Insight into the Epidemiology of Leptospirosis: A Review of Leptospira Isolations from “Unconventional” Hosts
Abstract
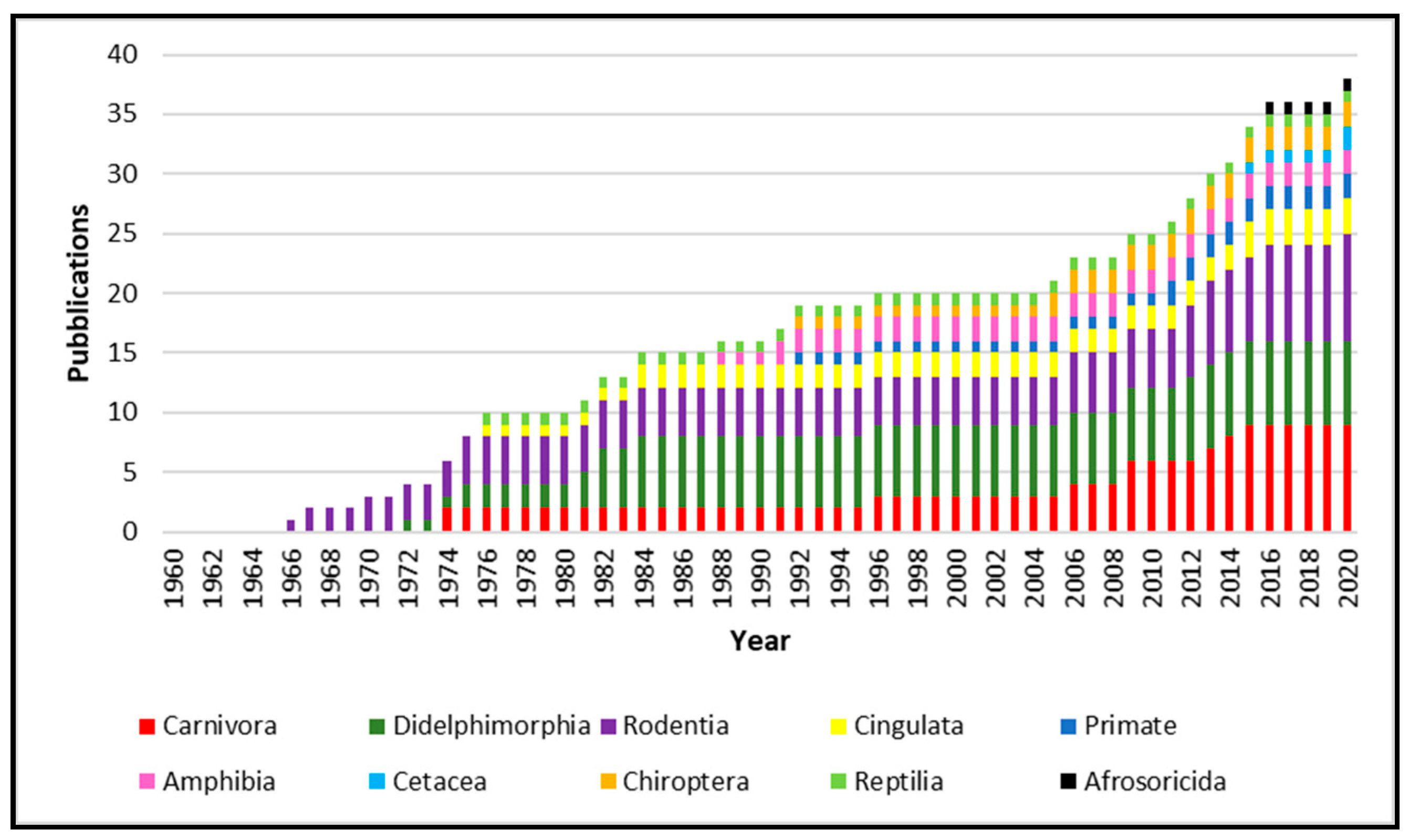
Simple Summary
Abstract
1. Introduction
2. Leptospira Isolation on “Unconventional” Host
2.1. Carnivora
2.2. Cetacea
2.3. Didelphimorphia
2.4. Cingulata
2.5. Rodentia
2.6. Afrosoricida
2.7. Chiroptera
2.8. Primates
2.9. Reptilia
2.10. Amphibia
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Adler, B.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Goldstein, S.F.; Charon, N.W. Motility of the spirochete leptospira. Cell Motil. Cytoskelet. 1988, 9, 101–110. [Google Scholar] [CrossRef]
- Adler, B. Leptospira and Leptospirosis. In Current Topics in Microbiology and Immunology; Springer: Berlin, Germany, 2015; ISBN 978-3-662-45059-8. [Google Scholar]
- Kmety, E.; Dikken, H. Classification of the Species Leptospira Interrogans and History of its Serovars; University Press Groningen: Groningen, The Netherlands, 1993. [Google Scholar]
- Guglielmini, J.; Bourhy, P.; Schiettekatte, O.; Zinini, F.; Brisse, S.; Picardeau, M. Genus-wide Leptospira core genome multilocus sequence typing for strain taxonomy and global surveillance. PLoS Negl. Trop. Dis. 2019, 13, e0007374. [Google Scholar] [CrossRef]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.N.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Gangadhar, N.L.; Mohandoss, N.; Thirumalesh, S.R.A.; Dhar, M.; Shome, R.; Krishnamoorthy, P.; Prabhudas, K.; Rahman, H. Characterization of leptospira isolates from animals and humans: Phylogenetic analysis identifies the prevalence of intermediate species in India. Springerplus 2013, 2, 362. [Google Scholar] [CrossRef]
- Barragan, V.; Chiriboga, J.; Miller, E.; Olivas, S.; Birdsell, D.; Hepp, C.; Hornstra, H.; Schupp, J.M.; Morales, M.; Gonzalez, M.; et al. High Leptospira Diversity in Animals and Humans Complicates the Search for Common Reservoirs of Human Disease in Rural Ecuador. PLoS Negl. Trop. Dis. 2016, 10, e0004990. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Levett, P.N.; Haake, D.A. Leptospira Species (Leptospirosis). In Principles and Practice of Infectious Diseases; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 2010; pp. 3059–3065. [Google Scholar]
- Leptospirosis. In OIE Terrestrial Manual 2018; World Organization for Animal Health: Paris, France, 2018; pp. 503–516.
