Semen Quality of Rasa Aragonesa Rams Carrying the FecXR Allele of the BMP15 Gene
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Collection and Analyses
2.3. Testicular Measurements
2.4. Artificial Inseminations (AI)
2.5. Statistical Analyses
3. Results
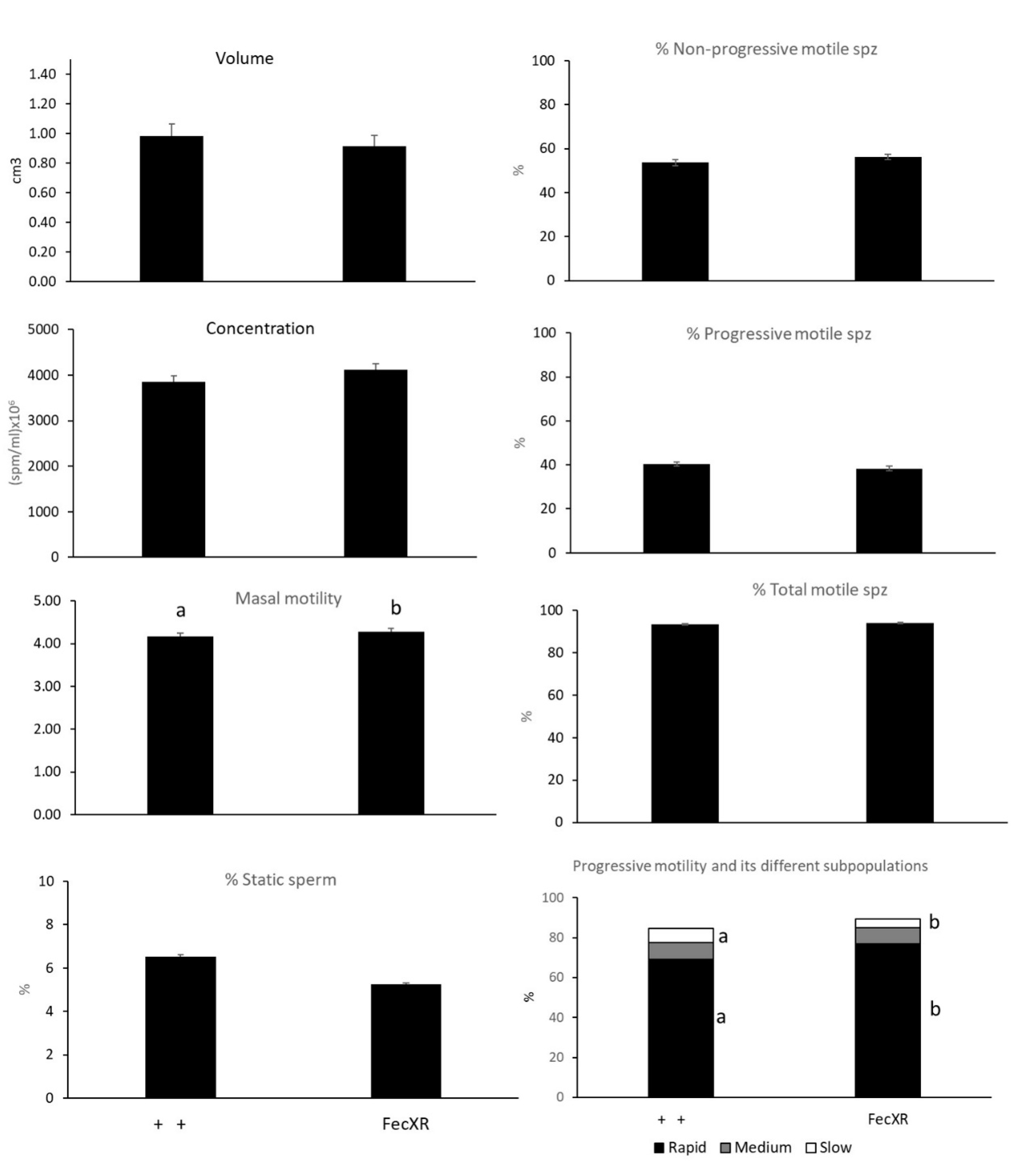
3.1. Semen Quality
3.2. Testicular Measurements
3.3. Reproductive Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vadhana, E.; Santhosh, A.; Pooja, G.S.; Vani, A.; Kumar, S. FecB: A major gene governing fecundity in sheep. J. Entomol. Zool. Stud. 2019, 7, 270–274. [Google Scholar]
- Galloway, S.M.; McNatty, K.P.; Cambridge, L.M.; Laitinen, M.P.E.; Juengel, J.L.; Jokiranta, T.S.; McLaren, R.J.; Luiro, K.; Dodds, K.G.; Montgomery, G.W.; et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat. Genet. 2000, 25, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.K.; Shimasaki, S. Molecular biology and physiological role of the oocyte factor, BMP-15. Mol. Cell. Endocrinol. 2005, 29, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, G.W.; Galloway, S.M.; Davis, G.H.; McNatty, K.P. Genes controlling ovulation rate in sheep. Reproduction 2001, 121, 843–852. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef]
- Davis, G.H. Major genes affecting ovulation rate in sheep. Genet. Sel. Evol. 2005, 37, 11–23. [Google Scholar] [CrossRef]
- Bodin, L.; Di Pasquale, E.; Fabre, S.; Bontoux, M.; Monget, P.; Persani, L.; Mulsant, P.A. A novel mutation in the bone morphogenetic protein 15 gene causing defective protein secretion is associated with both increased ovulation rate and sterility in Lacaune sheep. Endocrinology 2007, 148, 393–400. [Google Scholar] [CrossRef]
- Monteagudo, L.V.; Ponz, R.; Tejedor, M.T.; Laviña, A.; Sierra, I. A 17 bp deletion in the Bone Morphogenetic Protein 15 (BMP15) gene is associated to increased prolificacy in the Rasa Aragonesa sheep breed. Anim. Reprod. Sci. 2009, 110, 139–146. [Google Scholar] [CrossRef]
- Demars, J.; Fabre, S.; Sarry, J.; Rossetti, R.; Gilbert, H.; Persani, L.; Tosser-Klopp, G.; Mulsant, P.; Nowak, Z.; Drobik, W.; et al. Genome-wide association studies identify two novel BMP15 mutations responsible for an atypical hyperprolificacy phenotype in sheep. PLoS Genet. 2013, 9, e1003482. [Google Scholar] [CrossRef]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef]
