Application of Effective Day Degrees in the Assessment of Stable Isotope Patterns in Developing Seahorses under Different Temperatures
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broodstock
2.2. Fed Seahorses
- -
- First feeding (days 0 to 5): Single daily dose of cultivated copepods Acartia tonsa and Tisbe sp. (1:1; 0.6 copepods mL−1).
- -
- Transitional feeding (days 6 to 10): Daily dose of copepods (0.3 copepods mL−1) and Great Salt Lake Artemia nauplii (1 Artemia mL−1).
- -
- Artemia feeding (days 11 to 30): Three daily doses of Artemia nauplii and 24 h enriched Artemia metanauplii (1:1; 1 Artemia mL−1).
2.3. Unfed Seahorses
2.4. Bioethics
2.5. Sampling, Analyses and Data Treatment
3. Results
3.1. Growth, Survival and Condition of Juveniles
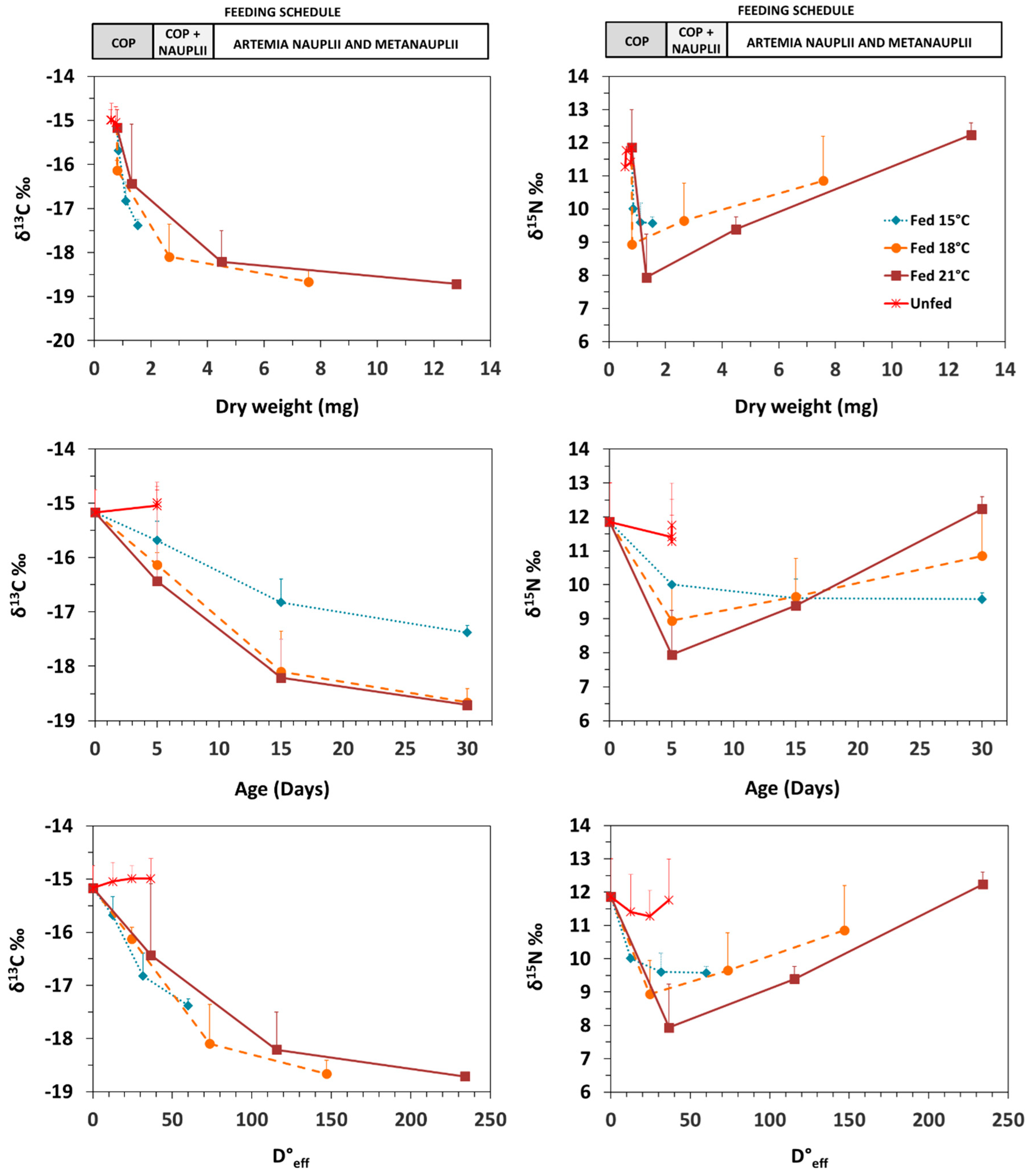
3.2. Isotopic Patterns with Ontogeny and Feeding Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Govoni, J.J.; Boehlert, G.W.; Watanabe, Y. The physiology of digestion in fish larvae. Environ. Biol. Fishes. 1986, 16, 59–77. [Google Scholar] [CrossRef]
- Conceição, L.E.C.; Morais, S.; Rønnestad, I. Tracers in fish larvae nutrition: A review of methods and applications. Aquaculture 2007, 267, 62–75. [Google Scholar] [CrossRef]
- Rønnestad, I.; Rojas-García, C.R.; Tonheim, S.K.; Conceição, L.E.C. In vivo studies of digestion and nutrient assimilation in marine fish larvae. Aquaculture 2001, 201, 161–175. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. δ13C measurements as indicators of carbon flow in marine and fresh-water ecosystems. Contrib. Mar. Sci. 1984, 27, 13–47. [Google Scholar]
- Michener, R.H.; Shell, D.M. Stable isotope ratios as tracers in marine aquatic food webs. In Stable Isotopes in Ecology and Environmental Science; Lajtha, K., Michener, R.H., Eds.; Blackwell scientific publications: Oxford, UK, 1994; Volume 1, pp. 138–157. [Google Scholar]
- Minagawa, M.; Wada, E. Stepwise enrichment of 15N along food-chains further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006; p. 308. [Google Scholar]
- Schroeder, G.L. Stable isotope ratios as naturally occurring tracers in the aquaculture food web. Aquaculture 1983, 30, 203–210. [Google Scholar] [CrossRef]
- Schlechtriem, C.; Focken, U.; Becker, K. Stable isotopes as a tool for nutrient assimilation studies on larval fish feeding on live food. Aquat. Ecol. 2004, 38, 93–100. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Cañavate, J.P.; Zerolo, R.; Le Vay, L. Natural carbon stable isotope ratios as indicators of the relative contribution of live and inert diets to growth in larval Senegalese sole (Solea senegalensis). Aquaculture 2008, 280, 190–197. [Google Scholar] [CrossRef]
