Immune Responses of Asian Seabass Lates calcarifer to Dietary Glycyrrhiza uralensis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Animals
2.3. Experimental Design and Sampling
2.4. Gene Expression Analysis
2.5. Statistical Analysis
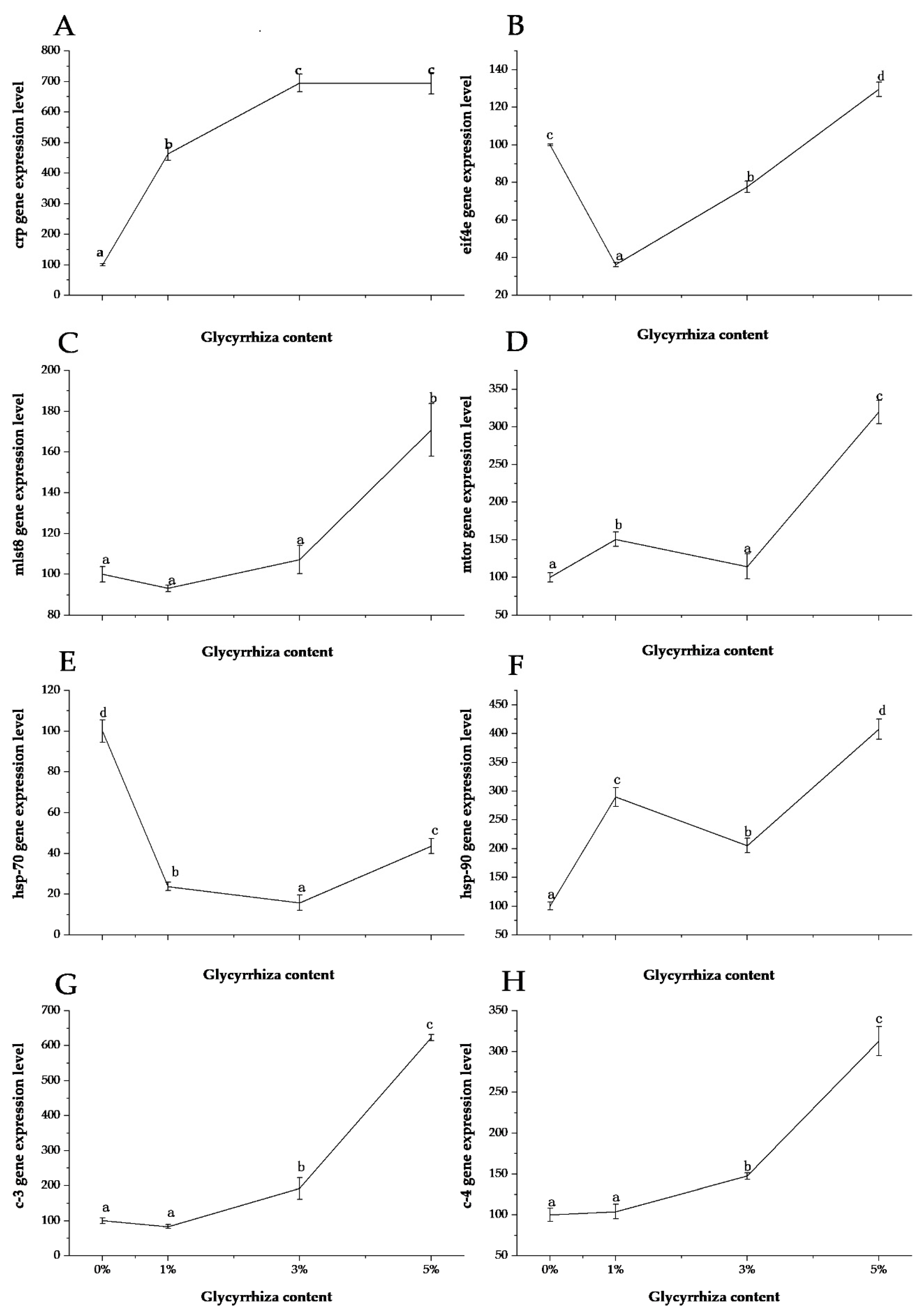
