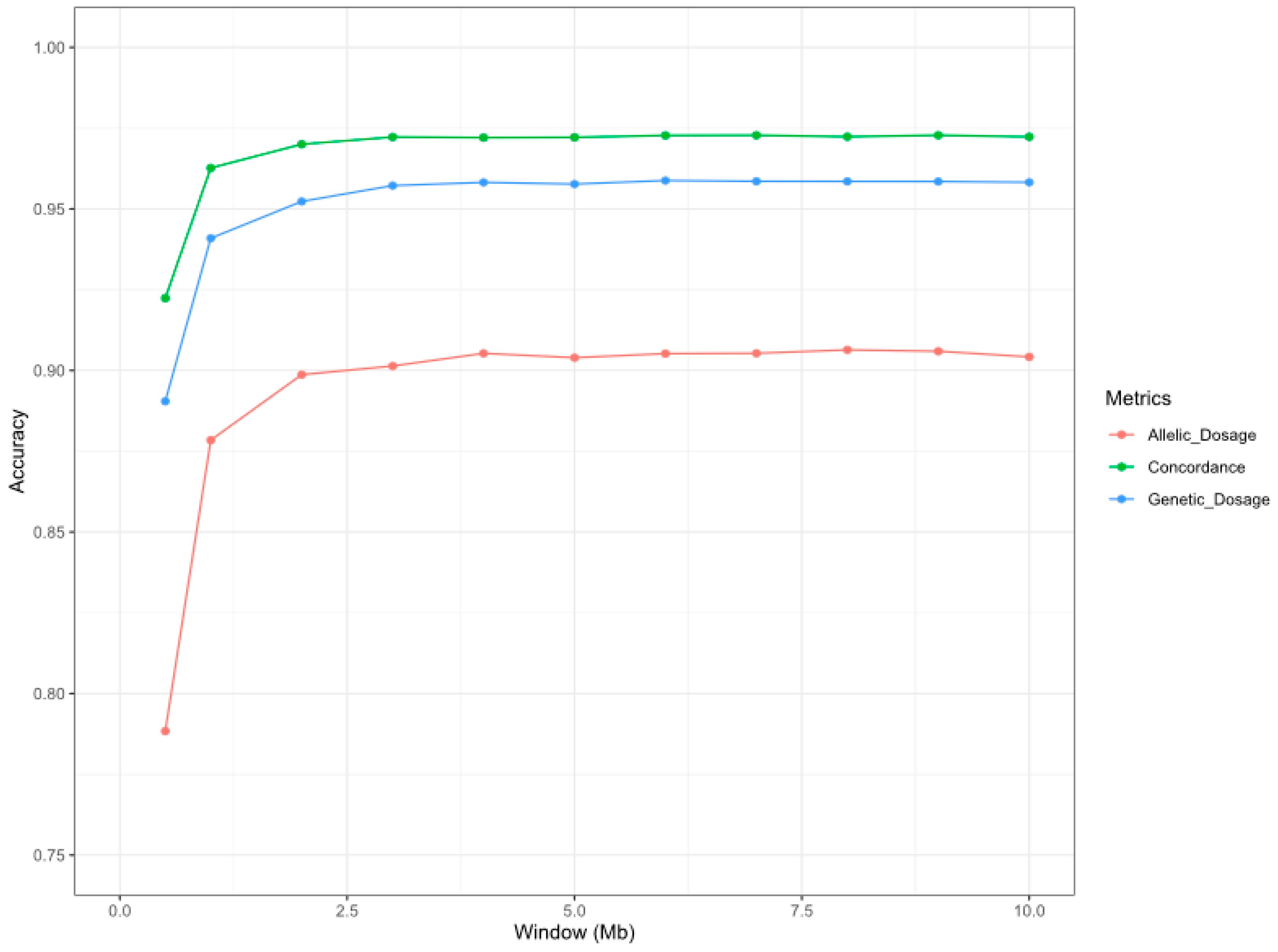
3.2. Imputation Results
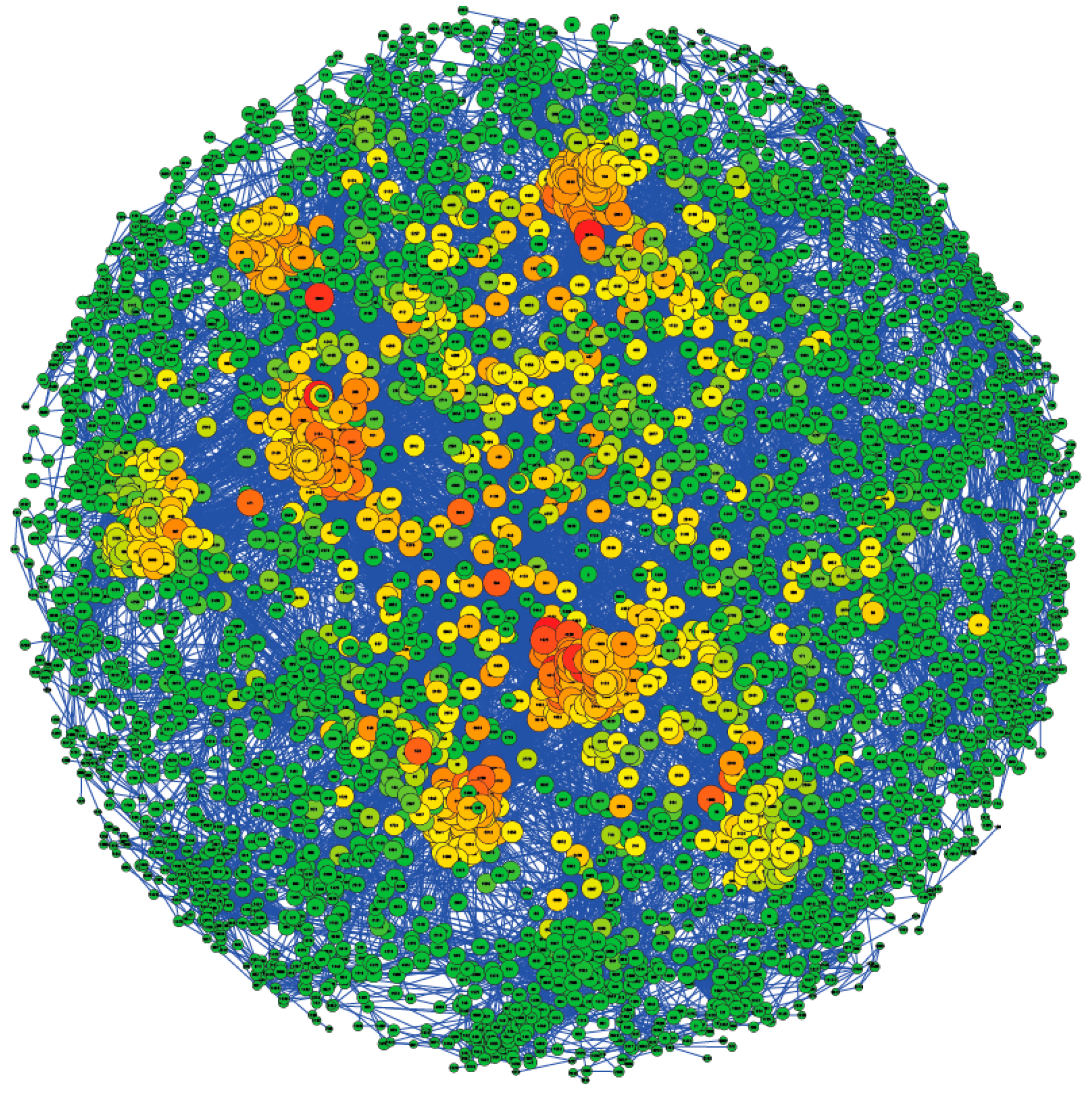
3.3. Population Structure and Effective Population Size
3.4. Parentage Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dodds, K.G.; Tate, M.L.; Sise, J.A. Genetic evaluation using parentage information from genetic markers. J. Anim. Sci. 2005, 83, 2271–2279. [Google Scholar] [CrossRef]
- Geldermann, H.; Pieper, U.; Weber, W.E. Effect of misidentification on the estimation of breeding value and heritability in cattle. J. Anim. Sci. 1986, 63, 1759–1768. [Google Scholar] [CrossRef]
- Heaton, M.P.; Leymaster, K.A.; Kalbfleisch, T.S.; Kijas, J.W.; Clarke, S.M.; McEwan, J.; Maddox, J.F.; Basnayake, V.; Petrik, D.T.; Simpson, B.; et al. SNPs for parentage testing and traceability in globally diverse breeds of sheep. PLoS ONE 2014, 9, e94851. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Ardren, W.R. Methods of parentage analysis in natural populations. Mol. Ecol. 2003, 12, 2511–2523. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Small, C.M.; Paczolt, K.A.; Ratterman, N.L. A practical guide to methods of parentage analysis. Mol. Ecol. Resour. 2010, 10, 6–30. [Google Scholar] [CrossRef]
- Chambers, G.K.; MacAvoy, E.S. Microsatellites: Consensus and controversy. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, 455–476. [Google Scholar] [CrossRef]
- Strucken, E.; Lee, S.H.; Lee, H.K.; Song, K.; Gibson, J.; Gondro, C. How many markers are enough? Factors influencing parentage testing in different livestock populations. J. Anim. Breed. Genet. 2016, 133, 13–23. [Google Scholar] [CrossRef]