- Musso, D.; La Scola, B. Laboratory diagnosis of leptospirosis: A challenge. J. Microbiol. Immunol. Infect. 2013, 46, 245–252. [Google Scholar] [CrossRef]
- Arent, Z.J.; Ellis, W.A. Leptospirosis. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 854–862. ISBN 9781119350927. [Google Scholar]
- Hornsby, R.L.; Alt, D.P.; Nally, J.E. Isolation and propagation of leptospires at 37 °C directly from the mammalian host. Sci. Rep. 2020, 10, 9620. [Google Scholar] [CrossRef]
- Bertelloni, F.; Cilia, G.; Turchi, B.; Pinzauti, P.; Cerri, D.; Fratini, F. Epidemiology of leptospirosis in North-Central Italy: Fifteen years of serological data (2002–2016). Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Bertelloni, F.; Cilia, G. Leptospira Infection in Wild Animals; Nova Science Publisher: Hauppauge, NY, USA, 2020; ISBN 978-1-53618-222-4. [Google Scholar]
- Cilia, G.; Bertelloni, F.; Mignone, W.; Spina, S.; Berio, E.; Razzuoli, E.; Vencia, W.; Franco, V.; Cecchi, F.; Bogi, S.; et al. Molecular detection of Leptospira spp. in wild boar (Sus scrofa) hunted in Liguria region (Italy). Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101410. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Bertelloni, F.; Pinzauti, P.; Cerri, D. Seroprevalence of Leptospira spp. and Borrelia burgdorferi sensu lato in Italian horses. Ann. Agric. Environ. Med. 2012, 19, 237–240. [Google Scholar] [PubMed]
- Cilia, G.; Bertelloni, F.; Piredda, I.; Ponti, M.N.; Turchi, B.; Cantinle, C.; Parisi, F.; Pinzauti, P.; Armani, A.; Palmas, B.; et al. Presence of pathogenic Leptospira spp. in the reproductive system and fetuses of wild boars (Sus scrofa) in Italy. PLoS Negl. Trop. Dis. 2020, 14, e0008982. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.A. Animal Leptospirosis. In Leptospira and Leptospirosis; Springer: Berlin, Germany, 2015; pp. 99–137. [Google Scholar]
- Cilia, G.; Bertelloni, F.; Angelini, M.; Cerri, D.; Fratini, F. Leptospira Survey in Wild Boar (Sus scrofa) Hunted in Tuscany, Central Italy. Pathogens 2020, 9, 377. [Google Scholar] [CrossRef]
- Blasdell, K.R.; Morand, S.; Perera, D.; Firth, C. Association of rodent-borne Leptospira spp. with urban environments in Malaysian Borneo. PLoS Negl. Trop. Dis. 2019, 13, e0007141. [Google Scholar] [CrossRef]
- Mori, M.; Bourhy, P.; Le Guyader, M.; van Esbroeck, M.; Djelouadji, Z.; Septfons, A.; Kodjo, A.; Picardeau, M. Pet rodents as possible risk for leptospirosis, Belgium and France, 2009 to 2016. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef]
- Bertelloni, F.; Mazzei, M.; Cilia, G.; Forzan, M.; Felicioli, A.; Sagona, S.; Bandecchi, P.; Turchi, B.; Cerri, D.; Fratini, F. Serological Survey on Bacterial and Viral Pathogens in Wild Boars Hunted in Tuscany. Ecohealth 2020, 17, 85–93. [Google Scholar] [CrossRef]
- Vale-Goncalves, H.M.; Cabral, J.A.; Faria, M.C.; Nunes-Pereira, M.; Faria, A.S.; Veloso, O.; Vieira, M.L.; Paiva-Cardoso, M.N. Prevalence of Leptospira antibodies in wild boars (Sus scrofa) from Northern Portugal: Risk factor analysis. Epidemiol. Infect. 2015, 143, 2126–2130. [Google Scholar] [CrossRef]
- Vengust, G.; Lindtner-Knific, R.; Zele, D.; Bidovec, A. Leptospira antibodies in wild boars (Sus scrofa) in Slovenia. Eur. J. Wildl. Res. 2008, 54, 749–752. [Google Scholar] [CrossRef]
- Boqvist, S.; Bergström, K.; Magnusson, U. Prevalence of antibody to six Leptospira Servovars in Swedish wild boars. J. Wildl. Dis. 2012, 48, 492–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cilia, G.; Bertelloni, F. Leptospira Infection in Wild Boar (Sus scrofa). In Leptospira Infection in Wild Animals; Fratini, F., Bertelloni, F., Cilia, G., Eds.; Nova Science Publisher: Hauppauge, NY, USA, 2020; pp. 53–77. [Google Scholar]
- Arent, Z.; Frizzell, C.; Gilmore, C.; Allen, A.; Ellis, W.A. Leptospira interrogans serovars Bratislava and Muenchen animal infections: Implications for epidemiology and control. Vet. Microbiol. 2016, 190, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.; Ellis, W.A.; Montgomery, J.; Gilmore, C.; Regalla, J.; Brem, S. Microbiological and serological study of leptospirosis in horses at slaughter: First isolations. Res. Vet. Sci. 2004, 76, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Farina, R.; Cerri, D.; Renzoni, G.; Andreani, E.; Mani, P.; Ebani, V.; Pedrini, A.; Nuvoloni, R. Leptospira interrogans in the genital tract of sheep. Research on ewes and rams experimentally infected with serovar hardjo (hardjobovis). New Microbiol. 1996, 19, 235–242. [Google Scholar] [PubMed]
- Cerri, D.; Ebani, V.V.; Fratini, F.; Pinzauti, P.; Andreani, E. Epidemiology of leptospirosis: Observations on serological data obtained by a “diagnostic laboratory for leptospirosis” from 1995 to 2001. New Microbiol. 2003, 26, 383–389. [Google Scholar] [PubMed]
- Chikeka, I.; Dumler, J.S. Neglected bacterial zoonoses. Clin. Microbiol. Infect. 2015, 21, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Vijayachari, P.; Sugunan, A.P.; Shriram, A.N. Leptospirosis: An emerging global public health problem. J. Biosci. 2008, 33, 557–569. [Google Scholar] [CrossRef]
- Hartskeerl, R.; Collares-Pereira, M.; Ellis, W.A. Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin. Microbiol. Infect. 2011, 17, 494–501. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Wang, Y.; Chang, Y.F.; Zhang, Y.; Jiang, X.; Zhuang, X.; Zhu, Y.; Zhang, J.; Zeng, L.; et al. Whole genome sequencing revealed host adaptation-focused genomic plasticity of pathogenic Leptospira. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Cinco, M. New insights into the pathogenicity of leptospires: Evasion of host defences. New Microbiol. 2010, 33, 283–292. [Google Scholar]