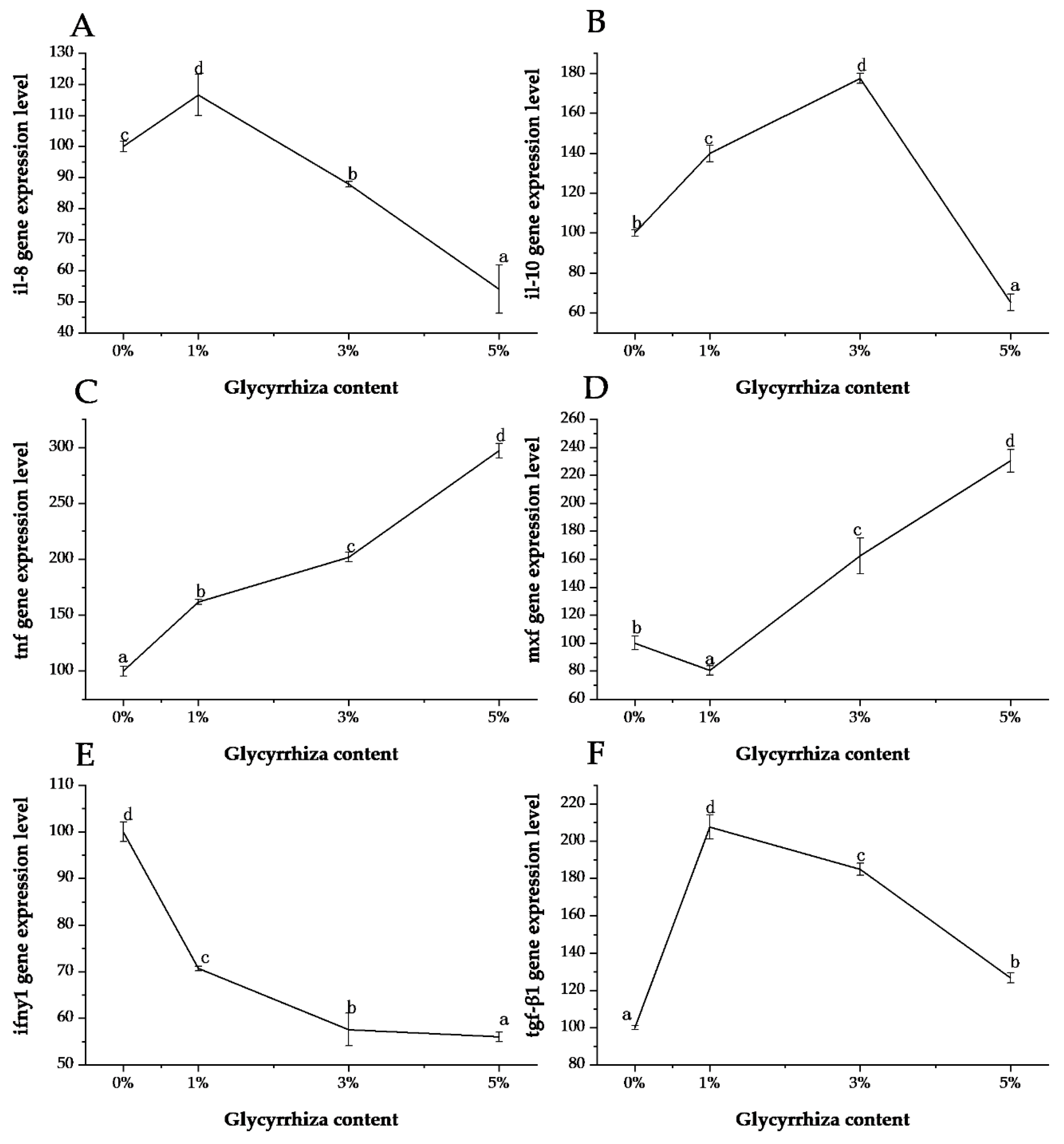
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Cultured Aquatic Species Information Programme. Lates calcarifer (Block, 1790). 2019. Available online: http://www.fao.org/fishery/culturedspecies/Lates_calcarifer/en (accessed on 10 July 2020).
