A Single Dose of Synbiotics and Vitamins at Birth Affects Piglet Microbiota before Weaning and Modifies Post-Weaning Performance
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Experimental Design
2.2. Diets, Feeding and Composition of the Supplement
2.3. Measurements, Sampling and Volatile Fatty Acid Analysis
2.4. DNA Extraction and Microbiota Profiling
2.5. Statistical Analyses
3. Results
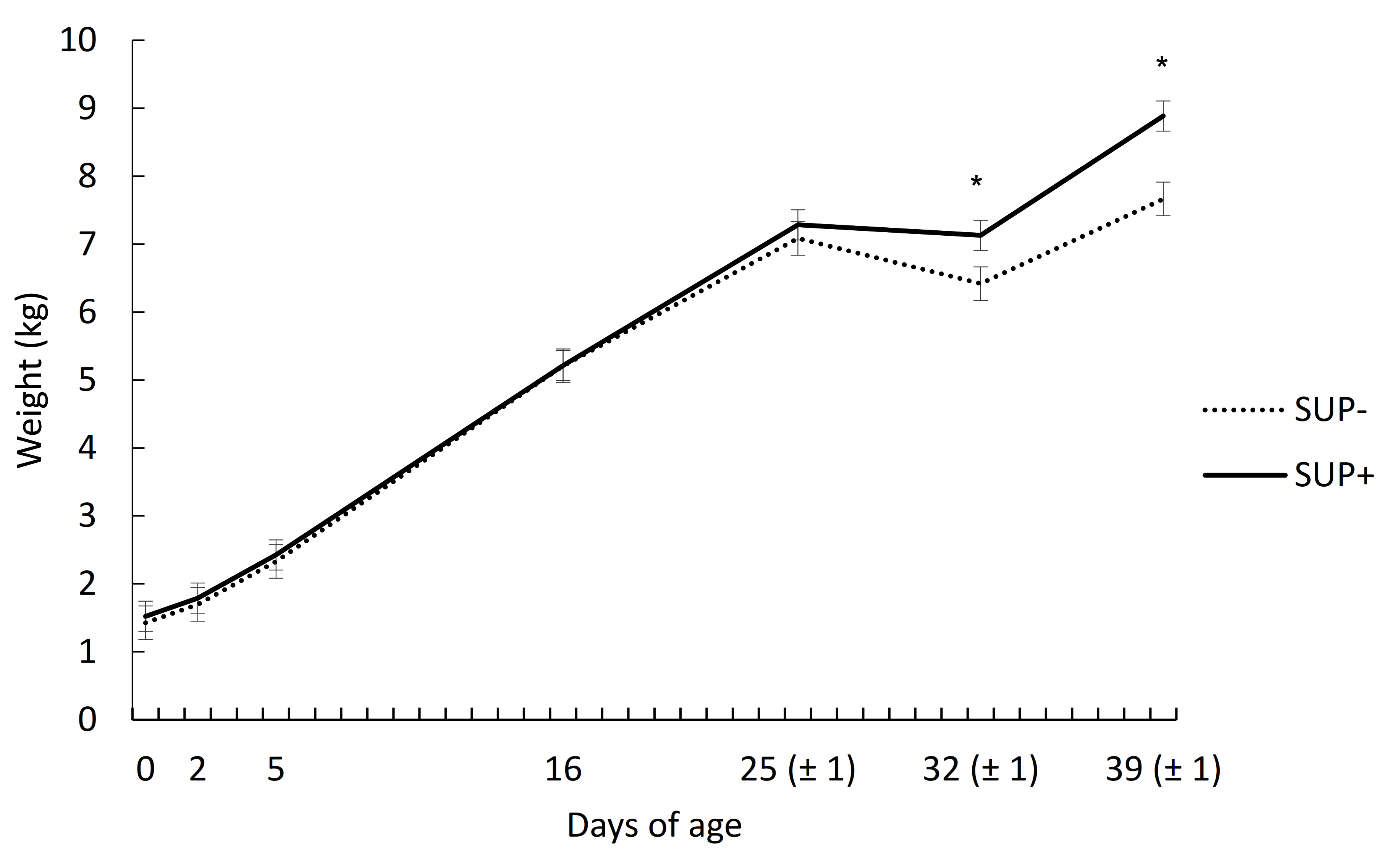
3.1. Growth Performance and Diarrhea
3.2. Production of Volatile Fatty Acids
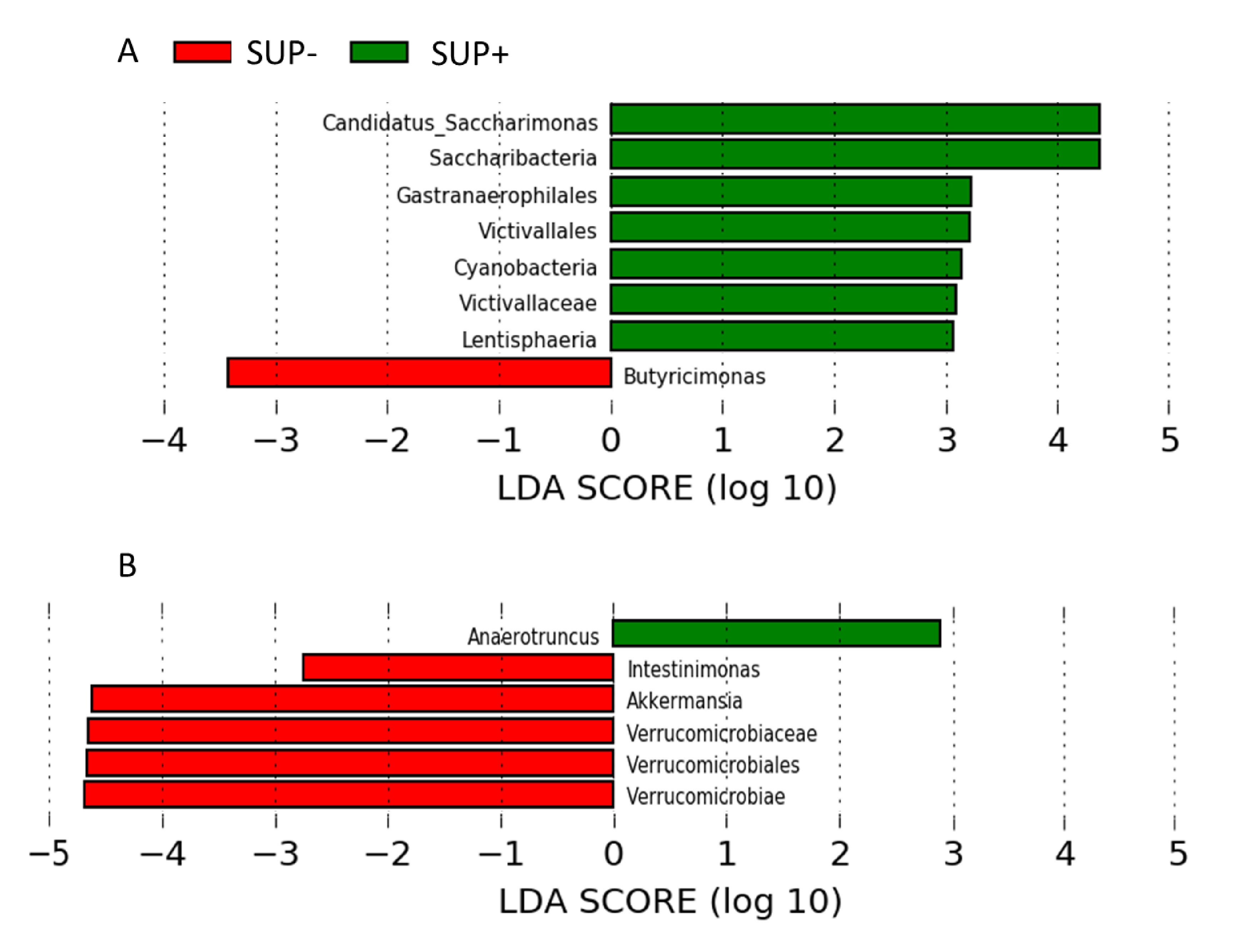
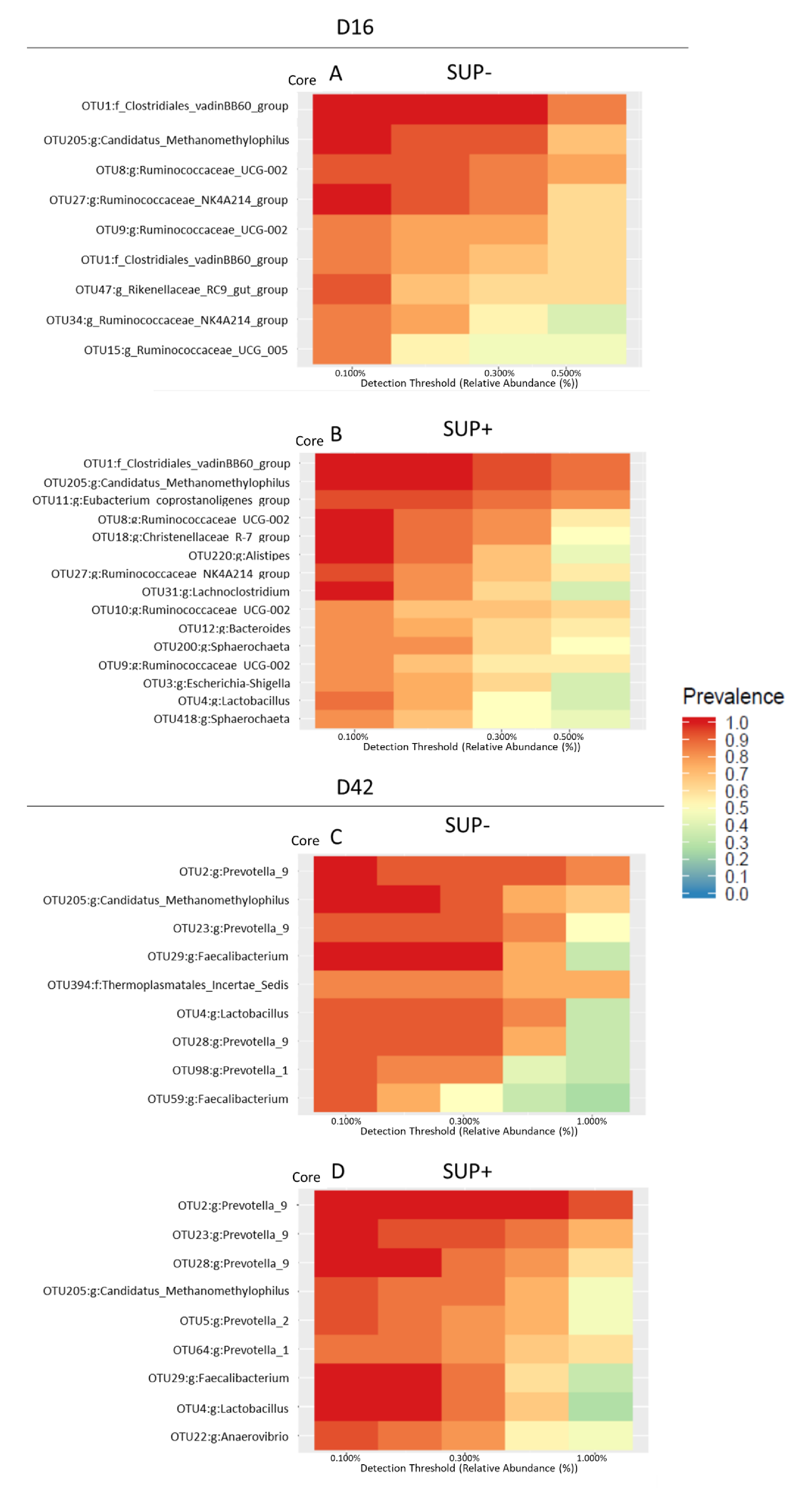
3.3. Microbial Diversity and Microbiota Composition in the Feces
4. Discussion
4.1. Effect of Supplementation on Growth Performance and Health
4.2. Effect of Supplementation on Fecal Microbiota and Fermentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliviero, C.; Junnikkala, S.; Peltoniemi, O. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 2019, 54, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Quesnel, H.; Brossard, L.; Valancogne, A.; Quiniou, N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal 2008, 2, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Björkman, S.; Oliviero, C.; Rajala-Schultz, P.J.; Soede, N.M.; Peltoniemi, O.A.T. The effect of litter size, parity and farrowing duration on placenta expulsion and retention in sows. Theriogenology 2017, 92, 36–44. [Google Scholar] [CrossRef]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Le Cozler, Y.; Pichodo, X.; Roy, H.; Guyomarc h, C.; Pellois, H.; Quiniou, N.; Louveau, I.; Lebret, B.; Lefaucheur, L.; Gondret, F. Influence du poids individuel et de la taille de la portée à la naissance sur la survie du porcelet, ses performances de croissance et d’abattage et la qualité de la viande. Journ. Rech. Porc. 2004, 36, 443–449. [Google Scholar]
- Le Dividich, J. The neonatal and weaner pig: Management to reduce variation. In Manipulating Pig Production VII; Cranwell, P.D., Ed.; Australasian Pig Science Association: Werribee, Australia, 1999; pp. 135–155. [Google Scholar]
- Everaert, N.; Van Cruchten, S.; Weström, B.; Bailey, M.; Van Ginneken, C.; Thymann, T.; Pieper, R. A review on early gut maturation and colonization in pigs, including biological and dietary factors affecting gut homeostasis. Anim. Feed. Sci. Technol. 2017, 233, 89–103. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Probert, H.M.; Smejkal, C.W.; Gibson, G.R. Using probiotics and prebiotics to improve gut health. Drug Discov. 2003, 8, 692–700. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Corino, C.; Modina, S.C.; Di Giancamillo, A.; Chiapparini, S.; Rossi, R. Seaweeds in Pig Nutrition. Animals 2019, 9, 1126. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Chattha, K.S.; Vlasova, A.N.; Saif, L.J. Prenatal vitamin A deficiency impairs adaptive immune responses to pentavalent rotavirus vaccine (RotaTeq®) in a neonatal gnotobiotic pig model. Vaccine 2014, 32, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Pinelli-Saavedra, A. Vitamin E in immunity and reproductive performance in pigs. Reprod. Nutr. Dev. 2003, 43, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Ratti, S.; Pastorelli, G.; Crotti, A.; Corino, C. The effect of dietary vitamin E and verbascoside on meat quality and oxidative stability of Longissimus Dorsi muscle in medium-heavy pigs. Food Res. Int. 2014, 65, 88–94. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant Functions of Vitamins. Ann. N. Y. Acad. Sci. 1992, 669, 7–20. [Google Scholar] [CrossRef]
- Agroscope 2017. Fütterungsempfehlungen und Nährwerttabellen fur Schweine (Feeding Recommendations and Nutrient Tables for Pigs). Agroscope, Posieux, Switzerland. Available online: http://www.agroscope.admin.ch/futtermitteldatenbank/04834 (accessed on 5 February 2018).
