Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Housing and Diets
2.2. Seaweed Preparation
2.3. Layer Performance
2.4. Collection of Blood Samples and Chemical Analysis
2.5. Hematology Sample Preparation
2.6. Statistical Analyses
3. Results
3.1. Layer Production Performance
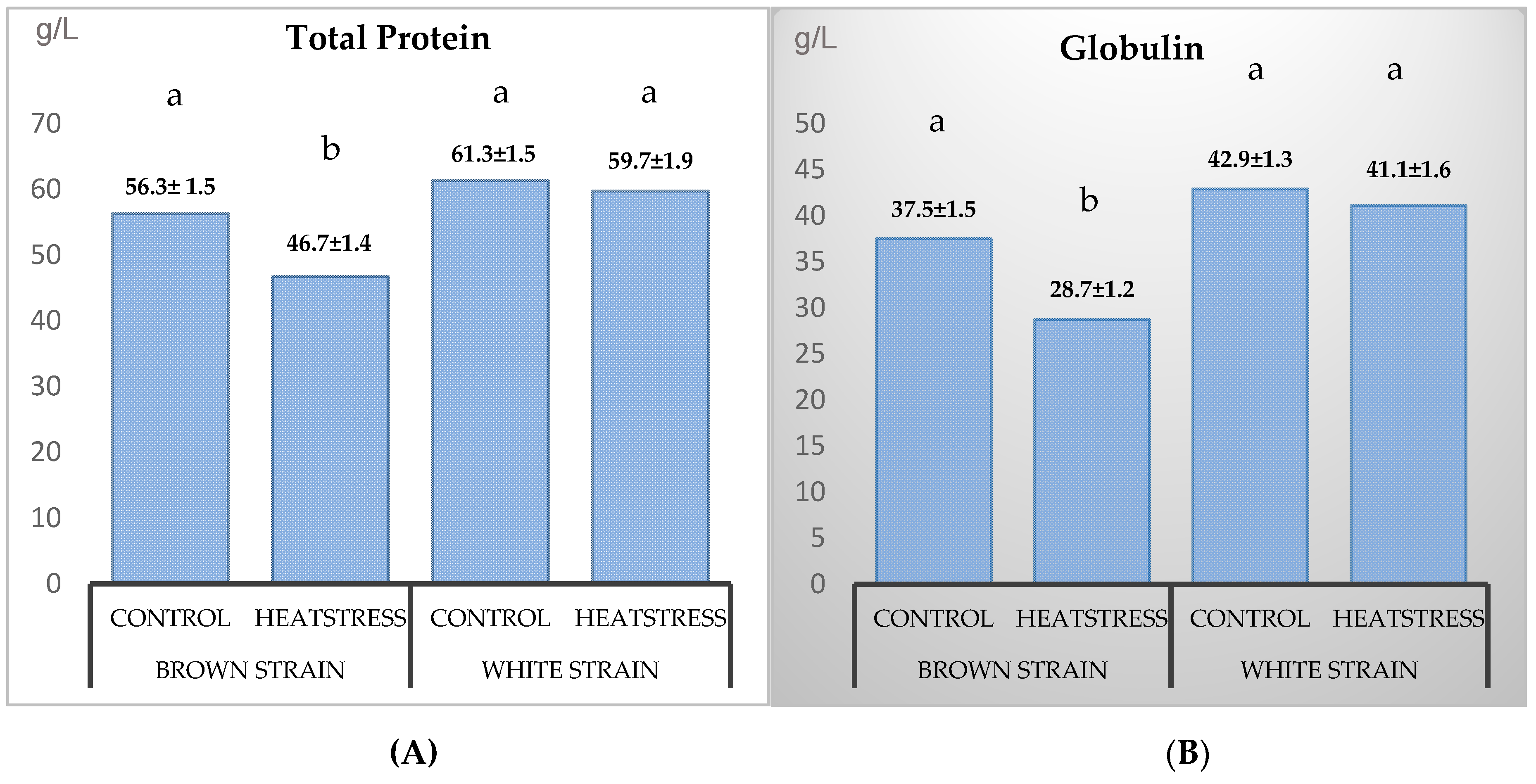
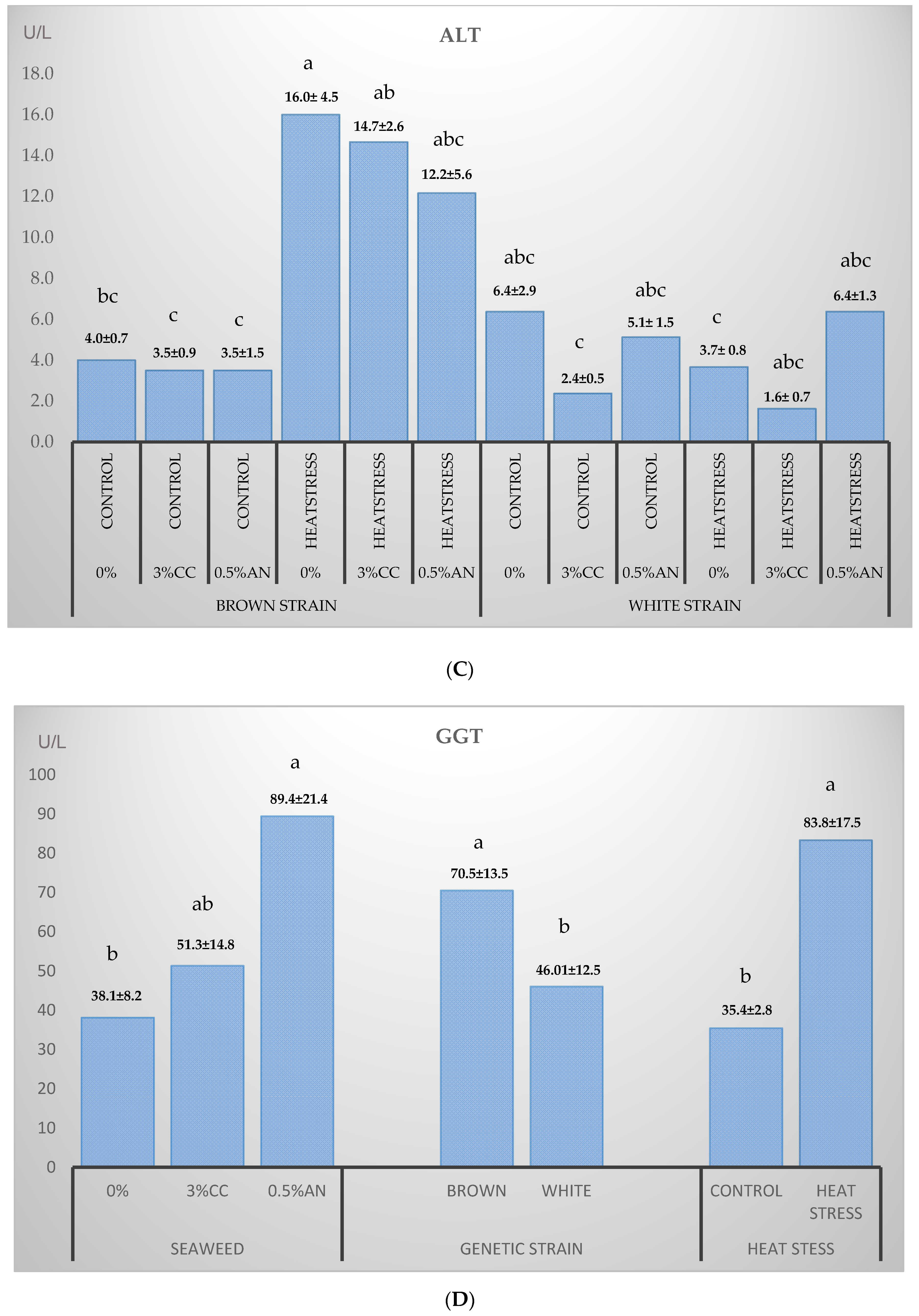
3.2. Blood Plasma Biochemistry
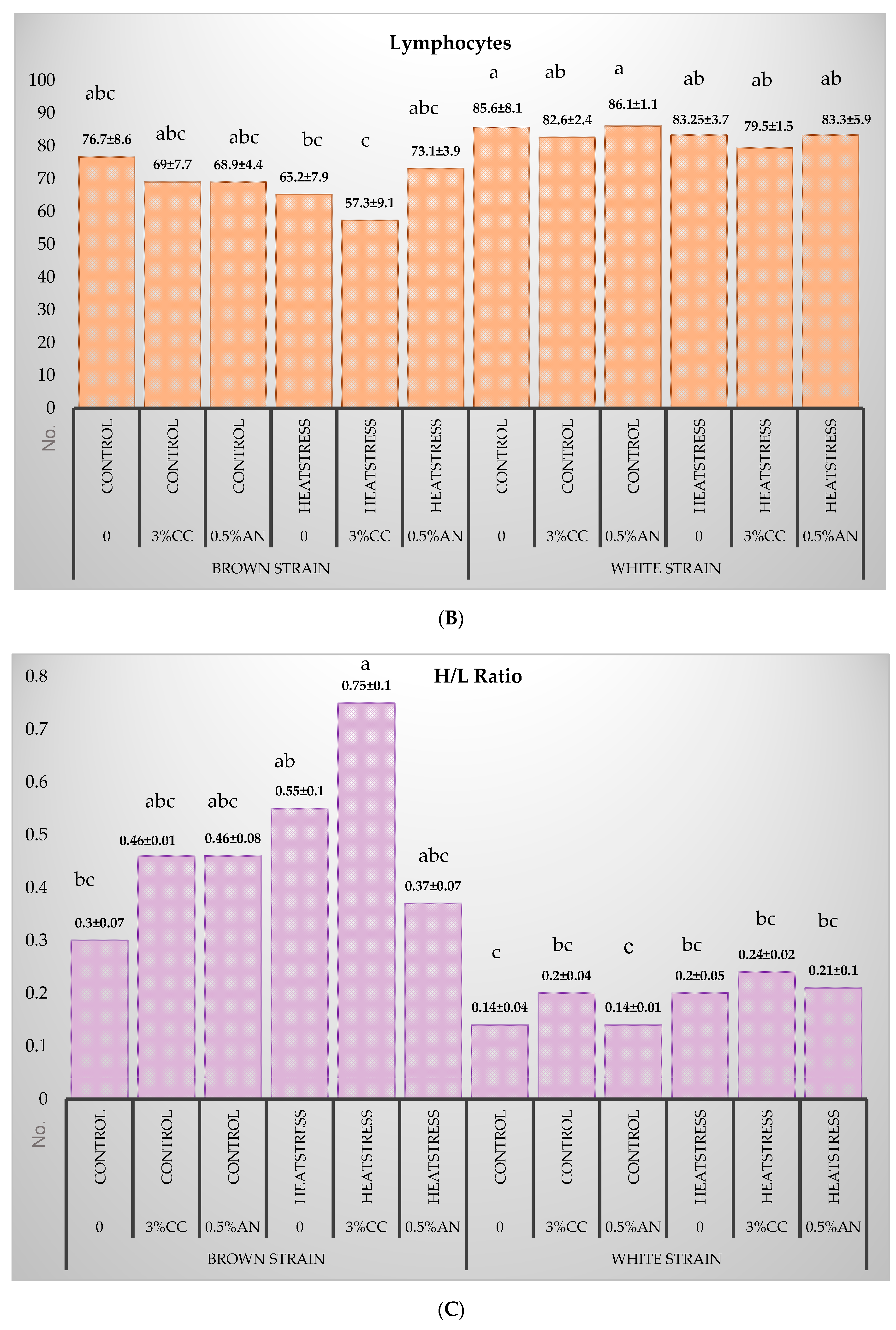
3.3. Hematology Analysis
4. Discussion
4.1. Layer Performance
4.2. Blood Biochemistry
4.3. Hematological Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | Chondrus crispus |
| AN | Ascophyllum nodosum |
| H/L | Heterophil/lymphocyte |
| APRI | Institute of the Dalhousie Agricultural Campus |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| GGT | Gamma-glutamyltransferase |
| ALP | Alkaline phosphatase |
| GLDH | Glutamate dehydrogenase |
| ANOVA | Analysis of Variance |
| SAS | Statistical analysis system |
References
- Tomar, S.K.; Anand, S.; Sharma, P.; Sangwan, V.; Mandal, S. Role of probiotics, prebiotics, synbiotics and postbiotics in inhibition of pathogens. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2015; pp. 717–732. [Google Scholar]