- Espinosa-Martínez, D.V.; Sánchez-Montes, D.S.; León-Paniagua, L.; Ríos-Muñoz, C.A.; Berzunza-Cruz, M.; Becker, I. New Wildlife Hosts of Leptospira interrogans in Campeche, Mexico. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Bogomolni, A.L.; Gast, R.J.; Ellis, J.C.; Dennett, M.; Pugliares, K.R.; Lentell, B.J.; Moore, M.J. Victims or vectors: A survey of marine vertebrate zoonoses from coastal waters of the Northwest Atlantic. Dis. Aquat. Organ. 2008, 81, 13–38. [Google Scholar] [CrossRef] [PubMed]
- Jobbins, S.E.; Alexander, K.A. Evidence of Leptospira sp. infection among a diversity of African wildlife species: Beyond the usual suspects. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V. Leptospira Infection in Amphibians and Reptiles. In Leptospira Infection in Wild Animals; Fratini, F., Bertelloni, F., Cilia, G., Eds.; Nova Science Publisher: Hauppauge, NY, USA, 2020; pp. 205–218. [Google Scholar]
- Thibeaux, R.; Girault, D.; Bierque, E.; Soupé-Gilbert, M.-E.; Rettinger, A.; Douyère, A.; Meyer, M.; Iraola, G.; Picardeau, M.; Goarant, C. Biodiversity of Environmental Leptospira: Improving Identification and Revisiting the Diagnosis. Front. Microbiol. 2018, 9, 816. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Fratini, F.; della Buona, E.; Bertelloni, F. Preliminary Evaluation of In Vitro Bacteriostatic and Bactericidal Effect of Salt on Leptospira spp. Vet. Sci. 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Dupouey, J.; Faucher, B.; Edouard, S.; Richet, H.; Kodjo, A.; Drancourt, M.; Davoust, B. Human leptospirosis: An emerging risk in Europe? Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 77–83. [Google Scholar] [CrossRef]
- Bertelloni, F.; Cilia, G.; Fratini, F. Bacteriostatic and Bactericidal Effect of Tigecycline on Leptospira spp. Antibiotics 2020, 9, 467. [Google Scholar] [CrossRef]
- Liegeon, G.; Delory, T.; Picardeau, M. Antibiotic susceptibilities of livestock isolates of leptospira. Int. J. Antimicrob. Agents 2018, 51, 693–699. [Google Scholar] [CrossRef]
- Ressner, R.A.; Griffith, M.E.; Beckius, M.L.; Pimentel, G.; Miller, R.S.; Mende, K.; Fraser, S.L.; Galloway, R.L.; Hospenthal, D.R.; Murray, C.K. Antimicrobial susceptibilities of geographically diverse clinical human isolates of Leptospira. Antimicrob. Agents Chemother. 2008, 52, 2750–2754. [Google Scholar] [CrossRef]
- Gulland, F.M.D.; Koski, M.; Lowenstine, L.J.; Colagross, A.; Morgan, L.; Spraker, T. Leptospirosis in California sea lions (Zalophus californianus) stranded along the central California coast, 1981–1994. J. Wildl. Dis. 1996, 32, 572–580. [Google Scholar] [CrossRef]
- Zuerner, R.L.; Cameron, C.E.; Raverty, S.; Robinson, J.; Colegrove, K.M.; Norman, S.A.; Lambourn, D.; Jeffries, S.; Alt, D.P.; Gulland, F. Geographical dissemination of Leptospira interrogans serovar Pomona during seasonal migration of California sea lions. Vet. Microbiol. 2009, 137, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Brown, R.J.; Skilling, D.E.; DeLong, R.L. Leptospira pomona and reproductive failure in California sea lions. J. Am. Vet. Med. Assoc. 1974, 165, 996–998. [Google Scholar] [PubMed]
- Smith, A.W.; Prato, C.M.; Gilmartin, W.G.; Brown, R.J.; Keyes, M.C. A Preliminary Report on Potentially Pathogenic Microbiological Agents Recently Isolated from Pinnipeds. J. Wildl. Dis. 1974, 10, 54–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piredda, I.; Ponti, M.N. Leptospirosis in Marine Mammals. In Leptospira Infection in Wild Animals; Fratini, F., Bertelloni, F., Cilia, G., Eds.; Nova Science Publisher: Hauppauge, NY, USA, 2020; pp. 9–52. [Google Scholar]
- Prager, K.C.; Greig, D.J.; Alt, D.P.; Galloway, R.L.; Hornsby, R.L.; Palmer, L.J.; Soper, J.; Wu, Q.; Zuerner, R.L.; Gulland, F.M.D.; et al. Asymptomatic and chronic carriage of Leptospira interrogans serovar Pomona in California sea lions (Zalophus californianus). Vet. Microbiol. 2013, 164, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Norman, S.A.; DiGiacomo, R.F.; Gulland, F.M.D.; Meschke, J.S.; Lowry, M.S. Risk factors for an outbreak of leptospirosis in California sea lions (Zalophus californianus) in California, 2004. J. Wildl. Dis. 2008, 44, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Brown, R.J.; Skilling, D.E.; Bray, H.L.; Keyes, M.C. Naturally-occurring leptospirosis in northern fur seals (Callorhinus ursinus). J. Wildl. Dis. 1977, 13, 144–148. [Google Scholar] [CrossRef]
- Godínez, C.R.; De Romillo, B.Z.; Aurioles-Gamboa, D.; Verdugo-Rodríguez, A.; Rodríguez-Reyes, E.A.; De la Peña-Moctezuma, A. Antibodies against Leptospira interrogans in California sea lion pups from gulf of California. J. Wildl. Dis. 1999, 35, 108–111. [Google Scholar] [CrossRef]
- Delaney, M.A.; Colegrove, K.M.; Spraker, T.R.; Zuerner, R.L.; Galloway, R.L.; Gulland, F.M.D. Isolation of Leptospira from a Phocid: Acute Renal Failure and Mortality from Leptospirosis in Rehabilitated Northern Elephant Seals (Mirounga angustirostris), California, USA. J. Wildl. Dis. 2014, 50, 621–627. [Google Scholar] [CrossRef]
- Stamper, M.A.; Gulland, F.M.D.; Spraker, T. Leptospirosis in rehabilitated Pacific harbor seals from California. J. Wildl. Dis. 1998, 34, 407–410. [Google Scholar] [CrossRef]
- Colegrove, K.M.; Lowenstine, L.J.; Gulland, F.M.D. Leptospirosis in northern elephant seals (Mirounga angustirostris) stranded along the California coast. J. Wildl. Dis. 2005, 41, 426–430. [Google Scholar] [CrossRef]
- Kik, M.J.L.; Goris, M.G.; Bos, J.H.; Hartskeerl, R.A.; Dorrestein, G.M. An outbreak of leptospirosis in seals (Phoca vitulina) in captivity. Vet. Q. 2006, 28, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.; Lipscomb, T.P.; Gulland, F.M. An additional case of leptospirosis in a harbor seal. J. Wildl. Dis. 1999, 35, 150. [Google Scholar] [CrossRef] [PubMed]