- Ou, Y. Study on artificial breeding of Lates calcarifer (Bloch). Ocean Fish. 2008, 12, 30–32. [Google Scholar]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S.; Nagao, N. Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch, 1790). Aquaculture 2019, 512, 11. [Google Scholar] [CrossRef]
- Talpur, A.D.; Ikhwanuddin, M. Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 2012, 364, 6–12. [Google Scholar] [CrossRef]
- Dong, H.T.; Jitrakorn, S.; Kayansamruaj, P.; Pirarat, N.; Rodkhum, C.; Rattanarojpong, T.; Senapin, S.; Saksmerprome, V. Infectious spleen and kidney necrosis disease (ISKND) outbreaks in farmed barramundi (Lates calcarifer) in Vietnam. Fish Shellfish Immunol. 2017, 68, 65–73. [Google Scholar] [CrossRef]
- Dong, H.T.; Taengphu, S.; Sangsuriya, P.; Charoensapsri, W.; Phiwsaiya, K.; Sornwatana, T.; Khunrae, P.; Rattanarojpong, T.; Senapin, S. Recovery of Vibrio harveyi from scale drop and muscle necrosis disease in farmed barramundi, Lates calcarifer in Vietnam. Aquaculture 2017, 473, 89–96. [Google Scholar] [CrossRef]
- Syed Raffic Ali, S.; Ambasankar, K.; Praveena, P.E.; Nandakumar, S.; Saiyad Musthafa, M. Effect of dietary prebiotic inulin on histology, immuno-haematological and biochemical parameters of Asian seabass (Lates calcarifer). Aquac. Res. 2018, 49, 2732–2740. [Google Scholar] [CrossRef]
- Ali, S.S.R.; Ambasankar, K.; Praveena, P.E.; Nandakumar, S.; Syamadayal, J. Effect of dietary fructooligosaccharide supplementation on growth, body composition, hematological and immunological parameters of Asian seabass (Lates calcarifer). Aquac. Int. 2017, 25, 837–848. [Google Scholar]
- Shiu, Y.L.; Lin, H.L.; Chi, C.C.; Yeh, S.P.; Liu, C.H. Effects of hirami lemon, Citrus depressa Hayata, leaf meal in diets on the immune response and disease resistance of juvenile barramundi, Lates calcarifer (block), against Aeromonas hydrophila. Fish Shellfish Immunol. 2016, 55, 332–338. [Google Scholar] [CrossRef]
- Devakumar, C.; Chinnasamy, A. Dietary administration of natural immunostimulants on growth performance, haematological, biochemical parameters and disease resistance of Asian Sea bass Lates calcarifer (Bloch, 1790). Aquac. Res. 2017, 48, 1131–1145. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Liu, C.; Xue, Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquac. Res. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.S. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 2011, 317, 1–15. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Wen, W. The immunoregulative effects of liquorice extract on crucian. Acta Hydrobiol. Sinica 2007, 31, 655–660. [Google Scholar]
- Wang, Q.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S.; et al. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ai, Q.; Mai, K.; Xu, W.; Wang, J.; Zuo, R. Effects of dietary supplementation of glycyrrhizic acid on growth performance, survival, innate immune response and parasite resistance in juvenile large yellow croaker, Larimichthys crocea (Richardson). Aquac. Res. 2015, 46, 86–94. [Google Scholar] [CrossRef]
- Zhenhua, M. Culture Biology and Processing of Lates calarifer; China Agricultural Press: Beijing, China, 2019. [Google Scholar]