- Kulshreshtha, G.; Rathgeber, B.; MacIsaac, J.; Boulianne, M.; Brigitte, L.; Stratton, G.; Thomas, N.; Critchley, A.; Hafting, J.; Prithiviraj, B. Feed supplementation with red Seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, Reduce Salmonella Enteritidis in laying hens. Front. Microbiol. 2017, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Y.; Hu, L.; Liu, S.; Yu, H.; Xing, R.; Li, R.; Wang, X.; Li, P. In vitro prebiotic effects of seaweed polysaccharides. J. Oceanol. Limnol. 2018, 36, 926–932. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, P.; Stanton, C. Prebiotics from Seaweeds: An Ocean of Opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Gudiel-Urbano, M.; Goñi, I. Effect of edible seaweeds (Undaria pinnatifida and Porphyra ternera) on the metabolic activities of intestinal microflora in rats. Nutr. Res. 2002, 22, 323–331. [Google Scholar] [CrossRef]
- Manilal, A.S.; Sujith, S.; Seghal Kiran, G.; Selvin, J.; Shakir, C.; Gandhimathi, R.; Nataraja Panikkar, M.V. Biopotentials of seaweeds collected from southwest coast of India. J. Mar. Sci. Technol. 2009, 17, 67–73. [Google Scholar]