- Glover, K.A.; Hansen, M.M.; Lien, S.; Als, T.D.; Høyheim, B.; Skaala, Ø. A comparison of SNP and STR loci for delineating population structure and performing individual genetic assignment. BMC Genet. 2010, 11, 2. [Google Scholar] [CrossRef]
- Carta, A.; Casu, S.; Salaris, S. Invited review: Current state of genetic improvement in dairy sheep. J. Dairy Sci. 2009, 92, 5814–5833. [Google Scholar] [CrossRef]
- Zhang, P.; Zhan, X.; Rosenberg, N.A.; Zöllner, S. Genotype imputation reference panel selection using maximal phylogenetic diversity. Genetics 2013, 195, 319–330. [Google Scholar] [CrossRef]
- Cesarani, A.; Gaspa, G.; Correddu, F.; Cellesi, M.; Dimauro, C.; Macciotta, N.P.P. Genomic selection of milk fatty acid composition in Sarda dairy sheep: Effect of different phenotypes and relationship matrices on heritability and breeding value accuracy. J. Dairy Sci. 2019, 102, 3189–3203. [Google Scholar] [CrossRef] [PubMed]
- Lillehammer, M.; Sonesson, A.K.; Klemetsdal, G.; Blichfeldt, T.; Meuwissen, T.H.E. Genomic selection strategies to improve maternal traits in Norwegian White Sheep. J. Anim. Breed. Genet. 2020, 137, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.; Clarke, S.M.; McEwan, J.C.; Miller, S.P.; Pickering, N.K.; Bain, W.E.; Dodds, K.G.; Sargolzaei, M.; Schenkel, F. Prediction of genomic breeding values for growth, carcass and meat quality traits in a multi-breed sheep population using a HD SNP chip. BMC Genet. 2017, 18, 7. [Google Scholar] [CrossRef]