- Greig, D.J.; Gulland, F.M.D.; Smith, W.A.; Conrad, P.A.; Field, C.L.; Fleetwood, M.; Harvey, J.T.; Ip, H.S.; Jang, S.; Packham, A.; et al. Surveillance for zoonotic and selected pathogens in harbor seals phoca vitulina from central California. Dis. Aquat. Organ. 2014, 111, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Hueffer, K.; Holcomb, D.; Ballweber, L.R.; Gende, S.M.; Blundell, G.; O’Hara, T.M. Serologic surveillance of pathogens in a declining harbor seal (Phoca vitulina) population in Glacier Bay National Park, Alaska, USA and a reference site. J. Wildl. Dis. 2011, 47, 984–988. [Google Scholar] [CrossRef]
- Bauer, K.L.; Goertz, C.E.C.; Belovarac, J.A.; Walton, R.W.; Lawrence Dunn, J.; Tuomi, P. Infectious disease and toxicological monitoring of stranded pacific harbor seals (Phoca vitulina richardsi) in cook inlet as surrogates for monitoring endangered belugas (Delphinapterus leucas). J. Zoo Wildl. Med. 2016, 47, 770–780. [Google Scholar] [CrossRef]
- Koizumi, N.; Uchida, M.; Makino, T.; Taguri, T.; Kuroki, T.; Muto, M.; Kato, Y.; Watanabe, H. Isolation and characterization of Leptospira spp. from raccoons in Japan. J. Vet. Med. Sci. 2009, 71, 425–429. [Google Scholar] [CrossRef]
- Schnurrenberger, P.R.; Hanson, L.E.; Martin, R.J. Leptospirosis: Long-term surveillance on an Illinois farm. Am. J. Epidemiol. 1970, 92, 223–239. [Google Scholar] [CrossRef]
- Mikaelian, I.; Higgins, R.; Lequient, M.; Major, M.; Lefebvre, F.; Martineau, D. Leptospirosis in raccoons in Quebec: 2 case reports and seroprevalence in a recreational area. Can. Vet. J. 1997, 38, 440–442. [Google Scholar]
- Mitchell, M.A.; Hungerford, L.L.; Nixon, C.; Esker, T.; Sullivan, J.; Koerkenmeier, R.; Dubey, J.P. Serologic survey for selected infectious disease agents in raccoons from Illinois. J. Wildl. Dis. 1999, 35, 347–355. [Google Scholar] [CrossRef]
- Warshawsky, B.; Lindsay, L.R.; Artsob, H. Leptospira infections in trappers from Ontario. Can. J. Infect. Dis. 2000, 11, 47–51. [Google Scholar] [CrossRef][Green Version]
- Scialfa, E.; Brihuega, B.; Venzano, A.; Morris, W.E.; Bolpe, J.; Schettino, M. First Isolation of Leptospira interrogans from Lycalopex griseus (South American Gray Fox) in Argentina Shows New MLVA Genotype. J. Wildl. Dis. 2013, 49, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Candela, M.G.; López-Bao, J.V.; Pereira, M.; Jiménez, M.Á.; León-Vizcaíno, L. Leptospirosis in Wild and Domestic Carnivores in Natural Areas in Andalusia, Spain. Vector Borne Zoonotic Dis. 2009, 9, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Żmudzki, J.; Arent, Z.; Jabłoński, A.; Nowak, A.; Zębek, S.; Stolarek, A.; Bocian, Ł.; Brzana, A.; Pejsak, Z. Seroprevalence of 12 serovars of pathogenic Leptospira in red foxes (Vulpes vulpes) in Poland. Acta Vet. Scand. 2018, 60, 34. [Google Scholar] [CrossRef] [PubMed]
- Slavica, A.; Dezdek, D.; Konjevic, D.; Cvetnic, Z.; Sindicic, M.; Stanin, D.; Habus, J.; Turk, N. Prevalence of Leptospiral Antibodies in the Red Fox (Vulpes vulpes) Population of Croatia. Vet. Med. 2011, 56, 209–213. [Google Scholar] [CrossRef]
- Martino, P.E.; Montenegro, J.L.; Preziosi, J.A.; Venturini, C.; Bacigalupe, D.; Stanchi, N.O.; Bautista, E.L. Serological survey of selected pathogens of free-ranging foxes in southern Argentina, 1998–2001. OIE Rev. Sci. Tech. 2004, 23, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Zamora, J.; Riedemann, S. Wild animals as reservoirs of leptospirosis in Chile: Revision of studies in the country. Arch. Med. Vet. 1999, 31, 151–156. [Google Scholar] [CrossRef]
- Da Silva, F.J.; dos Santos, C.E.P.; Silva, T.R.; Silva, G.C.P.; Loffler, S.G.; Brihuega, B.; Alarcon, M.F.F.; Curci, V.C.M.; Mathias, L.A.; Mathias, L.A. Search of leptospires and of antibodies against leptospires in animals and human beings in farms in Pantanal and Caatinga Brazilian biomes. Brazilian J. Vet. Res. Anim. Sci. 2015, 52, 234. [Google Scholar] [CrossRef]
- Grune Loffler, S.; Rago, V.; Martínez, M.; Uhart, M.; Florin-Christensen, M.; Romero, G.; Brihuega, B. Isolation of a Seawater Tolerant Leptospira spp. from a Southern Right Whale (Eubalaena australis). PLoS ONE 2015, 10, e0144974. [Google Scholar] [CrossRef]
- Piredda, I.; Palmas, B.; Noworol, M.; Tola, S.; Longheu, C.; Bertasio, C.; Scaltriti, E.; Denurra, D.; Cherchi, M.; Picardeau, M.; et al. Isolation of Leptospira interrogans from a Bottlenose Dolphin (Tursiops truncatus) in the Mediterranean Sea. J. Wildl. Dis. 2020. [Google Scholar] [CrossRef]
- Smith, A.W.; Skilling, D.E.; Benirschke, K.; Albert, T.F.; Barlough, J.E. Serology and virology of the bowhead whale (Balaena mysticetus L.). J. Wildl. Dis. 1987, 23, 92–98. [Google Scholar] [CrossRef]
- Mathews, P.D.; Da Silva, V.M.F.; Rosas, F.C.W.; D’Affonseca Neto, J.A.; Lazzarini, S.M.; Ribeiro, D.C.; Dubey, J.P.; Vasconcellos, S.A.; Gennari, S.M. Occurrence of antibodies to toxoplasma gondii and Lepstospira spp. in manatees (Trichechus inunguis) of the Brazilian Amazon. J. Zoo Wildl. Med. 2012, 43, 85–88. [Google Scholar] [CrossRef]
- Sulzner, K.; Kreuder Johnson, C.; Bonde, R.K.; Auil Gomez, N.; Powell, J.; Nielsen, K.; Luttrell, M.P.; Osterhaus, A.D.M.E.; Aguirre, A.A. Health Assessment and Seroepidemiologic Survey of Potential Pathogens in Wild Antillean Manatees (Trichechus manatus manatus). PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Mathews Delgado, P.; Sanchez Perea, N.; Biffi Garcia, C.; García Davila, C.R. Detection of infection with Leptospira spp. in manatees (Trichechus inunguis) of the Peruvian Amazon. Lat. Am. J. Aquat. Mamm. 2015, 10, 58. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Sarmiento, A.M.; Carvalho, V.L.; Meirelles, A.C.O.; Gravena, W.; Marigo, J.; Sacristán, C.; Costa-Silva, S.; Groch, K.R.; Dos Santos Silva, N.; Neto, J.S.F.; et al. Survey of Brucella spp. and Leptospira spp. Antibodies in cetaceans and manatees of the Amazon basin and Atlantic Ocean, Brazil. Dis. Aquat. Organ. 2018, 132, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santa Rosa, C.A.; Sulzer, C.R.; Pestana de Castro, A.F. A new leptospiral serotype in the bataviae group, isolated in Sao Paulo, Brazil. Am. J. Vet. Res. 1972, 33, 1719–1721. [Google Scholar]