- Fu, Z.; Yang, R.; Chen, X.; Qin, J.G.; Gu, Z.; Ma, Z. Dietary non-protein energy source regulates antioxidant status and immune response of barramundi (Lates calcarifer). Fish Shellfish Immunol. 2019, 95, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Zou, J.; Secombes, C.J. Advances in fish cytokine biology give clues to the evolution of a complex network. Curr. Pharm. Des. 2006, 12, 3051–3069. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.J.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef]
- Watts, M.; Munday, B.L.; Burke, C.M. Immune responses of teleost fish. Australian Vet. J. 2001, 79, 570–574. [Google Scholar] [CrossRef]
- Basu, N.; Todgham, A.E.; Ackerman, P.A.; Bibeau, M.R.; Nakano, K.; Schulte, P.M.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, H.; Cao, X. Research and Utilization Statue of Licorice. Chin. Agric. Sci. Bull. 2011, 27, 290–295. [Google Scholar]
- Kobayashi, M.; Fujita, K.; Katakura, T.; Utsunomiya, T.; Pollard, R.B.; Suzuki, F. Inhibitory effect of glycyrrhizin on experimental pulmonary metastasis in mice inoculated with b16 melanoma. Anticancer Res. 2002, 22, 4053–4058. [Google Scholar]
- Zhang, M.; Shen, Y. Advances in studies on the cardioprotectiio of glycyrrhizic acid compound and flavones. Drugs Clin. 2012, 27, 429–434. [Google Scholar]
- Wang, F.; Su, Y. Pharmacological effect and clinical Application of lradix Glycyrrhizae. Lishizhen Med. Mater. Med. Res. 2002, 13, 303–304. [Google Scholar]
- Zhu, Y.L.; Xie, Q.M.; Chen, J.Q.; Zhang, S.J. Inhibition of flavone from Glycyrrhiza uralensis on capsaicin-induced cough reflex in guinea pig. Chin. Tradit. Herb. Drugs 2006, 37, 1048–1051. [Google Scholar]
- Huang, Q.; Ma, Z. Pharmacological research progress of glycyrrhizin acid. Drug Eval. Res. 2011, 34, 384–387. [Google Scholar]
- Liu, Q. An overview of the chemical constituents and pharmacological effects of licorice. Chin. Med. Mod. Distance Educ. China 2011, 9, 84. [Google Scholar]
- Zhang, M.; Shen, Y. Advances in study on glycyrrhizic acid and its derivatives in anti-inflammatory and antiallergy. Drugs Clin. 2011, 26, 359–364. [Google Scholar]
- Weng, Q.; Li, Z.; Lu, K.; Wang, L.; Zhang, C.; Song, K. Effects of fermented licorice root under the stress of nitrite on blood indexes and antioxidant capacity of rhizoid grouper. Feed Res. 2019, 42, 24–27. [Google Scholar]
- Wang, W.B.; Fang, P.; Lin, X.T.; Xia, L.; Qi, C.B.; Wang, J.G.; Sun, J. Effect of Liquorice Extracts on the Resistance of Carassius auratus to Stress and Pathogen Infection. Freshw. Fish. 2007, 270, 3–6. [Google Scholar]
- Chen, C.; Chen, X.; Chen, C. Effect of feeding glycyrrhizine on the resistance of Chinese soft-shelled turtle (Peiodiscus sinesis) against Aromonas hydrophila. J. Huazhong Agric. Univ. 2000, 19, 577–580. [Google Scholar]
- Chen, X.; Mai, K.; Zhang, W.; Wang, X.; Ai, Q.; Xu, W.; Liufu, Z.; Ma, H.; Tan, B. Effects of Dietary Glycyrrhizin on Growth and Nonspecific Immunity of White Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2010, 41, 665–674. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.P.; Shaheen, A.; Yao, H.; Abbass, A. Feeding Glycyrrhiza glabra (liquorice) and Astragalus membranaceus (AM) alters innate immune and physiological responses in yellow perch (Perca flavescens). Fish Shellfish Immunol. 2016, 54, 374–384. [Google Scholar] [CrossRef]