- Xia, B.; Gao, Q.F.; Dong, S.L.; Wang, F. Carbon stable isotope turnover and fractionation in grass carp Ctenopharyngodon idella tissues. Aquat. Biol. 2013, 19, 207–2016. [Google Scholar] [CrossRef]
- Hobson, K.A.; Alisauskas, R.T.; Clark, R.G. Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: Implications for isotopic analyses of diet. Condor 1993, 95, 388–394. [Google Scholar] [CrossRef]
- Herzka, S.Z.; Holt, G.J. Changes in isotopic composition of red drum (Sciaenops ocellatus) larvae in response to dietary shifts: Potential applications to settlement studies. Can. J. Fish. Aquat. Sci. 2000, 57, 137–147. [Google Scholar] [CrossRef]
- Gaye-Siessegger, J.; Focken, U.; Muetzel, S.; Abel, H.J.; Becker, K. Feeding level and individual metabolic rate affect δ13C and δ15N values in carp: Implications for food web studies. Oecologia 2004, 138, 175–183. [Google Scholar] [CrossRef]
- Barnes, C.; Sweeting, C.J.; Jennings, S.; Barry, J.T.; Polunin, N.V.C. Effect of temperature and ration size on carbon and nitrogen stable isotope trophic fractionation. Funct. Ecol. 2007, 21, 356–362. [Google Scholar] [CrossRef]
- Ofelio, C.; Díaz, A.O.; Radaelli, G.; Planas, M. Histological characterization of early developmental stages in the seahorse Hippocampus guttulatus. J. Fish. Biol. 2018, 93, 72–87. [Google Scholar] [CrossRef]
- Fry, B.; Arnold, C. Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 1982, 172, 21–34. [Google Scholar] [CrossRef]
- Hobson, K.A.; Clark, R.G. Assessing avian diets using stable isotopes I: Turnover of 13C in tissues. Condor 1992, 94, 181–188. [Google Scholar] [CrossRef]
- Hesslein, R.H.; Hallard, K.A.; Ramlal, P. Replacement of sulphur, carbon and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C and δ15N. Can. J. Fish. Aquat. Sci. 1993, 50, 2071–2076. [Google Scholar] [CrossRef]
- Houde, E.D. Comparative growth, mortality, and energetics of marine fish larvae: Temperature and implied latitudinal effects. Fish. Bull. 1989, 87, 471–495. [Google Scholar]
- Hart, P.; Hutchinson, W.G.; Purser, J. Effects of photoperiod, temperature and salinity on hatchery-reared larvae of the greenback flounder (Rhombosolea tapirina Gfinther, 1862). Aquaculture 1996, 144, 303–311. [Google Scholar] [CrossRef]
- Weltzien, F.A.; Planas, M.; Fyhn, H.J. Temperature dependency of early growth of turbot (Scohthalmus maximus) and its implications for developmental progress. J. Exp. Mar. Biol. Ecol. 1999, 242, 201–210. [Google Scholar] [CrossRef]
- Wong, J.M.; Benzie, J.A.H. The effects of temperature, Artemia enrichment, stocking density and light on the growth of juvenile seahorses, Hippocampus whitei (Bleeker, 1855), from Australia. Aquaculture 2003, 228, 107–121. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, J.; Gao, Y.; Shen, L.; Cai, J.; Luo, J. The effect of temperature on gonad, embryonic development and survival rate of juvenile seahorses, Hippocampus kuda Bleeker. Aquaculture 2006, 254, 701–713. [Google Scholar] [CrossRef]