- Santa Rosa, C.A.; Sulzer, C.R.; Giorgi, W.; da Silva, A.S.; Yanaguita, R.M.; Lobao, A.O. Leptospirosis in wildlife in Brazil: Isolation of a new serotype in the pyrogenes group. Am. J. Vet. Res. 1975, 36, 1363–1365. [Google Scholar]
- Lins, Z.C.; Lopes, M.L. Isolation of Leptospira from wild forest animals in Amazonian Brazil. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 124–126. [Google Scholar] [CrossRef]
- Liceras de Hidalgo, J.L.; Sulzer, K.R. Six new leptospiral serovars isolated from wild animals in Peru. J. Clin. Microbiol. 1984, 19, 944–945. [Google Scholar] [CrossRef]
- Liceras de Hidalgo, J. Leptospirosis in Tingo María, Huánuco Department, Peru. II. Study in wild animals. Bol. Of. Sanit. Panam. 1981, 91, 47–55. [Google Scholar]
- Jorge, S.; Hartleben, C.P.; Seixas, F.K.; Coimbra, M.A.A.; Stark, C.B.; Larrondo, A.G.; Amaral, M.G.; Albano, A.P.N.; Minello, L.F.; Dellagostin, O.A.; et al. Leptospira borgpetersenii from free-living white-eared opossum (Didelphis albiventris): First isolation in Brazil. Acta Trop. 2012, 124, 147–151. [Google Scholar] [CrossRef]
- Cordeiro, F.; Sulzer, C.; Ramos, A. Leptospira interrogans in several wildlife species in Southeast Brazil. Pesqui. Vet. Bras. 1981, 1, 19–29. [Google Scholar]
- Brihuega, B.; Pavan, M.; Cairo, F.; Venzano, A.; Auteri, C.; Funes, D.; Romero, G.; Samartino, L. Pathogenic Leptospira in the kidney of Didelphys albiventris (weasel). Rev. Argent. Microbiol. 2007, 19, 19. [Google Scholar]
- Reilly, J.R. The susceptibility of five species of wild animals to experimental infection with Leptospira grippotyphosa. J. Wildl. Dis. 1970, 6, 289–294. [Google Scholar] [CrossRef]
- Schenk, J.A.P. Isolation of leptospira of the sero-group Hebdomadis of armadillos (Dasypus novemcinctus) captured in the State of Minas Gerais Brazil. Arq. Esc. Vet. UFMG 1976, 142, 468. [Google Scholar]
- Dalazen, G.T.; Filho, A.F.d.S.; Sarmiento, A.M.S.; Fuentes-Castillo, D.; Gattamorta, M.A.; Kluyber, D.; Desbiez, A.L.J.; Heinemann, M.B.; Matushima, E.R. Survey of leptospira spp. and brucella abortus in free-ranging armadillos from Pantanal, Brazil. J. Wildl. Dis. 2020, 56, 409–413. [Google Scholar] [CrossRef]
- da Silva, R.C.; Zetun, C.B.; Bosco, S.d.M.G.; Bagagli, E.; Rosa, P.S.; Langoni, H. Toxoplasma gondii and Leptospira spp. infection in free-ranging armadillos. Vet. Parasitol. 2008, 157, 291–293. [Google Scholar] [CrossRef]
- Mori, M.; Bakinahe, R. Leptospirosis in Wild Rodents: Besides the rattus genus. In Leptospira Infection in Wild Animals; Fratini, F., Bertelloni, F., Cilia, G., Eds.; Nova Science Publisher: Hauppauge, NY, USA, 2020; pp. 79–124. [Google Scholar]
- Fratini, F.; Turchi, B.; Ebani, V.V.; Bertelloni, F.; Galiero, A.; Cerri, D. The presence of Leptospira in coypus (Myocastor coypus) and rats (Rattus norvegicus) living in a protected wetland in Tuscany (Italy). Vet. Arh. 2015, 85, 407–414. [Google Scholar]
- Shotts, E.B.; Andrews, C.L.; Harvey, T.S. Leptospirosis in selected wild mammals of the Florida panhandle and southwestern Georgia. J. Am. Vet. Med. Assoc. 1975, 167, 587–589. [Google Scholar]
- Diesch, S.L.; Crawford, R.P.; McCulloch, W.F.; Top, F.H. Human Leptospirosis Acquired from Squirrels. New Engl. J. Med. 1967, 276, 838–842. [Google Scholar] [CrossRef]
- Masuzawa, T.; Okamoto, Y.; Une, Y.; Takeuchi, T.; Tsukagoshi, K.; Koizumi, N.; Kawabata, H.; Ohta, S.; Yoshikawa, Y. Leptospirosis in squirrels imported from United States to Japan. Emerg. Infect. Dis. 2006, 12, 1153–1155. [Google Scholar] [CrossRef]
- Tsai, H.J.; Huang, H.C.; Lin, C.M.; Lien, Y.Y.; Chou, C.H. Salmonellae and campylobacters in household and stray dogs in Northern Taiwan. Vet. Res. Commun. 2007, 31, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, A.C.; Guichón, L.M.; Benitez, V.V.; Romero, G.N.; Auteri, C.; Brihuega, B. First isolation of Leptospira interrogans from the arboreal squirrel Callosciurus erythraeus introduced in Argentina. Wildl. Biol. 2013, 19, 483–489. [Google Scholar] [CrossRef]
- Jorge, S.; Monte, L.G.; Coimbra, M.A.; Albano, A.P.; Hartwig, D.D.; Lucas, C.; Seixas, F.K.; Dellagostin, O.A.; Hartleben, C.P. Detection of Virulence Factors and Molecular Typing of Pathogenic Leptospira from Capybara (Hydrochaeris hydrochaeris). Curr. Microbiol. 2012, 65, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.Z.; Miraglia, F.; Marvulo, M.F.V.; Silva, J.C.R.; Paula, C.D.; Costa, B.L.P.; Morais, Z.M.; Ferreira, F.; Neto, J.S.F.; Dellagostin, O.A.; et al. Characterization of Leptospira santarosai Serogroup Grippotyphosa Serovar Bananal Isolated from Capybara (Hydrochaeris hydrochaeris) in Brazil. J. Wildl. Dis. 2016, 52, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Marvulo, M.F.V.; Silva, J.C.R.; Ferreira, P.M.; De Morais, Z.M.; Moreno, A.M.; Doto, D.S.; Paixão, R.; Baccaro, M.R.; Vasconcellos, S.A.; Neto, J.S.F. Experimental Leptospirosis in capybaras (Hydrochaeris hydrochaeris) infected with Leptospira interrogans serovar pomona. J. Zoo Wildl. Med. 2009, 40, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Bertelloni, F.; Coppola, F.; Turchi, B.; Biliotti, C.; Poli, A.; Parisi, F.; Felicioli, A.; Cerri, D.; Fratini, F. Isolation of Leptospira serovar Pomona from a crested porcupine (Hystrix cristata, L., 1758). Vet. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Mitchell, D.; Robertson, A.; Corner, A.H.; Boulanger, P. Some observations on the diagnosis and epidemiology of leptospirosis in swine. Can. J. Comp. Med. Vet. Sci. 1966, 30, 211–217. [Google Scholar]