- ANGRA. Available online: https://www.rasaaragonesa.com (accessed on 15 August 2020).
- Sierra, I. La raza ovina Rasa Aragonesa: Caracteres morfológicos y productivos. Anim. Genet. Resour. Inf. 1992, 10, 65–74. [Google Scholar]
- Chen, W.; Tian, Z.; Ma, L.; Gan, S.; Sun, W.; Chu, M. Expression analysis of BMPR1B, BMP15, GDF9, Smad1, Smad5, and Smad9 in rams with different fecundity. Pak. J. Zool. 2020, 52, 1665–1674. [Google Scholar] [CrossRef]
- Lahoz, B.; Blasco, M.E.; Sevilla, E.; Folch, J.; Roche, A.; Quintin, F.J.; Martínez-Royo, A.; Galeote, A.I.; Calvo, J.H.; Fantova, E.; et al. Fertility of Rasa Aragonesa rams carrying or not the FecXR allele of BMP15 gene when used in artificial insemination. In Proceedings of the European Association for Animal Production (EAAP), Barcelona, Spain, 24–27 August 2009. [Google Scholar]
- Kumar, D.; Joshi, A.; Naqvi, S.M.K.; Kumar, S.; Mishrac, A.K.; Maurya, V.P.; Arora, A.L.; Mittal, J.P.; Singh, V.K. Sperm motion characteristics of Garole × Malpura sheep evolved in a semi-arid tropical environment through introgression of FecB gene. Anim. Reprod. Sci. 2007, 100, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Maxwell, W.M.C. Handling and examination semen. In Salamon’S Artificial Insemination of Sheep and Goats; Salamon, S., Ed.; Butterworths: Sydney, Australia, 1987; pp. 93–106. [Google Scholar]
- Palacín, I.; Vicente-Fiel, S.; Santolaria, P.; Yániz, J.L. Standardization of CASA sperm motility assessment in the ram. Small Rumin. Res. 2013, 112, 128–135. [Google Scholar] [CrossRef]
- Macías, A.; Ferrer, L.M.; Ramos, J.J.; Lidón, I.; Rebollar, R.; Lacasta, D.; Tejedor, M.J. Technical Note: A new device for cervical insemination of sheep—Design and field test. J. Anim. Sci. 2017, 95, 5263–5269. [Google Scholar] [CrossRef]
- Trenberth, K.E. What are the Seasons? Bull. Am. Meteorol. Soc. 1983, 64, 11. [Google Scholar] [CrossRef]
- Kumar, D.; Naqvi, S.M.K.; Kumar, S. Sperm motion characteristics of FecBBB and FecBB + Garole × Malpura rams during the non-breeding season under hot semi-arid environment. Livest. Sci. 2012, 150, 337–341. [Google Scholar] [CrossRef]
- Aitkin, R.J. Motility parameters and fertility. In Control of Sperm Motility: Biological and Clinical Aspects; Gagnon, C., Ed.; CRS Press: Boca Raton, FL, USA, 1990; pp. 285–302. [Google Scholar]