- Htoo, J.K.; Araiza, B.A.; Sauer, W.C.; Rademacher, M.; Zhang, Y.; Cervantes, M.; Zijlstra, R.T. Effect of dietary protein content on ileal amino acid digestibility, growth performance, and formation of microbial metabolites in ileal and cecal digesta of early-weaned pigs. J. Anim. Sci. 2007, 85, 3303–3312. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 16, R79. [Google Scholar] [CrossRef]
- Wang, Y.B.; Du, W.; Fu, A.K.; Zhang, X.P.; Huang, Y.; Lee, K.H.; Yu, K.; Li, W.F.; Li, Y.L. Intestinal microbiota and oral administration of Enterococcus faecium associated with the growth performance of new-born piglets. Benef. Microbes 2016, 7, 529–538. [Google Scholar] [CrossRef]
- Kiros, T.G.; Luise, D.; Derakhshani, H.; Petri, R.; Trevisi, P.; D’Inca, R.; Auclair, E.; Van Kessel, A.G. Effect of live yeast Saccharomyces cerevisiae supplementation on the performance and cecum microbial profile of suckling piglets. PLoS ONE 2019, 14, e0219557. [Google Scholar] [CrossRef]
- Hancox, L.R.; Le Bon, M.; Richards, P.J.; Guillou, D.; Dodd, C.E.R.; Mellits, K.H. Effect of a single dose of Saccharomyces cerevisiae var. boulardii on the occurrence of porcine neonatal diarrhoea. Animal 2015, 9, 1756–1759. [Google Scholar] [CrossRef] [PubMed]
- Bajagai, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in Animal Nutrition: Production, Impact and Regulation; FAO Animal Production and Health Paper No. 179; FAO: Rome, Italy, 2016. [Google Scholar]
- Myhill, L.J.; Stolzenbach, S.; Hansen, T.V.A.; Skovgaard, K.; Stensvold, C.R.; Andersen, L.O.; Nejsum, P.; Mejer, H.; Thamsborg, S.M.; Williams, A.R. Mucosal Barrier and Th2 Immune Responses Are Enhanced by Dietary Inulin in Pigs Infected With Trichuris suis. Front. Immunol. 2018, 9, 2557. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A. Antioxidant Vitamins and Their Functions in Immune Responses. In Antioxidant Nutrients and Immune Functions; Bendich, A., Phillips, M., Tengerdy, R.P., Eds.; Springer: Boston, MA, USA, 1990; pp. 35–55. [Google Scholar] [CrossRef]
- Ross, A.C.; Chen, Q.; Ma, Y. Vitamin A and Retinoic Acid in the Regulation of B-Cell Development and Antibody Production. Vitam. Horm. 2011, 86, 103–126. [Google Scholar] [CrossRef]
- Matte, J.J.; Audet, I. Maternal perinatal transfer of vitamins and trace elements to piglets. Animal 2020, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C.; Engel, H.; Jensen, S.K.; Craig, A.M.; Traber, M.G. Lactating Sows and Suckling Piglets Preferentially Incorporate RRR-over All-rac-α-Tocopherol into Milk, Plasma and Tissues. J. Nutr. 2002, 132, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, A.; Shan, A.S. Effects of Ligustrum lucidum Fruits on Growth Performance, Antioxidation and Meat Quality in Arbor Acres Broilers. Asian Australas. J. Anim. Sci. 2009, 22, 700–705. [Google Scholar] [CrossRef]
- Tretola, M.; Luciano, A.; Ottoboni, M.; Baldi, A.; Pinotti, L. Influence of Traditional vs Alternative Dietary Carbohydrates Sources on the Large Intestinal Microbiota in Post-Weaning Piglets. Animals 2019, 9, 516. [Google Scholar] [CrossRef]
- Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal 2020, 14, 95–107. [Google Scholar] [CrossRef]
- Girard, M.; Hu, D.; Pradervand, N.; Neuenschwander, S.; Bee, G. Chestnut extract but not sodium salicylate decreases the severity of diarrhea and enterotoxigenic Escherichia coli F4 shedding in artificially infected piglets. PLoS ONE 2020, 15, e0214267. [Google Scholar] [CrossRef] [PubMed]
- Tretola, M.; Maghin, F.; Silacci, P.; Ampuero, S.; Bee, G. Effect of Supplementing Hydrolysable Tannins to a Grower–Finisher Diet Containing Divergent PUFA Levels on Growth Performance, Boar Taint Levels in Back Fat and Intestinal Microbiota of Entire Males. Animals 2019, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef]
- Bor, B.; Bedree, J.; Shi, W.; McLean, J.; He, X. Saccharibacteria (TM7) in the Human Oral Microbiome. J. Dent. Res. 2019, 98, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Q.; Wang, Y.; Wu, H.; Jin, X.; Yao, H.; Jin, D.; Liu, Y.; Wang, C. Gut microbiota was modulated by moxibustion stimulation in rats with irritable bowel syndrome. Chin. Med. 2018, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Li, P.; Hao, S.; Zhang, Y.; Kim, S.W.; Li, H.; Ma, X.; Gao, S.; He, L.; Wu, W.; et al. Dynamic Distribution of the Gut Microbiota and the Relationship with Apparent Crude Fiber Digestibility and Growth Stages in Pigs. Sci. Rep. 2015, 5, 9938. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Ding, H.; Zhu, H.; Zhang, C.; Yu, S.; Jie, H.; Zhang, Y.; Wu, B.; Liang, G.; Zhang, G.; et al. Study of Jianpi Mixture on Intestinal Microbiota of Diarrhea Irritable Bowel Syndrome Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 5241308. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef]
- Nie, Y.; Zhou, Z.; Guan, J.; Xia, B.; Luo, X.; Yang, Y.; Fu, Y.; Sun, Q. Dynamic changes of yak (Bos grunniens) gut microbiota during growth revealed by polymerase chain reaction-denaturing gradient gel electrophoresis and metagenomics. Asian Australas. J. Anim. Sci. 2017, 30, 957–966. [Google Scholar] [CrossRef][Green Version]
- De, R.; Mukhopadhyay, A.K.; Dutta, S. Metagenomic analysis of gut microbiome and resistome of diarrheal fecal samples from Kolkata, India, reveals the core and variable microbiota including signatures of microbial dark matter. Gut Pathog. 2020, 12, 2116–2130. [Google Scholar] [CrossRef]
- Salonen, A.; Salojärvi, J.; Lahti, L.; De Vos, W.M. The adult intestinal core microbiota is determined by analysis depth and health status. Clin. Microbiol. Infect. 2012, 18, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
| SUP− | SUP+ | SEM | p-Value | |
|---|---|---|---|---|
| Average daily gain (g/d) | ||||
| d 0–25 | 226 | 225 | 15.3 | 0.98 |
| d 25–32 | −96 | −22 | 23.6 | 0.04 |
| d 32–39 | 180 | 249 | 37.6 | 0.20 |
| d 0–39 | 159 | 185 | 8.8 | 0.05 |
| Occurrence of diarrhea (%) | ||||
| d 18–25 | 3.3 | 6.3 | 1.87 | 0.36 |
| d 25–32 | 26.4 | 12.5 | 4.62 | 0.03 |
| d 32–39 | 25.0 | 15.6 | 4.25 | 0.10 |
| Total | 18.5 | 11.7 | 2.30 | 0.02 |
| Days of diarrhea | 4.4 | 3.0 | 0.84 | 0.22 |