- Coppola, F.; Cilia, G.; Bertelloni, F.; Casini, L.; D’Addio, E.; Fratini, F.; Cerri, D.; Felicioli, A. Crested porcupine (Hystrix cristata L.): A new potential host for pathogenic Leptospira among semi-fossorial mammals. Comp. Immunol. Microbiol. Infect. Dis. 2020, 70, 101472. [Google Scholar] [CrossRef]
- Siti-Nurdyana, A.; Bahaman, A.; Sharma, R.; Azlan, C.; Abdul Razak, M. Serological prevalence of leptospiral infection in captive Malayan porcupines (Hystrix brachyura). J. Vet. Malaysia 2016, 28, 1–3. [Google Scholar]
- Fornazari, F.; Langoni, H.; Marson, P.M.; Nóbrega, D.B.; Teixeira, C.R. Leptospira reservoirs among wildlife in Brazil: Beyond rodents. Acta Trop. 2018, 178, 205–212. [Google Scholar] [CrossRef]
- Lagadec, E.; Gomard, Y.; Le Minter, G.; Cordonin, C.; Cardinale, E.; Ramasindrazana, B.; Dietrich, M.; Goodman, S.M.; Tortosa, P.; Dellagi, K. Identification of Tenrec ecaudatus, a Wild Mammal Introduced to Mayotte Island, as a Reservoir of the Newly Identified Human Pathogenic Leptospira mayottensis. PLoS Negl. Trop. Dis. 2016, 10, e0004933. [Google Scholar] [CrossRef] [PubMed]
- Desvars, A.; Naze, F.; Vourc’h, G.; Cardinale, E.; Picardeau, M.; Michault, A.; Bourhy, P. Similarities in Leptospira serogroup and species distribution in animals and humans in the Indian ocean island of Mayotte. Am. J. Trop. Med. Hyg. 2012, 87, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Desvars, A.; Cardinale, E.; Michault, A. Animal leptospirosis in small tropical areas. Epidemiol. Infect. 2011, 139, 167–188. [Google Scholar] [CrossRef]
- Matthias, M.A.; Mónica Díaz, M.; Campos, K.J.; Calderon, M.; Willig, M.R.; Pacheco, V.; Gotuzzo, E.; Gilman, R.H.; Vinetz, J.M. Diversity of Bat-Associated Leptospira in The Peruvian Amazon Inferred By Bayesian Phylogenetic Analysis Of 16s Ribosomal Dna Sequences. Am. J. Trop. Med. Hyg. 2005, 73, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Ahmed, A.; Rocha, T.; Vieira, M.L.; Paiva-Cardoso, M.d.N.; Mesquita, J.R.; Linden, H.; Goris, M.; Thompson, G.; Hartskeerl, R.A.; et al. Genetic diversity of pathogenic leptospires from wild, domestic and captive host species in Portugal. Transbound. Emerg. Dis. 2019. [Google Scholar] [CrossRef]
- Perolat, P.; Poingt, J.-P.; Vie, J.-C.; Jouaneau, C.; Baranton, G.; Gysin, J. Occurrence of Severe Leptospirosis in a Breeding Colony of Squirrel Monkeys. Am. J. Trop. Med. Hyg. 1992, 46, 538–545. [Google Scholar] [CrossRef]
- Szonyi, B.; Agudelo-Flórez, P.; Ramírez, M.; Moreno, N.; Ko, A.I. An outbreak of severe leptospirosis in capuchin (Cebus) monkeys. Vet. J. 2011, 188, 237–239. [Google Scholar] [CrossRef]
- Hyakutake, S.; de Biasi, P.; Santa Rosa, C.; Belluomini, H. Contribuiclo ao estudo epidemioldgico das leptospiroses em serpentes do Brasil. Rev. Inst. Med. Trop. Sao Paulo 1976, 18, 10–16. [Google Scholar]
- Ferris, D.H.; Rhoades, H.E.; Hanson, L.E.; Galton, M.; Mansfiels, M.E. Research into the nidality of Leptospira ballum in campestral hosts including the hog-nosed snake (Heterodon platyrhinus). Cornell Vet. 1961, 51, 405–419. [Google Scholar]
- Gravekamp, C.; Korver, H.; Montgomery, J.; Everard, C.O.; Carrington, D.; Ellis, W.A.; Terpstra, W.J. Leptospires isolated from toads and frogs on the Island of Barbados. Zentralbl. Bakteriol. 1991, 275, 403–411. [Google Scholar] [CrossRef]
- Everard, C.O.R.; Carrington, D.; Korver, H.; Everard, J.D. Leptospires in the marine toad (Bufo marinus) on Barbados. J. Wildl. Dis. 1988, 24, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.L.; Cilia, G. Bat-Leptospira: A New Key. In Leptospira Infection in Wild Animals; Fratini, F., Bertelloni, F., Cilia, G., Eds.; Nova Science Publisher: Hauppauge, NY, USA, 2020; pp. 151–172. [Google Scholar]
- Fennestad, K.L.; Borg-Petersen, C. Leptospirosis in Danish Wild Animals. J. Wildl. Dis. 1972, 8, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bessa, T.Á.F.; Spichler, A.; Berardis Chapola, É.G.; Husch, A.C.; De Almeida, M.F.; Sodré, M.M.; Mouriz Savani, E.S.M.; Veiga Sacramento, D.R.; Vinetz, J.M. The contribution of bats to leptospirosis transmission in São Paulo City, Brazil. Am. J. Trop. Med. Hyg. 2010, 82, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Saraullo, V.; Grune, S.; Martinez, M.; Moreira, C.; Alonso, M.L.; Pastorino, F.; Auteri, C.; Martinez, G.; Brihuega, B. Detection of pathogenic Leptospira . in renal tissue from bats (Mammalia: Chiroptera) of Buenos Aires Province using duplex PCR. In Proceedings of the II Congreso Internacional de Zoonosis IX Congreso Argentino de Zoonosis, Buenos Aires, Argentina, 5–7 June 2018; p. 253. [Google Scholar]
- Smythe, L.D.; Field, H.E.; Barnett, L.J.; Smith, C.S.; Dohnt, M.F.; Symonds, M.L.; Moore, M.R.; Rolfe, P.F. Leptospiral Antibodies in Flying Foxes in Australia. J. Wildl. Dis. 2002, 38, 182–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cox, T.E.; Smythe, L.D.; Leung, L.K.-P. Flying Foxes as Carriers of Pathogenic Leptospira species. J. Wildl. Dis. 2005, 41, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Zetun, C.; Hoffmann, J.; Silva, R.; Souza, L.; Langoni, H. Leptospira spp. and Toxoplasma gondii antibodies in vampire bats (Desmodus rotundus) in Botucatu region, SP, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 546–552. [Google Scholar] [CrossRef]