- Yániz, J.L.; Palacín, I.; Vicente-Fiel, S.; Sánchez-Nadal, J.A.; Santolaria, P. Sperm population structure in high and low field fertility rams. Anim. Reprod. Sci. 2015, 156, 128–134. [Google Scholar] [CrossRef]
- Gomendio, M.; Roldán, E.R.S. Implications of diversity in sperm size and function for sperm competition and fertility. Int. J. Dev. Biol. 2008, 52, 439–447. [Google Scholar] [CrossRef]
- Lahoz, B.; Alabart, J.L.; Jurado, J.J.; Calvo, J.H.; Martínez-Royo, A.; Fantova, E.; Folch, J. Effect of the FecXR polymorphism in the bone morphogenetic protein 15 gene on natural or equine chorionic gonadotropin-induced ovulation rate and litter size in Rasa Aragonesa ewes and implications for on-farm application. J. Anim. Sci. 2011, 89, 3522–3530. [Google Scholar] [CrossRef]
- Anel, L.; Kaabi, M.; Abroug, B.; Alvarez, M.; Anel, E.; Boixo, J.C.; de la Fuente, L.F.; Paz, P. Factors influencing the success of vaginal and laparoscopic artificial insemination in Churra ewes: A field assay. Theriogenology 2005, 63, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Abecia, J.A. Meteorological variables affect fertility rate afterintrauterine artificial insemination in sheep in a seasonal-dependent manner: A 7-year study. Int. J. Biometeorol. 2015, 59, 585–592. [Google Scholar] [CrossRef]
- Abecia, J.A.; Máñez, J.; Macias, A.; Laviña, A.; Palacios, C. Climate zone influences the effect of temperature on the day of artificial insemination on fertility in two Iberian sheep breeds. J. Anim. Behav. Biometeorol. 2017, 5, 124–131. [Google Scholar] [CrossRef]
- Palacín, I.; Yániz, J.L.; Fantova, E.; Blasco, M.E.; Quintín-Casorrán, F.J.; Sevilla-Mur, E.; Santolaria, P. Factors affecting fertility after cervical insemination with cooled semen in meat sheep. Anim. Reprod. Sci. 2012, 132, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Forcada, F.; Abecia, J.A.; Sierra, I. Seasonal changes in oestrous activity and ovulation rate in Rasa Aragonesa ewes maintained at two different body condition levels. Small Rumin. Res. 1992, 8, 313–324. [Google Scholar] [CrossRef]