- Helland, S.J.; Grisdale-Helland, B. Growth, feed utilization and body composition of juvenile Atlantic halibut (Hippoglossus hippoglossus) fed diets differing in the ratio between the macronutrients. Aquaculture 1998, 166, 49–56. [Google Scholar] [CrossRef]
- Ballou, S.P.; Lozanski, G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine 1992, 4, 361–368. [Google Scholar] [CrossRef]
- Minich, W.B.; Balasta, M.L.; Goss, D.J.; Rhoads, R.E. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: Increased cap affinity of the phosphorylated form. Proc. Natl. Acad. Sci. USA 1994, 91, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Cafferkey, R.; Young, P.R.; McLaughlin, M.M.; Bergsma, D.J.; Koltin, Y.; Sathe, G.M.; Faucette, L.; Eng, W.K.; Johnson, R.K.; Livi, G.P. Dominant missense mutations in a novel yeast protein related to mammalian phosphatidylinositol 3-kinase and VPS34 abrogate rapamycin cytotoxicity. Mol. Cell. Biol. 1993, 13, 6012–6023. [Google Scholar] [CrossRef]
- Ma, X.J.M.; Blenis, J. Molecular mechanisms of mtor-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Loewith, R.; Jacinto, E.; Wullschleger, S.; Lorberg, A.; Crespo, J.L.; Bonenfant, D.; Oppliger, W.; Jenoe, P.; Hall, M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 2002, 10, 457–468. [Google Scholar] [CrossRef]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I.; Georgopoulos, C.J.S. Heat Shock. In Book Reviews: Stress Proteins in Biology and Medicine; Cold Spring Harbor Laboratory: New York, NY, USA, 1990; Volume 249, pp. 572–573. [Google Scholar]
- Kiang, J.G.; Tsokos, G.C. Heat shock protein 70 kDa: Molecular biology, biochemistry, and physiology. Pharmacol. Therap. 1998, 80, 183–201. [Google Scholar] [CrossRef]
- Young, J.C.; Moarefi, I.; Hartl, F.U. Hsp90: A specialized but essential protein-folding tool. J. Cell Biol. 2001, 154, 267–273. [Google Scholar] [CrossRef]
- Laing, K.J.; Secombes, C.J. Chemokines. Dev. Comp. Immunol. 2004, 28, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Vodovotz, Y.; Nathan, C. Macrophage deactivation by interleukin 10. J. Exp. Med. 1991, 174, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Ai, Q.U.; Wang, C.R. Interferons and Their Current Progress. J. Xuzhou Norm. Univ. 2002, 20, 57–60. [Google Scholar]
- Franzen, P.; Heldin, C.H.; Miyazono, K. The GS domain of the transforming growth factor-beta type I receptor is important in signal transduction. Biochem. Biophys. Res. Commun. 1995, 207, 682–689. [Google Scholar] [CrossRef]
- Staeheli, P. Interferon-induced proteins and the antiviral state. Adv. Virus Res. 1990, 38, 147–200. [Google Scholar]
- Pitossi, F.; Blank, A.; Schröder, A.; Schwarz, A.; Hüssi, P.; Schwemmle, M.; Pavlovic, J.; Staeheli, P. A functional GTP-binding motif is necessary for antiviral activity of Mx proteins. J. Virol. 1993, 67, 6726–6732. [Google Scholar] [CrossRef]
| Ingredients | 0% Control Group | 1% Test Group | 3% Test Group | 5% Test Group |
|---|---|---|---|---|
| Fish Meal | 50 | 50 | 50 | 50 |
| Flour | 23 | 22 | 20 | 18 |
| Soybean Meal | 12.9 | 12.9 | 12.9 | 12.9 |
| Vitamin Premix | 0.5 | 0.5 | 0.5 | 0.5 |
| Mineral Premix | 0.5 | 0.5 | 0.5 | 0.5 |
| Fish Oil | 13 | 13 | 13 | 13 |
| Glycyrrhiza Meal | 0 | 1 | 3 | 5 |