- Bunnell, J.E.; Hice, C.L.; Watts, D.M.; Montrueil, V.; Tesh, R.B.; Vinetz, J.M. Detection of pathogenic Leptospira spp. infections among mammals captured in the Peruvian Amazon basin region. Am. J. Trop. Med. Hyg. 2000, 63, 255–258. [Google Scholar] [CrossRef][Green Version]
- Ballados-González, G.G.; Sánchez-Montes, S.; Romero-Salas, D.; Colunga Salas, P.; Gutiérrez-Molina, R.; León-Paniagua, L.; Becker, I.; Méndez-Ojeda, M.L.; Barrientos-Salcedo, C.; Serna-Lagunes, R.; et al. Detection of pathogenic Leptospira species associated with phyllostomid bats (Mammalia: Chiroptera) from Veracruz, Mexico. Transbound. Emerg. Dis. 2018, 65, 773–781. [Google Scholar] [CrossRef]
- Mayer, F.Q.; Dos Reis, E.M.; Bezerra, A.V.A.; Cerva, C.; Rosa, J.; Cibulski, S.P.; Lima, F.E.S.; Pacheco, S.M.; Rodrigues, R.O. Pathogenic Leptospira spp. in bats: Molecular investigation in Southern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2017, 52, 14–18. [Google Scholar] [CrossRef]
- Gomard, Y.; Dietrich, M.; Wieseke, N.; Ramasindrazana, B.; Lagadec, E.; Goodman, S.M.; Dellagi, K.; Tortosa, P. Malagasy bats shelter a considerable genetic diversity of pathogenic Leptospira suggesting notable host-specificity patterns. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Ayral, F.C.; Bicout, D.J.; Pereira, H.; Artois, M.; Kodjo, A. Distribution of Leptospira serogroups in cattle herds and dogs in France. Am. J. Trop. Med. Hyg. 2014, 91, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Bertelloni, F.; Cilia, G. Leptospira Infection in Non-Human Primates. In Leptospira Infection in Wild Animals; Fratini, F., Bertelloni, F., Cilia, G., Eds.; Nova Science Publisher: Hauppauge, NY, USA, 2020; pp. 173–204. [Google Scholar]
- Romero, P.M.; Astudillo, H.M.; Sánchez, V.J.; González, G.L.; Varela, A.N. Títulos de anticuerpos contra Leptospira sp., en primates del zoológico Matecaña, Pereira, Colombia. Rev. MVZ Córdoba 2012, 17, 3224–3230. [Google Scholar] [CrossRef][Green Version]
- Lilenbaum, W.; Monteiro, R.; Ristow, P.; Fraguas, S.; Cardoso, V.; Fedullo, L.P. Leptospirosis antibodies in mammals from Rio de Janeiro Zoo, Brazil. Res. Vet. Sci. 2002, 73, 319–321. [Google Scholar] [CrossRef]
- Ullmann, L.S.; Neto, R.N.D.; Teixeira, R.H.F.; Nunes, A.V.; Silva, R.C.; Pereira-Richini, V.B.; Langoni, H. Epidemiology of leptospirosis at Sorocaba Zoo, São Paulo state, Southeastern Brazil. Pesqui. Vet. Bras. 2012, 32, 1174–1178. [Google Scholar] [CrossRef]
- González Astudillo, V.; Hernández, D.W.; Stadlin, J.P.; Bernal, L.A.; Rodríguez, D.A.L.; Hernández, M.A. Comparative seroprevalence of Leptospira interrogans in Colombian mammals along a climatic gradient. J. Zoo Wildl. Med. 2012, 43, 768–775. [Google Scholar] [CrossRef]
- Moreno-Beas, E.; Abalos, P.; Hidalgo-Hermoso, E. Seroprevalence of nine leptospira interrogans serovars in wild carnivores, ungulates, and primates from a zoo population in a metropolitan region of Chile. J. Zoo Wildl. Med. 2015, 46, 774–778. [Google Scholar] [CrossRef]
- De Souza Júnior, M.F.; Lobato, Z.I.P.; Lobato, F.C.F.; Moreira, É.C.; de Oliveira, R.R.; Leite, G.G.; Freitas, T.D.; de Assis, R.A. Presença de anticorpos da classe IgM de Leptospira interrogans em animais silvestres do Estado do Tocantins, 2002. Rev. Soc. Bras. Med. Trop. 2006, 39, 292–294. [Google Scholar] [CrossRef]
- Baitchman, E.J.; Calle, P.P.; James, S.B.; Linn, M.J.; Raphael, B.L. Leptospirosis in Wied’s marmosets (Callithrix kuhlii). J. Zoo Wildl. Med. 2006, 37, 182–185. [Google Scholar] [CrossRef]
- Almeida, D.S.; Dos Santos, A.C.; da Silva, C.L.R.; Oriá, A.P.; Oliveira, A.V.D.; Libório, F.A.; Athanazio, D.A.; Pinna, M.H. Evidence of leptospiral exposure in neotropical primatesrescued from illegal trade and a Zoo in Bahia, Brazil. Pesqui. Vet. Bras. 2016, 36, 864–868. [Google Scholar] [CrossRef]
- Pinna, M.H.; Martins, G.; Pinheiro, A.C.O.; Almeida, D.S.; Oriá, A.P.; Lilenbaum, W. Detection of anti-Leptospira antibodies in captive nonhuman primates from Salvador, Brazil. Am. J. Primatol. 2012, 74, 8–11. [Google Scholar] [CrossRef]
- Ebani, V.V. Domestic reptiles as source of zoonotic bacteria: A mini review. Asian Pac. J. Trop. Med. 2017, 10, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.C.S.; Santos, A.L.Q.; Lima, A.M.C.; Gomes, D.O.; Brites, V.L.C. Anti-Leptospira spp. antibodies in Crotalus durissus collilineatus kept in captivity and its zoonotic relevance. Acta Trop. 2016, 158, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Stanchi, N.O.; Grisolia, C.S.; Martino, P.E.; Peluso, F. Presence of antileptospira antibodies in ophidia in Argentina. Rev. Argent. Microbiol. 1986, 18, 127–130. [Google Scholar] [PubMed]

| Animal | Leptospira Isolation | Year | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Class | Order | Family | Species | Common Name | Country | Samples | Serogroup | Serovar | ||
| Mammalia | Carnivora | Otariidae | Zalophus californianus | California Sea Lion | USA | K | Pomona | Pomona | 1996 | [50] |
| - | - | - | K | Pomona | Pomona | 2009 | [115] | |||
| - | - | - | U | Pomona | Pomona | 1974 | [52] | |||
| - | - | - | U | Pomona | Pomona | 2009 | [115] | |||
| - | - | - | P | Pomona | - | 1974 | [53] | |||
| - | - | - | Callorhinus ursinus | Fur Seal | USA | U | Pomona | Pomona | 1974 | [52] |
| - | - | - | L | Pomona | Pomona | 1974 | [52] | |||
| - | - | Phocidae | Phoca vitulina | Harbor Seal | NL | B | Ict | Ict | 2006 | [61] |
| - | - | - | Mirounga angustirostris | Northern Elephant Seal | USA | K | Pomona | Pomona | 2014 | [58] |
| - | - | Procyonidae | Procyon lotor | Raccoon | JPN | K | Ict | Cop/Ict | 2009 | [66] |
| - | - | - | K | Hebdomadis | Hebdomadis | 2009 | [66] | |||
| - | - | Canidae | Lycalopex griseus | South American Gray Fox | ARG | K | Ict | Ict | 2013 | [71] |
| - | - | - | Cerdocyon thous | Maikong | BRA | K | Pomona | - | 2015 | [77] |