- Planas, M.; Blanco, A.; Chamorro, A.; Valladares, S.; Pintado, J. Temperature-induced changes of growth and survival in the early development of the seahorse Hippocampus guttulatus. J. Exp. Mar. Biol. Ecol. 2012, 438, 154–162. [Google Scholar] [CrossRef]
- Bosley, K.L.; Witting, D.A.; Chambers, R.C.; Wainright, S.C. Estimating turnover rates of carbon and nitrogen in recently metamorphosed winter flounder Pseudopleuronectes americanus with stable isotopes. Mar. Ecol. Prog. Ser. 2002, 236, 233–240. [Google Scholar] [CrossRef]
- Witting, D.A.; Chambers, R.C.; Bosley, K.L.; Wainright, S.C. Experimental evaluation of ontogenetic diet transitions in summer flounder (Paralichthys dentatus), using stable isotopes as diet tracers. Can. J. Fish. Aquat. Sci. 2004, 61, 2069–2084. [Google Scholar] [CrossRef]
- Planas, M.; Chamorro, A.; Quintas, P.; Vilar, A. Establishment and maintenance of threatened long-snouted seahorse, Hippocampus guttulatus, broodstock in captivity. Aquaculture 2008, 283, 19–28. [Google Scholar] [CrossRef]
- Blanco, A.; Chamorro, A.; Planas, M. Implications of physical key factors in the early rearing of the long-snouted seahorse Hippocampus guttulatus. Aquaculture 2014, 433, 214–222. [Google Scholar] [CrossRef]
- Blanco, A.; Planas, M. Mouth growth and prey selection in juveniles of the European long-snouted seahorse, Hippocampus Guttulatus. J. World Aquacult. Soc. 2015, 46, 596–607. [Google Scholar] [CrossRef]
- Lourie, S.A.; Vincent, A.C.J.; Hall, H.J. Seahorses: An Identification Guide to the World’s Species and their Conservation; Project Seahorse: London, UK, 1999; p. 214. [Google Scholar]
- Logan, J.M.; Jardine, T.D.; Miller, T.J.; Bunn, S.E.; Cunjak, R.A.; Lutcavage, M.E. Lipid corrections in carbon and nitrogen stable isotope analyses: Comparison of chemical extraction and modelling methods. J. Anim. Ecol. 2008, 77, 838–846. [Google Scholar] [CrossRef]
- Post, D.M.; Craig, A.; Layman, D.; Albrey Arrington, D.; Takimoto, G.; Quatrocchi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia. 2007, 152, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Lin, Q.; Chen, Q.; Shen, L.; Lu, J. Effect of starvation on the initiation of feeding, growth and survival rate of juvenile seahorses, Hippocampus trimaculatus Leach and Hippocampus kuda Bleeker. Aquaculture 2007, 271, 469–478. [Google Scholar] [CrossRef]
- Blanco, A.; Quintas, P.; Planas, M. Catabolic sources in the early development of the long-snouted seahorse Hippocampus guttulatus under starving conditions. In Proceedings of the 5th International Husbandry Symposium, The Husbandry, Management and Conservation of Syngnathids, Chicago, IL, USA, 2–4 November 2011. [Google Scholar]