| Choline Chloride | 0.1 | 0.1 | 0.1 | 0.1 |
| Dry Ingredients | ||||
| Crude Protein | 41.44 | 41.31 | 41.06 | 40.81 |
| Crude Lipid | 17.53 | 17.51 | 17.46 | 17.41 |
| Crude Ash | 9.26 | 9.22 | 9.13 | 9.05 |
| Total Energy | 20.28 | 20.12 | 19.79 | 19.46 |
| Sample | Gene Abbreviation | Primer Sequence (5′–3′) | Amplicon Size (bp) | Accession No. |
|---|---|---|---|---|
| Head Kidney | il-8 | F: TCTGACTGTTCCTGAGGCTATC | 92 | XM_018695863 |
| R: GACGTCCAATGGGCTTTCT | ||||
| il-10 | F: TGCTGCCGTTTTGTGGAG | 194 | XM_018686737 | |
| R: ACCGTGCTCAGGTAAAAGTCC | ||||
| tgfβ1 | F: TACCTCGCTTCCCGTTTC | 105 | XM_018665504 | |
| R: CTGCTCATCCTCAGTCCCTC | ||||
| tnf | F: AAGGACTCCGCTGAGAAAAC | 241 | XM_018699809 | |
| R: TGAACGATGCCTGGCTGTA | ||||
| ifn-γ1 | F: TACCAGGAGCAGGACAAGC | 134 | NM_001360734 | |
| R: TCGTCAGGCAGCGAACTT | ||||
| mxf | F: GGTGGACAAAGGCACAGAA | 215 | AY821518 | |
| R: GTTTAGGAACGGTGGCATG | ||||
| Liver | crp | F: ACCGAACTGAAGACCACGAT | 106 | HQ652974 |
| R: TGGGGCACCTCAAACAAA | ||||
| c-3 | F: AAATGCTGCCATCGTTCC | 175 | XM_018679796 | |
| R: CCAGTGACCTTCAGACCAAA | ||||
| c-4 | F: CGAGGTTGAACGAAAAGGAC | 97 | XM_018688206 | |
| R: CACAGCAAGCAAAGCCACT | ||||
| mtor | F: GTTTCTTCCGCTCCATTTC | 110 | XM_018675222 | |
| R: CAGGGCTTCATTCACTTCA | ||||
| mlst-8 | F: TGATTCAACACTATTAGCCACA | 212 | XM_018687802 | |
| R: TTTCCACGCACCACAGG | ||||
| eif4e | F: TGACGACTACAGCGATGAT | 183 | XM_018697729 | |
| R: GTGTCTGCGTGGGATTG | ||||
| hsp-70 | F: CTGGAGTCCTACGCTTTCAA | 204 | HQ646109 | |
| R: CTTGCTGATGATGGGGTTAC | ||||
| hsp-90 | F: ACGATGATGAGCAGTATGCC | 201 | XM018661637 | |
| R: CAAACAGGGTGATGGGGTA | ||||
| Head Kidney and Liver | β-actin | F: AACCAAACGCCCAACAACT | 112 | XM_018667666 |
| R: ATAACTGAAGCCATGCCAATG |
| Productivity Index | Experimental Diets | |||
|---|---|---|---|---|
| 0% | 1% | 3% | 5% | |
| WG (g fish−1) | 10.74 ± 0.35 a | 14.91 ± 0.06 b | 13.91 ± 3.34 b | 16.33 ± 2.47 b |
| SGR (% d−1) | 1.04 ± 0.05 a | 1.31 ± 0.08 ab | 1.23 ± 0.21 ab | 1.37 ± 0.21 b |
| Survival (%) | 73.33 ± 16.67 a | 79.25 ± 11.15 ab | 98.89 ± 1.92 b | 95.56 ± 5.09 b |
| FI (g fish−1·d−1) | 0.76 ± 0.06 | 0.86 ± 0.06 | 0.84 ± 0.08 | 0.90 ± 0.09 |
| HIS (%) | 2.32 ± 0.28 | 2.12 ± 0.31 | 2.49 ± 0.43 | 1.86 ± 0.32 |
| IPF (%) | 5.76 ± 1.42 b | 5.51 ± 0.50 b | 4.09 ± 1.35 b | 2.90 ± 1.01 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Han, M.; Fu, Z.; Wang, Y.; Zhao, W.; Yu, G.; Ma, Z. Immune Responses of Asian Seabass Lates calcarifer to Dietary Glycyrrhiza uralensis. Animals 2020, 10, 1629. https://doi.org/10.3390/ani10091629
Yang R, Han M, Fu Z, Wang Y, Zhao W, Yu G, Ma Z. Immune Responses of Asian Seabass Lates calcarifer to Dietary Glycyrrhiza uralensis. Animals. 2020; 10(9):1629. https://doi.org/10.3390/ani10091629
Chicago/Turabian StyleYang, Rui, Mingyang Han, Zhengyi Fu, Yifu Wang, Wang Zhao, Gang Yu, and Zhenhua Ma. 2020. "Immune Responses of Asian Seabass Lates calcarifer to Dietary Glycyrrhiza uralensis" Animals 10, no. 9: 1629. https://doi.org/10.3390/ani10091629
APA StyleYang, R., Han, M., Fu, Z., Wang, Y., Zhao, W., Yu, G., & Ma, Z. (2020). Immune Responses of Asian Seabass Lates calcarifer to Dietary Glycyrrhiza uralensis. Animals, 10(9), 1629. https://doi.org/10.3390/ani10091629