- Di Stasio, L. ISAG Panels of Markers for Parentage Verification. Available online: http://www.isag.us/Docs/consignmentforms/02_PVpanels_LPCGH.doc (accessed on 22 September 2020).
- McClure, M.C.; Sonstegard, T.S.; Wiggans, G.; Van Tassell, C. Imputation of microsatellite alleles from dense SNP genotypes for parental verification. Front. Genet. 2012, 3, 140. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Browning, B.L.; Zhou, Y.; Browning, S.R. A one-penny imputed genome from next-generation reference panels. Am. J. Hum. Genet. 2018, 103, 338–348. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Mitra, I.; Mousavi, N.; Fotsing, S.F.; Gymrek, M. A reference haplotype panel for genome-wide imputation of short tandem repeats. Nat. Commun. 2018, 9, 4397. [Google Scholar] [CrossRef]
- VanRaden, P.M. Efficient methods to compute genomic predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Reverter, A.; Dominik, S.; Ferraz, J.B.S.; Corrigan, L.; Porto-Neto, L.R. Pedigromics: A network-inspired approach to visualise and analyse pedigree structures. Assoc. Adv. Anim. Breed. Genet. 2019, 23, 540–543. [Google Scholar]
- Caraux, G.; Pinloche, S. PermutMatrix: A graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 2005, 21, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Misztal, I.; Tsuruta, S.; Lourenco, D.; Aguilar, I.; Legarra, A.; Vitezica, Z. Manual for BLUPF90 Family of Programs. Available online: http://nce.ads.uga.edu/wiki/lib/exe/fetch.php?media=blupf90_all2.pdf (accessed on 15 October 2019).