- Sun, L.P.; Song, Y.P.; Du, Q.Z.; Song, L.W.; Tian, Y.Z.; Zhang, S.L.; Hua, G.H.; Yang, L.G. Polymorphisms in the bone morphogenetic protein 15 gene and their effect on sperm quality traits in Chinese Holstein bulls. Genet. Mol. Res. 2014, 13, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Ripoll, G.; Joy, M.; Folch, J.; Panea, B.; Calvo, J.H.; Alabart, J.L. Effects of the FecX R allele of BMP15 gene on the birth weight, growth rate and carcass quality of Rasa Aragonesa light lambs. Small Rumin. Res. 2012, 108, 45–53. [Google Scholar] [CrossRef]
- Davis, G.H.; Dodds, K.G.; McEwan, J.C.; Fennessy, P.F. Liveweight, fleece weight and prolificacy of Romney ewes carrying the Inverdale prolificacy gene (FecXI) located on the X-chromosome. Livest. Prod. Sci. 1993, 34, 83–91. [Google Scholar] [CrossRef]
- Kheradmand, A.; Babaei, H.; Abshenas, J. Comparative evaluation of the effect of antioxidants on the chilled-stored ram semen. Iran. J. Vet. Res. 2006, 7, 40–45. [Google Scholar]
- Avdi, M.; Banos, G.; Stefos, K.; Chemineau, P. Seasonal variation in testicular volume and sexual behavior of Chios and Serres rams. Theriogenology 2004, 62, 275–282. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Varsakeli, S.; Alexopoulos, C.; Amarantidis, I. Seasonal variation in semen characteristics of Chios and Friesian rams in Greece. Small Rumin. Res. 2000, 37, 125–130. [Google Scholar] [CrossRef]
- Casao, A.; Vega, S.; Palacín, I.; Pérez-Pe, R.; Laviña, A.; Quintín, F.J.; Sevilla, E.; Abecia, J.A.; Cebrián-Pérez, J.A.; Forcada, F.; et al. Effects of Melatonin Implants During Non-Breeding Season on Sperm Motility and Reproductive Parameters in Rasa Aragonesa Rams. Reprod. Dom. Anim. 2010, 45, 425–432. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Short, V. Seasonal Breeding: Nature’s Contraceptive. Recent Prog. Horm. Res. 1980, 36, 1–52. [Google Scholar] [PubMed]
- Malpaux, B.; Viguié, C.; Skinner, D.C.; Thiéry, J.C.; Pelletier, J.; Chemineau, P. Seasonal breeding in sheep: Mechanism of action of melatonin. Anim. Reprod. Sci. 1996, 42, 109–117. [Google Scholar] [CrossRef]
- Lincoln, G.A. Significance of seasonal cycles in prolactin secretion in male mammals. In Perspectives in Andrology; Serio, M., Ed.; Raven Press: New York, NY, USA, 1989; pp. 299–306. [Google Scholar]
| Spring | Summer | Autumn | Winter | |||||
|---|---|---|---|---|---|---|---|---|
| ++ | FecXR | ++ | FecXR | ++ | FecXR | ++ | FecXR | |
| Sperm count (×106) | 4020 ± 311 | 3665 ± 533 | 4381 ± 91 | 4456 ± 364 | 3465 ± 553 | 3297 ± 375 | 3352 ± 338 | 3525 ± 458 |
