Myeloperoxidase Inhibition Decreases the Expression of Collagen and Metallopeptidase in Mare Endometria under In Vitro Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mares and Retrieval of Endometrium
2.2. In Vitro Culture of Endometrial Explants
2.3. Endometrial Explant Viability Assay
2.4. Total RNA Extraction, Synthesis of cDNA and qPCR
2.5. Western Blot Analysis
2.6. Zymography
2.7. Statistical Analysis
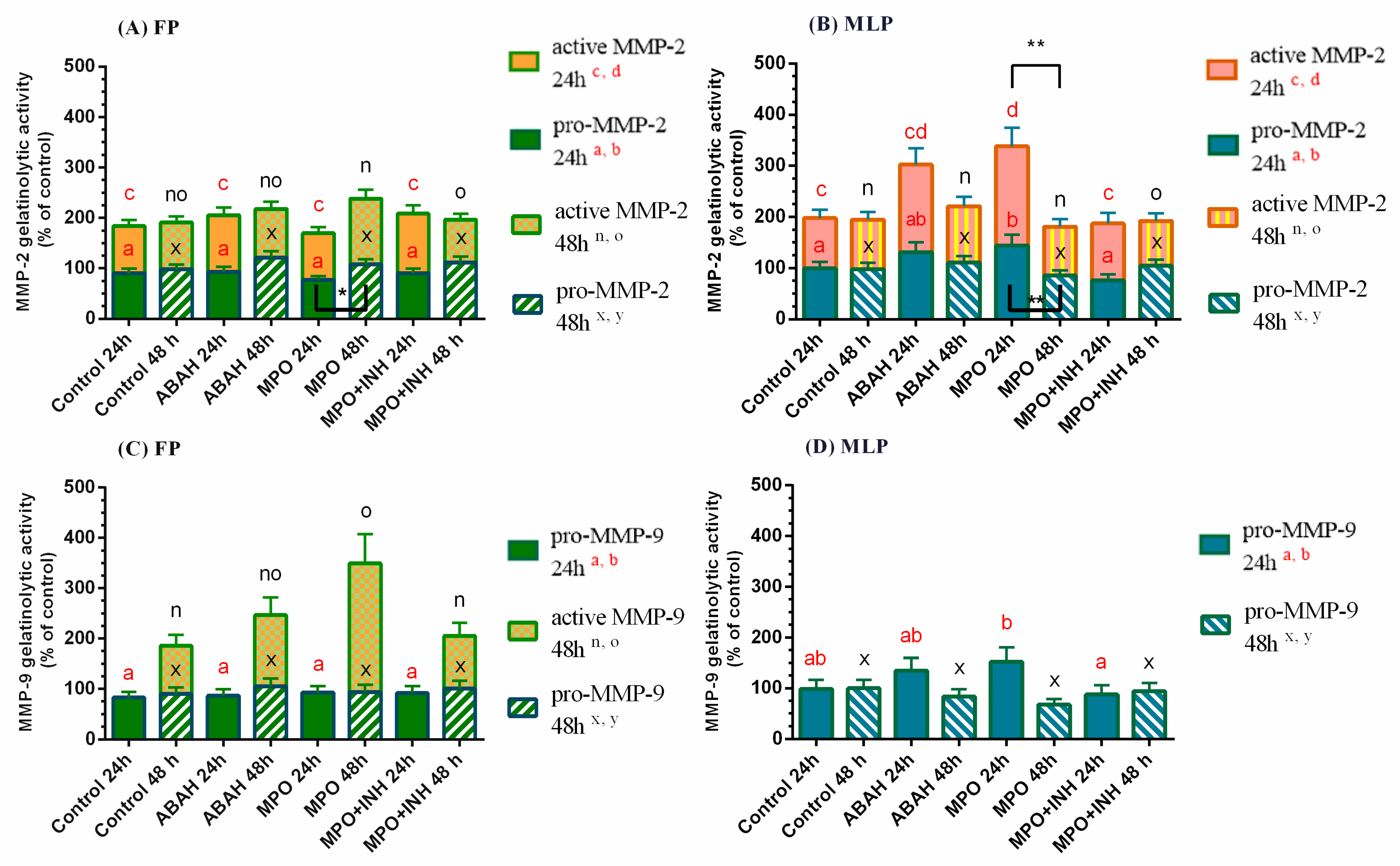
3. Results
3.1. The Effect of ABAH on the Inhibition of COL1 Induced by MPO
3.2. The Influence of MPO and ABAH on MMP Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and Cardiovascular Disease. ATVB 2005, 25, 1102–1111. [Google Scholar] [CrossRef]
- Liu, W.-Q.; Zhang, Y.-Z.; Wu, Y.; Zhang, J.-J.; Li, T.-B.; Jiang, T.; Xiong, X.-M.; Luo, X.-J.; Ma, Q.-L.; Peng, J. Myeloperoxidase-Derived Hypochlorous Acid Promotes Ox-LDL-Induced Senescence of Endothelial Cells through a Mechanism Involving β-Catenin Signaling in Hyperlipidemia. Biochem. Biophys. Res. Commun. 2015, 467, 859–865. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and Foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Teng, T.-S.; Ji, A.; Ji, X.-Y.; Li, Y.-Z. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Brinkmann, V. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. Myeloperoxidase in Human Neutrophil Host Defence: Myeloperoxidase in Human Neutrophil Host Defence. Cell Microbiol. 2014, 16, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Kotilainen, T.; Huhtinen, M.; Katila, T. Sperm-Induced Leukocytosis in the Equine Uterus. Theriogenology 1994, 41, 629–636. [Google Scholar] [CrossRef]
- Katila, T. Onset and Duration of Uterine Inflammatory Response of Mares after Insemination with Fresh Semen. Biol. Reprod. 1995, 52, 515–517. [Google Scholar] [CrossRef]
- Troedsson, M.H.T.; Liu, I.K.M.; Thurmond, M. Function of Uterine and Blood-Derived Polymorphonuclear Neutrophils in Mares Susceptible and Resistant to Chronic Uterine Infection: Phagocytosis and Chemotaxis1. Biol. Reprod. 1993, 49, 507–514. [Google Scholar] [CrossRef][Green Version]
- Troedsson, M.H.T. Breeding-Induced Endometritis in Mares. Vet. Clin. North Am. Equine Pract. 2006, 22, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.S.; Foster, D.N. Seminal DNase Frees Spermatozoa Entangled in Neutrophil Extracellular Traps. Biol. Reprod 2005, 73, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.S.; Lovaas, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, D.N. Species-Specific Interaction of Seminal Plasma on Sperm-Neutrophil Binding. Anim. Reprod. Sci. 2009, 114, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Rebordão, M.R.; Carneiro, C.; Alexandre-Pires, G.; Brito, P.; Pereira, C.; Nunes, T.; Galvão, A.; Leitão, A.; Vilela, C.; Ferreira-Dias, G. Neutrophil Extracellular Traps Formation by Bacteria Causing Endometritis in the Mare. J. Reprod. Immunol. 