- Ponsard, S.; Averbuch, P. Should growing and adult animals fed on the same diet show different delta 15N values? Rapid Commun. Mass. Sp. 1999, 13, 1305–1310. [Google Scholar] [CrossRef]
- Zimmer, A.M.; Wright, P.A.; Wood, C.M. Ammonia and urea handling by early life stages of fishes. J. Exp. Biol. 2017, 220, 3843–3855. [Google Scholar] [CrossRef]
- Brett, J.R.; Groves, T.D.D. Physiological energetics. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press: New York, NY, USA, 1979; Volume 8, pp. 279–352. [Google Scholar]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment, 5th ed.; Cambridge University Press: New York, NY, USA, 1997; p. 607. [Google Scholar]
- Adams, T.S.; Sterner, R.W. The effect of dietary nitrogen content on trophic level 15N enrichment. Limnol. Oceanogr. 2000, 45, 601–607. [Google Scholar] [CrossRef]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia. 2003, 136, 169–182. [Google Scholar] [CrossRef]
- Gaye-Siessegger, J.; Focken, U.; Abel, H.J.; Becker, K. Starvation and low feeding levels result in an enrichment of 13C in lipids and 15N in protein of Nile tilapia Oreochromis niloticus L. J. Fish. Biol. 2007, 71, 90–100. [Google Scholar] [CrossRef]
- Gannes, L.Z.; O’Brien, D.M.; Martínez del Rio, C. Stable isotopes in animal ecology: Assumptions, caveats, and a call for more laboratory experiments. Ecology 1997, 78, 1271–1276. [Google Scholar] [CrossRef]
- Schlechtriem, C.; Focken, U.; Becker, K. Digestion and assimilation of the free-living nematode Punugrellus redivivus fed to first feeding coregonid larvae: Evidence from histological and isotopic studies. J. World Aquacul. Soc. 2005, 36, 24–31. [Google Scholar] [CrossRef]
- Jomori, R.K.; Ducatti, C.; Carneiro, D.J.; Portella, M. Stable carbon (δ13C) and nitrogen (δ15N) isotopes as natural indicators of live and dry food in Piaractus mesopotamicus (Holmberg, 1887) larval tissue. Aquac. Res. 2008, 39, 370–381. [Google Scholar] [CrossRef]
- Uriarte, A.; García, A.; Ortega, A.; de la Gándara, F.; Quintanilla, J.; Laiz-Carrión, R. Isotopic discrimination factors and nitrogen turnover rates in reared Atlantic bluefin tuna larvae (Thunnus thynnus): Effects of maternal transmission. Sci. Mar. 2016, 80, 447–456. [Google Scholar] [CrossRef]
- Olive, P.J.W.; Pinnegar, J.K.; Polunin, N.V.C.; Richards, G.; Welch, R. Isotope trophic-step fractionation: A dynamic equilibrium model. J. Anim. Ecol. 2003, 72, 608–617. [Google Scholar] [CrossRef]
- Power, M.; Guiguer, K.; Barton, D.R. Effects of temperature on isotopic enrichment in Daphnia magna: Implications for aquatic food-web studies. Rapid Commun. Mass. Sp. 2003, 17, 1619–1625. [Google Scholar] [CrossRef]