| - | Cetacea | Balaenidae | Eubalaena australia | Southern Right Whale | ARG | K | - | Manara | 2015 | [78] |
| - | - | Delphinidae | Tursiops truncatus | Common Bottlenose Dolphin | ITA | K | Pomona | Pomona | 2020 | [79] |
| - | Didelphimorphia | Didelphidae | Didelphis marsupialis | Common Opossum | BRA | K | Bataviae | Brasiliensis | 1972 | [85] |
| - | - | - | K | Grip | Grip | 1975 | [86] | |||
| - | - | - | K | Sejroe | Ballum | 1984 | [87] | |||
| - | - | - | K | Mini | Szwajzak | 2975 | [86] | |||
| - | - | - | K | Ict | Ict | 1975 | [86] | |||
| - | - | - | PER | K | Autumnalis | Autumnalis | 1981 | [89] | ||
| - | - | - | K | Cynopteri | Tingomaria | 1982 | [88,89] | |||
| - | - | - | K | Hebdomadis | Georgia | 1091 | [89] | |||
| - | - | - | K | Djasiman | Huallaga | 1984 | [88] | |||
| - | - | - | K | Sejroe | Rupa rupa | 1984 | [88] | |||
| - | - | - | Didelphis albiventris | White-Eared Opossum | BRA | U | Borg | Castellonis | 2012 | [90] |
| - | - | - | K | Panama | - | 1981 | [91] | |||
| - | - | - | K | Pomona | - | 1981 | [91] | |||
| - | - | - | Philander opossum | Gray Four-Eyed Opossum | BRA | K | Pyrogenes | Guaratuba | 1975 | [86] |
| - | - | - | K | Ballum | - | 1981 | [91] | |||
| - | - | - | K | Grip | - | 1981 | [91] | |||
| - | - | - | PER | K | Pomona | Pomona | 1981 | [89] | ||
| - | - | - | K | Cynopteri | Tingomaria | 1981 | [89] | |||
| - | - | - | K | Hebdomadis | Georgia | 1981 | [89] | |||
| - | - | - | K | Tarassovi | Luis | 1982 | [88,89] | |||
| K | Bataviae | Roja | 1984 | [88] | ||||||
| K | Ict | Machiguenga | 1984 | [88] | ||||||
| - | - | - | - | - | - | - | - | - | - | - |
| - | Cingulata | Dasypodidae | Dasypus novemcinctus | Nine-Banded Armadillo | BRA | K | Autumnalis | - | 1984 | [87] |
| - | - | - | K | Cynopteri | - | 1976 | [94] | |||
| - | - | - | K | Hebdomadis | - | 1975 | [87,94] | |||
| - | - | - | K | Pomona | - | 1976 | [94] | |||
| - | - | - | Euphractus sexcinctus | Six-Banded Armadillo | BRA | U | Pomona | - | 2015 | [77] |
| - | Rodentia | Caviidae | Hydrochoerus hydrochaeris | Capybara | BRA | K | Ict | Ict/Cop | 2012 | [104] |
| - | - | - | K | Grip | Bananal | 2016 | [105] | |||
| - | - | Sciuridae | Callosciurus erythraeus | Red-Bellied Tree Squirrel | ARG | K | Ict | Ict | 2013 | [103] |
| - | - | - | K | Canicola | Canicola | 2013 | [103] | |||
| - | - | - | Glaucomys volans | Southern Flying Squirrel | JPN | K | Grip | - | 2006 | [101] |
| - | - | - | Callosciurus flavimanus | Pallas’s Squirrel | TWN | K | Javanica | - | 2007 | [102] |
| - | - | - | Sciurus niger | Fox squirrel | USA | K | Ballum | - | 1975 | [99] |
| - | - | - | Grip | - | 1967 | [99,100] | ||||
| - | - | Erethizontidae | Erethizon dorsatum | North American Porcupine | CAN | B | Pomona | Pomona | 1966 | [108] |
| - | - | - | U | Pomona | Pomona | 1966 | [108] | |||
| - | - | Hystricidae | Hystrix crsitata | Crested Porcupine | ITA | K | Pomona | Pomona | 2020 | [107] |
| - | Afrosoricida | Tenrecidae | Tenrec ecaudatus | Tailless Tenrec | MD | K | Mayottensis | - | 2016 | [112] |
| - | Chiroptera | Pteropodidae | Pteropus seychellensis comorensis | Seychelles Flying Fox | MD | K | Grip | - | 2016 | [112] |
| - | - | - | Phyllostomus hastatus | Greater Spear-Nosed Bat | PER | K | Ict | Ict | 2005 | [115] |
| - | - | - | Mimon crenulatum | Striped Hairy-Nosed Bat | PER | K | Grip | Grip | 2005 | [115] |
| - | - | - | Promops nasutus; | Brown Mastiff Bat | PER | K | Grip | Grip | 2005 | [115] |
| - | Primates | Lemuridae | Lemur catta | Ring-tailed Lemur | PT | B | Ict | Cop | 2019 | [116] |
| - | - | Cebidae | Callithrix jacchius | Common Marmoset | PT | B | Ict | Cop | 2019 | [116] |
| - | - | - | Saimiri sciureus | Squirrel Monkey | FG | B | Ict | Cop | 1992 | [117] |
| - | - | - | Cebus capuchinus | White-Faced Capuchin Monkeys | COL | B | Ict | Cop/Ict | 2011 | [118] |
| - | - | - | Cebus apella | Tufted Capuchin Monkeys | PT | B | Ict | Cop/Ict | 2011 | [116,118] |
| Reptilia | Squamata | Viperidae | Bothrops pradoi | Prado’s Lancehead Snake | BRA | K | Andaman | Andamana | 1976 | [119] |
| - | - | Colubridae | Heterodon platghrinus | Hognosed Snake | USA | K | Ballum | - | 1961 | [120] |
| Amphibia | Anura | Bufonidae | Bufo marinus | Marine Toad | BRB | K | Australis | Bim | 1991 | [121] |
| - | - | - | K | Australis | Bajan | 1991 | [121] | |||
| - | - | - | B | Autumnalis | Bim | 1988 | [122] | |||
| - | - | - | U | Autumnalis | Bim | 1988 | [122] | |||
| - | - | Eleutherodactylidae | Eleutherodactylus johnstonei | Whistling Frog | BRB | K | Autumnalis | Bim | 1991 | [121] |
| - | - | - | K | Autumnalis | Bim | 1991 | [121] | |||
| - | - | - | K | Australis | Bajan | 1991 | [121] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cilia, G.; Bertelloni, F.; Albini, S.; Fratini, F. Insight into the Epidemiology of Leptospirosis: A Review of Leptospira Isolations from “Unconventional” Hosts. Animals 2021, 11, 191. https://doi.org/10.3390/ani11010191
Cilia G, Bertelloni F, Albini S, Fratini F. Insight into the Epidemiology of Leptospirosis: A Review of Leptospira Isolations from “Unconventional” Hosts. Animals. 2021; 11(1):191. https://doi.org/10.3390/ani11010191
Chicago/Turabian StyleCilia, Giovanni, Fabrizio Bertelloni, Sara Albini, and Filippo Fratini. 2021. "Insight into the Epidemiology of Leptospirosis: A Review of Leptospira Isolations from “Unconventional” Hosts" Animals 11, no. 1: 191. https://doi.org/10.3390/ani11010191
APA StyleCilia, G., Bertelloni, F., Albini, S., & Fratini, F. (2021). Insight into the Epidemiology of Leptospirosis: A Review of Leptospira Isolations from “Unconventional” Hosts. Animals, 11(1), 191. https://doi.org/10.3390/ani11010191