2014, 106, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Manda, A.; Pruchniak, M.P.; Araźna, M.; Demkow, U.A. Neutrophil Extracellular Traps in Physiology and Pathology. Cent. Eur. J. Immunol. 2014, 1, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Parrilla-Hernandez, S.; Ponthier, J.; Franck, T.Y.; Serteyn, D.D.; Deleuze, S.C. High Concentrations of Myeloperoxidase in the Equine Uterus as an Indicator of Endometritis. Theriogenology 2014, 81, 936–940. [Google Scholar] [CrossRef]
- Nazhat, S.A.; Kitahara, G.; Kozuka, N.; Mido, S.; Sadawy, M.; Ali, H.E.-S.; Osawa, T. Associations of Periparturient Plasma Biochemical Parameters, Endometrial Leukocyte Esterase and Myeloperoxidase, and Bacterial Detection with Clinical and Subclinical Endometritis in Postpartum Dairy Cows. J. Vet. Med. Sci. 2018, 80, 302–310. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Constituents of Neutrophil Extracellular Traps Induce in Vitro Collagen Formation in Mare Endometrium. Theriogenology 2018, 113, 8–18. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Lukasik, K.; Szóstek-Mioduchowska, A.; Baclawska, A.; Rebordão, M.R.; Aguiar-Silva, J.; Pinto-Bravo, P.; Skarzynski, D.J.; Ferreira-Dias, G. Elastase Inhibition Affects Collagen Transcription and Prostaglandin Secretion in Mare Endometrium during the Estrous Cycle. Reprod. Dom. Anim. 2018, 53, 66–69. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Rebordão, M.R.; Szóstek-Mioduchowska, A.; Lukasik, K.; Gawronska-Kozak, B.; Telo da Gama, L.; Skarzynski, D.J.; Ferreira-Dias, G. The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium. Animals 2020, 10, 863. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Morazzo, S.; Rebordão, M.R.; Szóstek-Mioduchowska, A.; Lukasik, K.; Gawronska-Kozak, B.; Telo da Gama, L.; Skarzynski, D.J.; Ferreira-Dias, G. The Inhibition of Cathepsin G on Endometrial Explants With Endometrosis in the Mare. Front. Vet. Sci. 2020, 7, 582211. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.M. The aetiology, diagnosis, and classification of chronic degenerative endometritis. In Workshop on Equine Endometritis; Hughes, J.P., Ed.; Newmarket Press: New York, NY, USA, 1992; Volume 125, p. 186. [Google Scholar]
- Hoffmann, C.; Ellenberger, C.; Mattos, R.C.; Aupperle, H.; Dhein, S.; Stief, B.; Schoon, H.-A. The Equine Endometrosis: New Insights into the Pathogenesis. Anim. Reprod. Sci. 2009, 111, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Wagner, B.D.; Anthony, M.M.; Emmett, P.; Zemanick, E.T. Sputum Biomarkers of Inflammation and Lung Function Decline in Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Sly, P.D.; Gangell, C.L.; Chen, L.; Ware, R.S.; Ranganathan, S.; Mott, L.S.; Murray, C.P.; Stick, S.M. Risk Factors for Bronchiectasis in Children with Cystic Fibrosis. N. Engl. J. Med. 2013, 368, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.R.; Jones, B.E. Hepatitis C Virus and Oxidative Stress: A Dangerous Liaison. Future Virol. 2006, 1, 223–232. [Google Scholar] [CrossRef]