- Doncheva, N.T.; Assenov, Y.; Domingues, F.S.; Albrecht, M. Topological analysis and interactive visualization of biological networks and protein structures. Nat. Protoc. 2012, 7, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Perez, B. Strategies to Improve Results from Genomic Analyzes in Small Dairy Cattle Populations. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2019. [Google Scholar]
- Sharma, A.; Park, J.-E.; Park, B.; Park, M.-N.; Roh, S.H.; Jung, W.-Y.; Lee, S.H.; Chai, H.-H.; Chang, G.-W.; Cho, Y.-M.; et al. Accuracy of imputation of microsatellite markers from BovineSNP50 and BovineHD BeadChip in Hanwoo population of Korea. Genom. Inform. 2018, 16, 10–13. [Google Scholar] [CrossRef]
- Yoshida, G.M.; Carvalheiro, R.; Lhorente, J.P.; Correa, K.; Figueroa, R.; Houston, R.D.; Yánez, J.M. Accuracy of genotype imputation and genomic predictions in a two-generation farmed Atlantic salmon population using high-density and low-density SNP panels. Aquacculture 2018, 491, 147–154. [Google Scholar] [CrossRef]
- Bolormaa, S.; Gore, K.; van der Werf, J.H.J.; Hayes, B.J.; Daetwyler, H.D. Design of a low-density SNP chip for the main Australian sheep breeds and its effect on imputation and genomic prediction accuracy. Anim. Genet. 2015, 46, 544–556. [Google Scholar] [CrossRef]
- Druet, T.; Macleod, I.M.; Hayes, B.J. Toward genomic prediction from whole-genome sequence data: Impact of sequencing design on genotype imputation and accuracy of predictions. Heredity 2014, 112, 39–47. [Google Scholar] [CrossRef]
- Kijas, J.W.; Lenstra, J.A.; Hayes, B.; Boitard, S.; Neto, L.R.P.; Cristobal, M.S.; Servin, B.; McCulloch, R.; Whan, V.; Gietzen, K.J.; et al. Genome-wide analysis of the world’s sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 2012, 10, e1001258. [Google Scholar] [CrossRef]
- García-Gámez, E.; Sahana, G.; Gutiérrez-Gil, B.; Arranz, J.-J. Linkage disequilibrium and inbreeding estimation in Spanish Churra sheep. BMC Genet. 2012, 13, 43. [Google Scholar] [CrossRef]
- Prieur, V.; Clarke, S.M.; Brito, L.; McEwan, J.C.; Lee, M.; Brauning, R.; Dodds, K.G.; Auvray, B. Estimation of linkage disequilibrium and effective population size in New Zealand sheep using three different methods to create genetic maps. BMC Genet. 2017, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Relationship of genetic variation to population Size in Wildlife. Conserv. Biol. 1996, 10, 1500–1508. [Google Scholar] [CrossRef]
- Boichard, D.; Chung, H.; Dassonneville, R.; David, X.; Eggen, A.; Fritz, S.; Gietzen, K.J.; Hayes, B.J.; Lawley, C.T.; Sonstegard, T.S.; et al. Design of a bovine low-density SNP array optimized for imputation. PLoS ONE 2012, 7, e34130. [Google Scholar] [CrossRef] [PubMed]
| Microsatellite ID | CHR 1 | Position (bp) | Nº of Alleles | Range (bp) |
|---|---|---|---|---|
| INRA006 | 1 | 109478015 | 13 | 104–134 |
| INRA049 | 1 | 1952560108 | 9 | 134–166 |
| INRA023 | 1 | 86986507 | 14 | 194–220 |
| FCB20 | 2 | 153680836 | 14 | 87–115 |
| AE129 | 5 | 78045895 | 6 | 135–161 |
| SPS113 | 7 | 23419543 | 11 | 126–152 |
| ILSTS005 | 7 | 92854099 | 12 | 190–214 |
| ILSTS011 | 9 | 25256863 | 8 | 268–282 |