- Blanco, A.; Planas, M.; Moyano, F.J. Ontogeny of digestive enzymatic capacities in juvenile seahorses Hippocampus guttulatus fed on different live diets. Aquac. Res. 2015, 47, 3558–3569. [Google Scholar] [CrossRef]
- Ofelio, C.; Cohen, S.; Adriaens, D.; Radaelli, G.; Díaz, A.O. Histochemistry of goblet cells and micro-computed tomography to study the digestive system in the long-snouted seahorse Hippocampus guttulatus. Aquaculture 2019, 502, 400–409. [Google Scholar] [CrossRef]
- Olivotto, I.; Planas, M.; Simoes, N.; Holt, G.J.; Avella, A.M.; Calado, R. Advances in breeding and rearing marine ornamentals. J. World Aquacul. Soc. 2011, 42, 135–166. [Google Scholar] [CrossRef]
- Dou, S.Z.; Masuda, R.; Tanaka, M.; Tuskamoto, K. Effects of temperature and delayed initial feeding on the survival and growth of Japanese flounder larvae. J. Fish Biol. 2005, 66, 362–377. [Google Scholar] [CrossRef]
- Herzka, S.Z.; Holt, S.A.; Holt, G.J. Documenting the settlement history of individual fish larvae using stable isotope ratios. Model development and validation. J. Exp. Mar. Biol. Ecol. 2001, 265, 49–79. [Google Scholar] [CrossRef]
- Fry, F.E.J. The effect of environmental factors on the physiology of fish. In Fish Physiology, Environmental Relations and Behavior; Hoar, W.S., Randall, D.J., Eds.; Academic Press: New York, NY, USA, 1971; pp. 1–98. [Google Scholar]
- Bloomfield, A.L.; Elsdon, T.S.; Walther, B.D.; Gier, E.J.; Gillanders, B.M. Temperature and diet affect carbon and nitrogen isotopes of fish muscle: Can amino acid nitrogen isotopes explain effects? J. Exp. Mar. Biol. Ecol. 2011, 399, 48–59. [Google Scholar] [CrossRef]
- Fauconneau, B.; Arnal, M. In vivo protein synthesis in different tissues and the whole body of rainbow trout (Salmo gairdnerii R.). Influence of environmental temperature. Comp. Biochem. Phys. A 1985, 82, 179–187. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Vincent, A.C.J. The International Trade in Seahorses; TRAFFIC International: Cambridge, UK, 1996; p. 163. [Google Scholar]
- Foster, S.J.; Vincent, A.C.J. Life history and ecology of seahorses: Implications for conservation and management. J. Fish. Biol. 2004, 65, 1–61. [Google Scholar] [CrossRef]
- Lourie, S.A.; Foster, S.J.; Cooper, E.W.T.; Vincent, A.C.J. A Guide to the Identification of Seahorses; University of British Columbia and World Wildlife Fund: Washington, DC, USA, 2004; p. 114. [Google Scholar]
- Arbones, B.; Castro, C.G.; Alonso-Pérez, F.; Figueiras, F.G. Phytoplankton size structure and water column metabolic balance in a coastal upwelling system: The Ría de Vigo, NW Iberia. Aquat. Microb. Ecol. 2008, 50, 169–179. [Google Scholar] [CrossRef]