- Pulli, B.; Ali, M.; Iwamoto, Y.; Zeller, M.W.G.; Schob, S.; Linnoila, J.J.; Chen, J.W. Myeloperoxidase–Hepatocyte–Stellate Cell Cross Talk Promotes Hepatocyte Injury and Fibrosis in Experimental Nonalcoholic Steatohepatitis. Antioxid. Redox Signal. 2015, 23, 1255–1269. [Google Scholar] [CrossRef]
- Kettle, A.J.; Gedye, C.A.; Winterbourn, C.C. Mechanism of Inactivation of Myeloperoxidase by 4-Aminobenzoic Acid Hydrazide. Biochem. J. 1997, 321, 503–508. [Google Scholar] [CrossRef]
- Lazarević-Pasti, T.; Leskovac, A.; Vasić, V. Myeloperoxidase Inhibitors as Potential Drugs. Curr. Drug. Metab. 2015, 16, 168–190. [Google Scholar] [CrossRef]
- Kim, H.; Wei, Y.; Lee, J.Y.; Wu, Y.; Zheng, Y.; Moskowitz, M.A.; Chen, J.W. Myeloperoxidase Inhibition Increases Neurogenesis after Ischemic Stroke. J. Pharmacol. Exp. Ther. 2016, 359, 262–272. [Google Scholar] [CrossRef]
- Hair, P.S.; Sass, L.A.; Krishna, N.K.; Cunnion, K.M. Inhibition of Myeloperoxidase Activity in Cystic Fibrosis Sputum by Peptide Inhibitor of Complement C1 (PIC1). PLoS ONE 2017, 12, e0170203. [Google Scholar] [CrossRef]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9): The next Decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. In Advance Pharmacol; Elsevier: Amsterdam, The Netherlands, 2018; Volume 81, pp. 241–330. [Google Scholar] [CrossRef]
- Aresu, L.; Benali, S.; Giannuzzi, D.; Mantovani, R.; Castagnaro, M.; Falomo, M.E. The Role of Inflammation and Matrix Metalloproteinases in Equine Endometriosis. J. Vet. Sci. 2012, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Centeno, L.A.M.; Bastos, H.B.A.; Bueno, V.L.C.; Trentin, J.M.; Fiorenza, M.F.; Fiala-Rechsteiner, S.; Kretzmann, N.A.; Mattos, R.C.; Rubin, M.I.B. Gene Expression of MMP-1, MMP-2 and TNF-α in the Endometrium of Mares With Different Degrees of Fibrosis. J. Equine Vet. Sci. 2018, 66, 143–144. [Google Scholar] [CrossRef]
- Walter, I.; Handler, J.; Miller, I.; Aurich, C. Matrix metalloproteinase 2 (MMP-2) and tissue transglutaminase (TG 2) are expressed in periglandular fibrosis in horse mares with endometrosis. Histol. Histopathol. 2005, 20, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Szóstek-Mioduchowska, A.Z.; Baclawska, A.; Okuda, K.; Skarzynski, D.J. Effect of Proinflammatory Cytokines on Endometrial Collagen and Metallopeptidase Expression during the Course of Equine Endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef] [PubMed]