| ILSTS008 | 9 | 45990219 | 2 | 168–170 |
| McM042 | 9 | 51865313 | 8 | 81–107 |
| CSRD247 | 14 | 15564041 | 19 | 205–257 |
| INRA063 | 14 | 39826970 | 18 | 167–207 |
| SPS115 | 15 | 23269440 | 12 | 237–255 |
| MAF65 | 15 | 30901387 | 9 | 119–137 |
| MAF214 | 16 | 33667802 | 16 | 183–269 |
| CP49 | 17 | 14434435 | 25 | 76–136 |
| HSC | 20 | 25764806 | 17 | 263–297 |
| INRA132 | 20 | 4668849 | 17 | 146–180 |
| INRA172 | 22 | 20603037 | 12 | 126–172 |
| CHR | Position | Microsatellite | Conc. 1 | GD 2 | AD 3 | Min AD 3 | Max AD 3 | Naive Conc. | Random Conc. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 86986507 | INRA023 | 0.98 | 0.97 | 0.97 | 0.93 | 0.99 | 0.28 | 0.13 |
| 1 | 109478015 | INRA006 | 0.93 | 0.87 | 0.84 | 0.60 | 1.00 | 0.48 | 0.11 |
| 1 | 195256010 | INRA049 | 0.97 | 0.97 | 0.88 | 0.32 | 0.98 | 0.44 | 0.16 |
| 2 | 153680836 | FCB20 | 0.96 | 0.94 | 0.89 | 0.52 | 1.00 | 0.26 | 0.10 |
| 5 | 78045895 | AE129 | 0.96 | 0.96 | 0.88 | 0.13 | 1.00 | 0.47 | 0.20 |
| 7 | 23419543 | SPS113 | 0.95 | 0.93 | 0.79 | 0.16 | 0.94 | 0.34 | 0.16 |
| 7 | 92854099 | ILSTS005 | 0.99 | 0.97 | 0.97 | 0.97 | 0.97 | 0.41 | 0.11 |
| 9 | 25256863 | ILSTS011 | 0.98 | 0.96 | 0.92 | 0.83 | 0.97 | 0.50 | 0.18 |
| 9 | 45990219 | ILSTS008 | 0.97 | 0.86 | 0.80 | 0.37 | 0.97 | 0.67 | 0.61 |
| 9 | 51865313 | McM042 | 0.97 | 0.97 | 0.93 | 0.70 | 0.98 | 0.48 | 0.17 |
| 14 | 15564041 | CSRD247 | 0.99 | 0.97 | 0.97 | 0.94 | 1.00 | 0.34 | 0.07 |
| 14 | 39826970 | INRA063 | 0.97 | 0.95 | 0.82 | 0.47 | 0.98 | 0.33 | 0.09 |
| 15 | 23269440 | SPS115 | 0.96 | 0.95 | 0.90 | 0.67 | 1.00 | 0.33 | 0.14 |
| 15 | 30901387 | MAF65 | 0.98 | 0.97 | 0.92 | 0.75 | 1.00 | 0.36 | 0.18 |
| 16 | 33667802 | MAF214 | 0.98 | 0.98 | 0.86 | 0.32 | 1.00 | 0.54 | 0.09 |
| 17 | 14434435 | CP49 | 0.98 | 0.97 | 0.92 | 0.84 | 0.98 | 0.39 | 0.06 |
| 20 | 4668849 | INRA132 | 0.98 | 0.97 | 0.95 | 0.84 | 0.97 | 0.29 | 0.11 |
| 20 | 25764806 | HSC | 0.98 | 0.98 | 0.95 | 0.77 | 0.99 | 0.54 | 0.09 |
| 22 | 20603037 | INRA172 | 0.96 | 0.95 | 0.91 | 0.72 | 1.00 | 0.35 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marina, H.; Suarez-Vega, A.; Pelayo, R.; Gutiérrez-Gil, B.; Reverter, A.; Esteban-Blanco, C.; Arranz, J.J. Accuracy of Imputation of Microsatellite Markers from a 50K SNP Chip in Spanish Assaf Sheep. Animals 2021, 11, 86. https://doi.org/10.3390/ani11010086
Marina H, Suarez-Vega A, Pelayo R, Gutiérrez-Gil B, Reverter A, Esteban-Blanco C, Arranz JJ. Accuracy of Imputation of Microsatellite Markers from a 50K SNP Chip in Spanish Assaf Sheep. Animals. 2021; 11(1):86. https://doi.org/10.3390/ani11010086
Chicago/Turabian StyleMarina, Héctor, Aroa Suarez-Vega, Rocío Pelayo, Beatriz Gutiérrez-Gil, Antonio Reverter, Cristina Esteban-Blanco, and Juan José Arranz. 2021. "Accuracy of Imputation of Microsatellite Markers from a 50K SNP Chip in Spanish Assaf Sheep" Animals 11, no. 1: 86. https://doi.org/10.3390/ani11010086
APA StyleMarina, H., Suarez-Vega, A., Pelayo, R., Gutiérrez-Gil, B., Reverter, A., Esteban-Blanco, C., & Arranz, J. J. (2021). Accuracy of Imputation of Microsatellite Markers from a 50K SNP Chip in Spanish Assaf Sheep. Animals, 11(1), 86. https://doi.org/10.3390/ani11010086