- Curtis, J.M.R. Validation of a method for estimating realized annual fecundity in a multiple spawner, the long-snouted seahorse (Hippocampus guttulatus), using underwater visual census. Fish. Bull. 2007, 105, 327–336. [Google Scholar]

| Treatment | Temp | Day | D°eff | n | Survival | Dry Weight (mg) | Weight Change (mg) | SL (mm) | Size Change (mm) | C:N | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (%) | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | ||||
| Onset | 15 | 0 | 0 | 10 | 100 | 0.80 | 0.18 | − | − | 15.30 | 0.69 | − | − | 2.80 | 0.05 |
| Fed | 15 | 5 | 12.5 | 4 | 94 | 0.86 | 0.12 | 0.06 | 0.18 | 17.22 | 0.86 | 1.92 | 0.16 | 2.92 | 0.11 |
| 15 | 15 | 31.5 | 4 | 44 | 1.11 | 0.39 | 0.31 | 0.21 | 18.08 | 1.70 | 2.78 | 1.01 | 2.85 | 0.02 | |
| 15 | 30 | 60.0 | 4 | 22 | 1.53 | 0.39 | 0.73 | 0.21 | 21.32 | 2.98 | 6.02 | 2.28 | 2.83 | 0.01 | |
| 18 | 5 | 24.5 | 4 | 100 | 0.81 | 0.42 | 0.01 | 0.24 | 17.84 | 0.42 | 2.53 | 0.28 | 2.92 | 0.06 | |
| 18 | 15 | 73.5 | 4 | 93 | 2.65 | 1.63 | 1.85 | 1.45 | 23.92 | 5.46 | 8.62 | 4.76 | 2.89 | 0.03 | |
| 18 | 30 | 147.0 | 4 | 86 | 7.57 | 7.28 | 6.77 | 7.10 | 30.49 | 12.98 | 15.19 | 12.29 | 2.92 | 0.07 | |
| 21 | 5 | 36.5 | 4 | 100 | 1.32 | 0.86 | 0.52 | 0.69 | 19.58 | 4.05 | 4.28 | 3.36 | 2.88 | 0.11 | |
| 21 | 15 | 115.5 | 4 | 96 | 4.49 | 2.45 | 3.69 | 2.27 | 29.37 | 4.53 | 14.07 | 3.83 | 2.86 | 0.01 | |
| 21 | 30 | 234.0 | 4 | 81 | 12.79 | 10.20 | 11.99 | 10.03 | 39.13 | 12.15 | 23.73 | 11.31 | 2.82 | 0.10 | |
| Unfed | 15 | 5 | 12.5 | 10 | 88 | 0.76 | 0.24 | −0.04 | 0.06 | 16.34 | 0.37 | 1.03 | 0.33 | 2.87 | 0.01 |
| 18 | 5 | 24,5 | 10 | 94 | 0.57 | 0.11 | −0.23 | 0.07 | 16.35 | 0.70 | 1.05 | 0.00 | 2.94 | 0.01 | |
| 21 | 5 | 36.5 | 10 | 89 | 0.61 | 0.02 | −0.20 | 0.16 | 16.16 | 0.70 | 0.86 | 0.01 | 2.81 | 0.08 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valladares, S.; Planas, M. Application of Effective Day Degrees in the Assessment of Stable Isotope Patterns in Developing Seahorses under Different Temperatures. Animals 2020, 10, 1571. https://doi.org/10.3390/ani10091571
Valladares S, Planas M. Application of Effective Day Degrees in the Assessment of Stable Isotope Patterns in Developing Seahorses under Different Temperatures. Animals. 2020; 10(9):1571. https://doi.org/10.3390/ani10091571
Chicago/Turabian StyleValladares, Sonia, and Miquel Planas. 2020. "Application of Effective Day Degrees in the Assessment of Stable Isotope Patterns in Developing Seahorses under Different Temperatures" Animals 10, no. 9: 1571. https://doi.org/10.3390/ani10091571
APA StyleValladares, S., & Planas, M. (2020). Application of Effective Day Degrees in the Assessment of Stable Isotope Patterns in Developing Seahorses under Different Temperatures. Animals, 10(9), 1571. https://doi.org/10.3390/ani10091571