- Szóstek-Mioduchowska, A.; Słowińska, M.; Pacewicz, J.; Skarzynski, D.J.; Okuda, K. Matrix Metallopeptidase Expression and Modulation by Transforming Growth Factor-Β1 in Equine Endometrosis. Sci. Rep. 2020, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Szóstek-Mioduchowska, A.Z.; Baclawska, A.; Rebordão, M.R.; Ferreira-Dias, G.; Skarzynski, D.J. Prostaglandins Effect on Matrix Metallopeptidases and Collagen in Mare Endometrial Fibroblasts. Theriogenology 2020, 153, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Roberto da Costa, R.P.; Ferreira-Dias, G.; Mateus, L.; Korzekwa, A.; Andronowska, A.; Platek, R.; Skarzynski, D.J. Endometrial Nitric Oxide Production and Nitric Oxide Synthases in the Equine Endometrium: Relationship with Microvascular Density during the Estrous Cycle. Domest. Anim. Endocrinol. 2007, 32, 287–302. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Impairment of the Antifibrotic Prostaglandin E2 Pathway May Influence Neutrophil Extracellular Traps–Induced Fibrosis in the Mare Endometrium. Domest. Anim. Endocrinol. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Kenney, R.M.; Doig, P.A. Equine endometrial biopsy. Current Therapy in Theriogenology 2: Diagnosis, Treatment, and Prevention of Reproductive Diseases in Small and Large Animals, 2nd ed.; David, A.M., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1986; pp. 723–729. [Google Scholar]
- Nash, D.; Lane, E.; Herath, S.; Martin Sheldon, I. Endometrial Explant Culture for Characterizing Equine Endometritis: Optimization of equine endometrial culture. Am. J. Reprod. Immunol. 2008, 59, 105–117. [Google Scholar] [CrossRef]
- Szóstek, A.Z.; Lukasik, K.; Galvão, A.M.; Ferreira-Dias, G.M.; Skarzynski, D.J. Impairment of the Interleukin System in Equine Endometrium During the Course of Endometrosis1. Biol. Reprod. 2013, 89, 79. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.V.; Sjögren, T.; Auchère, F.; Jenkins, D.W.; Thong, B.; Laughton, D.; Hemsley, P.; Pairaudeau, G.; Turner, R.; Eriksson, H.; et al. Potent Reversible Inhibition of Myeloperoxidase by Aromatic Hydroxamates. J. Biol. Chem. 2013, 288, 36636–36647. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of Housekeeping Genes for Normalizing RNA Expression in Real-Time PCR. BioTechniques 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Posch, A.; Kohn, J.; Oh, K.; Hammond, M.; Liu, N. V3 Stain-Free Workflow for a Practical, Convenient, and Reliable Total Protein Loading Control in Western Blotting. J. Vis. Exp. 2013, 82, e50948. [Google Scholar] [CrossRef] [PubMed]
- Manuel, J.A.; Gawronska-Kozak, B. Matrix Metalloproteinase 9 (MMP-9) Is Upregulated during Scarless Wound Healing in Athymic Nude Mice. Matrix Biol. 2006, 25, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Raykin, J.; Snider, E.; Bheri, S.; Mulvihill, J.; Ethier, C.R. A Modified Gelatin Zymography Technique Incorporating Total Protein Normalization. Anal. Biochem. 2017, 521, 8–10. [Google Scholar] [CrossRef]