| Volume (cm3) | 0.91 ± 0.04 | 0.86 ± 0.10 | 1.10 ± 0.02 | 0.99 ± 0.10 | 0.92 ± 0.09 | 0.84 ± 0.09 | 0.97 ± 0.03 | 0.97 ± 0.11 |
| Concentration (×106) | 4420 ± 140 | 4163 ± 256 | 3987 ± 146 | 4572 ± 209 | 3713 ± 252 | 4002 ± 242 | 3452 ± 241 | 3609 ± 143 |
| Mass motility (0–5) | 4.30 ± 0.00 | 4.28 ± 0.01 | 4.27 ± 0.03 | 4.29 ± 0.01 | 4.29 ± 0.01 | 4.28 ± 0.02 | 3.83 ± 0.42 * | 4.30 ± 0.00 |
| % Static spz | 3.43 ± 0.88 | 5.65 ± 2.24 | 8.33 ± 3.34 | 4.93 ± 0.90 | 5.89 ± 0.95 | 6.79 ± 1.80 | 7.44 ± 0.29 | 5.48 ± 0.61 |
| % NPM spz | 57.93 ± 0.88 | 54.99 ± 1.84 | 52.78 ± 3.58 | 53.93 ± 2.32 | 54.78 ± 3.16 | 58.55 ± 2.45 | 50.67 ± 4.10 | 57.57 ± 1.19 |
| % PM spz | 41.66 ± 3.01 | 40.71 ± 2.76 | 38.89 ± 4.08 | 41.13 ± 2.09 | 39.33 ± 2.22 | 34.67 ± 1.72 | 41.89 ± 4.37 | 36.95 ± 1.41 |
| % TM spz | 96.41 ± 1.04 | 93.87 ± 2.21 | 91.67 ± 3.34 | 95.07 ± 0.90 | 94.11 ± 0.95 | 93.21 ± 1.80 | 92.56 ± 0.29 | 94.38 ± 0.65 |
| % Rapid spz | 69.97 ± 5.27 | 75.50 ± 3.83 | 69.78 ± 8.42 * | 80.23 ± 2.21 | 76.33 ± 2.96 | 75.09 ± 3.68 | 60.89 ± 2.63 ** | 76.52 ± 2.69 |
| % Medium spz | 6.31 ± 0.94 | 8.43 ± 1.16 | 8.33 ± 1.20 | 7.17 ± 1.17 | 8.11 ± 1.46 | 8.33 ± 1.14 | 10.33 ± 2.19 | 8.52 ± 0.89 |
| % Slow spz | 18.69 ± 5.26 ** | 8.95 ± 1.79 | 11.78 ± 4.11 ** | 6.23 ± 0.54 | 9.00 ± 3.02 | 8.94 ± 1.83 | 20.67 ± 1.86 ** | 8.48 ± 1.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abecia, J.A.; Macías, Á.; Casao, A.; Burillo, C.; Martín, E.; Pérez-Pé, R.; Laviña, A. Semen Quality of Rasa Aragonesa Rams Carrying the FecXR Allele of the BMP15 Gene. Animals 2020, 10, 1628. https://doi.org/10.3390/ani10091628
Abecia JA, Macías Á, Casao A, Burillo C, Martín E, Pérez-Pé R, Laviña A. Semen Quality of Rasa Aragonesa Rams Carrying the FecXR Allele of the BMP15 Gene. Animals. 2020; 10(9):1628. https://doi.org/10.3390/ani10091628
Chicago/Turabian StyleAbecia, José Alfonso, Ángel Macías, Adriana Casao, Clara Burillo, Elena Martín, Rosaura Pérez-Pé, and Adolfo Laviña. 2020. "Semen Quality of Rasa Aragonesa Rams Carrying the FecXR Allele of the BMP15 Gene" Animals 10, no. 9: 1628. https://doi.org/10.3390/ani10091628
APA StyleAbecia, J. A., Macías, Á., Casao, A., Burillo, C., Martín, E., Pérez-Pé, R., & Laviña, A. (2020). Semen Quality of Rasa Aragonesa Rams Carrying the FecXR Allele of the BMP15 Gene. Animals, 10(9), 1628. https://doi.org/10.3390/ani10091628