| Day | 16 | 39 ± 1 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Group | SUP− | SUP+ | SUP− | SUP+ | SEM | SUP 1 | Day | SUP × Day |
| Total (mol/g) | 40.3 | 56.2 | 86.1 | 93.0 | 6.86 | 0.14 | < 0.001 | 0.40 |
| Proportion (%) | ||||||||
| acetate | 52.6 | 52.7 | 63.0 | 62.9 | 1.91 | 0.98 | < 0.001 | 0.96 |
| propionate | 17.2 | 17.9 | 22.3 | 20.7 | 0.91 | 0.63 | < 0.001 | 0.15 |
| butyrate | 15.1 | 15.3 | 10.6 | 12.1 | 1.36 | 0.47 | < 0.01 | 0.66 |
| isobutyrate | 4.71 | 4.3 | 1.1 | 1.2 | 0.26 | 0.52 | < 0.001 | 0.26 |
| valerate | 3.4 | 3.1 | 1.8 | 2.0 | 0.30 | 0.96 | < 0.001 | 0.63 |
| isovalerate | 7.0 | 6.6 | 1.1 | 1.1 | 0.46 | 0.65 | < 0.001 | 0.47 |
| Day | Taxonomic Rank | OTU 1 | Taxa | Log2 Fold CHANGE | p-Value |
|---|---|---|---|---|---|
| 16 | genus | 154 | Candidatus Saccharimonas | 6.33 | 0.023 |
| order | 508 | Gastranaerophilales | 2.37 | 0.011 | |
| family | 196 | Victivillaceae | 4.23 | 0.004 | |
| genus | 830 | Butyricimonas | −2.24 | 0.032 | |
| 42 | genus | 83 | Intestinimonas | −3.14 | 0.024 |
| genus | 122 | Akkermansia | −9.48 | 0.023 | |
| genus | 152 | Anaerotruncus | NA | 0.008 |
| OTU | Taxa | VFA | N | N Not 0 | CoE | p-Value | Q-Value |
|---|---|---|---|---|---|---|---|
| 994 | Ruminiclostridium_9 | Total | 56 | 37 | −7.5 × 10−4 | 2.3 × 10−5 | 0.017 |
| 994 | Ruminiclostridium_9 | Acetate | 56 | 41 | −9.6 × 10−4 | 2.2 × 10−5 | 0.002 |
| 292 | Bacteroides | Acetate | 56 | 18 | −1.2 × 10−4 | 1.3 × 10−4 | 0.011 |
| 60 | Ruminococcaceae_UCG−002 | Acetate | 56 | 56 | −1.4 × 10−3 | 3.6 × 10−4 | 0.023 |
| 558 | Alloprevotella | Acetate | 56 | 37 | 9.4 × 10−4 | 6.5 × 10−4 | 0.037 |
| 224 | Alistipes | Iso-butyrate | 56 | 42 | 6.7 × 10−3 | 4.2 × 10−5 | 0.004 |
| 94 | Thalassospira | Iso-butyrate | 56 | 26 | 6.9 × 10−3 | 3.1 × 10−4 | 0.021 |
| 994 | Ruminiclostridium_9 | Butyrate | 56 | 41 | −1.2 × 10−3 | 5.3 × 10−5 | 0.005 |
| 516 | Helicobacter | Propionate | 56 | 42 | 8.7 × 10−4 | 5.1 × 10−4 | 0.029 |
| 623 | Prevotella_2 | Valerate | 56 | 27 | 6.9 × 10−3 | 2.5 × 10−4 | 0.018 |
| 94 | Thalassospira | Iso-valerate | 56 | 26 | 6.9 × 10−3 | 4.9 × 10−4 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, M.; Tretola, M.; Bee, G. A Single Dose of Synbiotics and Vitamins at Birth Affects Piglet Microbiota before Weaning and Modifies Post-Weaning Performance. Animals 2021, 11, 84. https://doi.org/10.3390/ani11010084
Girard M, Tretola M, Bee G. A Single Dose of Synbiotics and Vitamins at Birth Affects Piglet Microbiota before Weaning and Modifies Post-Weaning Performance. Animals. 2021; 11(1):84. https://doi.org/10.3390/ani11010084
Chicago/Turabian StyleGirard, Marion, Marco Tretola, and Giuseppe Bee. 2021. "A Single Dose of Synbiotics and Vitamins at Birth Affects Piglet Microbiota before Weaning and Modifies Post-Weaning Performance" Animals 11, no. 1: 84. https://doi.org/10.3390/ani11010084
APA StyleGirard, M., Tretola, M., & Bee, G. (2021). A Single Dose of Synbiotics and Vitamins at Birth Affects Piglet Microbiota before Weaning and Modifies Post-Weaning Performance. Animals, 11(1), 84. https://doi.org/10.3390/ani11010084