- Aurich, C. Reproductive Cycles of Horses. Anim. Reprod. Sci. 2011, 124, 220–228. [Google Scholar] [CrossRef]
- Marth, C.D.; Firestone, S.M.; Glenton, L.Y.; Browning, G.F.; Young, N.D.; Krekeler, N. Oestrous Cycle-Dependent Equine Uterine Immune Response to Induced Infectious Endometritis. Vet. Res. 2016, 47, 110. [Google Scholar] [CrossRef]
- Ferreira-Dias, G.; Nequin, L.G.; King, S.S. Influence of Estrous Cycle Stage on Adhesion of Streptococcus Zooepidemicus to Equine Endometrium. Am. J. Vet. Res. 1994, 55, 1028–1031. [Google Scholar]
- Gebhardt, S.; Merkl, M.; Herbach, N.; Wanke, R.; Handler, J.; Bauersachs, S. Exploration of Global Gene Expression Changes During the Estrous Cycle in Equine Endometrium1. Biol. Reprod. 2012, 87, 136. [Google Scholar] [CrossRef] [PubMed]
- Stenbäck, F. Collagen Type III Formation and Distribution in the Uterus: Effects of Hormones and Neoplasm Development. Oncology 1989, 46, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.D.; Margaroli, C.; Horati, H.; Kilgore, M.B.; Veltman, M.; Liu, H.K.; Taurone, A.J.; Peng, L.; Guglani, L.; Uppal, K.; et al. Myeloperoxidase Oxidation of Methionine Associates with Early Cystic Fibrosis Lung Disease. Eur. Respir. J. 2018, 52, 1801118. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.; Baldus, S.; Klinke, A. Fibrosis in Atrial Fibrillation–Role of Reactive Species and MPO. Front. Physio. 2012, 3, 214. [Google Scholar] [CrossRef] [PubMed]
- Szóstek-Mioduchowska, A.Z.; Lukasik, K.; Skarzynski, D.J.; Okuda, K. Effect of Transforming Growth Factor -Β1 on α-Smooth Muscle Actin and Collagen Expression in Equine Endometrial Fibroblasts. Theriogenology 2019, 124, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Burner, U.; Obinger, C.; Paumann, M.; Furtmüller, P.G.; Kettle, A.J. Transient and Steady-State Kinetics of the Oxidation of Substituted Benzoic Acid Hydrazides by Myeloperoxidase. J. Biol. Chem. 1999, 274, 9494–9502. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Gomes, C.; Birk, B.; Williford, T.; Hernandez, T.R.; Haase, C.; Zbranek, R.; van Ravenzwaay, B.; Landsiedel, R. In Vitro-to-in Vivo Extrapolation (IVIVE) by PBTK Modeling for Animal-Free Risk Assessment Approaches of Potential Endocrine-Disrupting Compounds. Arch. Toxicol. 2019, 93, 401–416. [Google Scholar] [CrossRef]
- Clippinger, A.J.; Ahluwalia, A.; Allen, D.; Bonner, J.C.; Casey, W.; Castranova, V.; David, R.M.; Halappanavar, S.; Hotchkiss, J.A.; Jarabek, A.M.; et al. Expert Consensus on an in Vitro Approach to Assess Pulmonary Fibrogenic Potential of Aerosolized Nanomaterials. Arch. Toxicol. 2016, 90, 1769–1783. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef]
- Nascimento, G.C.; Rizzi, E.; Gerlach, R.F.; Leite-Panissi, C.R.A. Expression of MMP-2 and MMP-9 in the Rat Trigeminal Ganglion during the Development of Temporomandibular Joint Inflammation. Braz. J. Med. Biol. Res. 2013, 46, 956–967. [Google Scholar] [CrossRef]
- Accorsi-Mendonça, T.; Silva, E.J.N.L.; Marcaccini, A.M.; Gerlach, R.F.; Duarte, K.M.R.; Pardo, A.P.S.; Line, S.R.P.; Zaia, A.A. Evaluation of Gelatinases, Tissue Inhibitor of Matrix Metalloproteinase-2, and Myeloperoxidase Protein in Healthy and Inflamed Human Dental Pulp Tissue. J. Endod. 2013, 39, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Spallarossa, P.; Garibaldi, S.; Barisione, C.; Ghigliotti, G.; Altieri, P.; Tracchi, I.; Fabbi, P.; Barsotti, A.; Brunelli, C. Postprandial Serum Induces Apoptosis in Endothelial Cells: Role of Polymorphonuclear-Derived Myeloperoxidase and Metalloproteinase-9 Activity. Atherosclerosis 2008, 198, 458–467. [Google Scholar] [CrossRef] [PubMed]


| Gene (Accession Number) | Sequence 5′-3′ | Amplicon |
|---|---|---|
| RPL32 (XM_001492042.6) | Forward: AGCCATCTACTCGGCGTCA | 144 |
| Reverse: GTCAATGCCTCTGGGTTTCC | ||
| COL1A2 (XM_001492939.3) | Forward: CAAGGGCATTAGGGGACACA | 196 |
| Reverse: ACCCACACTTCCATCGCTTC | ||
| MMP2 (XM_001493281.2) | Forward: TCCCACTTTGATGACGACGA | 115 |
| Reverse: TTGCCGTTGAAGAGGAAAGG | ||
| MMP9 (NM_001111302.1) | Forward: GCGGTAAGGTGCTGCTGTTC | 177 |
| Reverse: GAAGCGGTCCTGGGAGAAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, A.; Fernandes, C.; Rebordão, M.R.; Szóstek-Mioduchowska, A.; Lukasik, K.; Pinto-Bravo, P.; Telo da Gama, L.; Jan Skarzynski, D.; Ferreira-Dias, G. Myeloperoxidase Inhibition Decreases the Expression of Collagen and Metallopeptidase in Mare Endometria under In Vitro Conditions. Animals 2021, 11, 208. https://doi.org/10.3390/ani11010208
Amaral A, Fernandes C, Rebordão MR, Szóstek-Mioduchowska A, Lukasik K, Pinto-Bravo P, Telo da Gama L, Jan Skarzynski D, Ferreira-Dias G. Myeloperoxidase Inhibition Decreases the Expression of Collagen and Metallopeptidase in Mare Endometria under In Vitro Conditions. Animals. 2021; 11(1):208. https://doi.org/10.3390/ani11010208
Chicago/Turabian StyleAmaral, Ana, Carina Fernandes, Maria Rosa Rebordão, Anna Szóstek-Mioduchowska, Karolina Lukasik, Pedro Pinto-Bravo, Luís Telo da Gama, Dariusz Jan Skarzynski, and Graça Ferreira-Dias. 2021. "Myeloperoxidase Inhibition Decreases the Expression of Collagen and Metallopeptidase in Mare Endometria under In Vitro Conditions" Animals 11, no. 1: 208. https://doi.org/10.3390/ani11010208
APA StyleAmaral, A., Fernandes, C., Rebordão, M. R., Szóstek-Mioduchowska, A., Lukasik, K., Pinto-Bravo, P., Telo da Gama, L., Jan Skarzynski, D., & Ferreira-Dias, G. (2021). Myeloperoxidase Inhibition Decreases the Expression of Collagen and Metallopeptidase in Mare Endometria under In Vitro Conditions. Animals, 11(1), 208. https://doi.org/10.3390/ani11010208