- Souza, B.W.S.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Cerna, M. Seaweed Proteins and Amino Acids as Nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 297–312. [Google Scholar]
- Abudabos, A.; Okab, A.; Aljumaah, R.; Samara, E.; Al-Haidary, K. Nutritional value of green seaweed (Ulva Lactuca) for broiler chickens. Ital. J. Anim. Sci. 2013, 12, 177–181. [Google Scholar] [CrossRef]
- Evans, F.D.; Critchley, A.T. Seaweeds for animal production use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Rathgeber, B.; Stratton, G.; Thomas, N.; Evans, F.; Critchley, A.; Hafting, J.; Prithiviraj, B. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult. Sci. J. 2014, 933, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdi, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Carrillo, D.S.; Casas, V.M.M.; Castro, G.M.I.; Perez-Gil, R.F.; Garcia, V.R. The use of Macrocystis pyrifera seaweed in broiler diets. Investig. Agrar. Prod. Sanid. Anim. 1990, 5, 137–142. [Google Scholar]
- El-DeeK, A.A.; Al-Harthi, M.A.; Abdalla, A.A.; Elbanoby, M.M. The use of brown algae meal in finisher broiler diets. Egypt Poult. 2011, 3, 767–781. [Google Scholar]
- Carrillo, S.; López, E.; Casas, M.M.; Avila, E.; Castillo, R.M.; Carranco, M.E.; Calvo, C.; Pérez-Gil, F. Potential use of seaweeds in the laying hen ration to improve the quality of n-3 fatty acid enriched eggs. J. Appl. Phycol. 2008, 20, 721–728. [Google Scholar] [CrossRef]
- Sahin, K.; Sahin, N.; Kucuk, O.; Hayirli, A.; Prasad, A.S. Role of dietary zinc in heat-stressed poultry: A review. Poult. Sci. J. 2009, 88, 2176–2183. [Google Scholar] [CrossRef]
- Lebman, D.A.; Coffman, R.L. Interleukin 4 causes isotype switching to IgE in T cellstimulated clonal B cell cultures. J. Exp. Med. 1988, 168, 853–862. [Google Scholar] [CrossRef]
- Savic, V.; Mikec, M.; Pavicic, P.; Tisijar, M. Effect of repeated heat stress on the humoral immune response and productivity of broiler chicks. Vet. Stanica 1993, 24, 195–202. [Google Scholar]
- Zulkifi, I.; Norma, M.T.; Israf, D.A.; Omar, A.R. The effect of early age feed restriction on subsequent response to high environmental temperatures in female broiler chickens. Poult. Sci. J. 2000, 79, 1401–1407. [Google Scholar] [CrossRef]
- El husseiny, O.; Creger, C.R. Effect of ambient temperature on mineral retention and balance of the broiler chicks. Poult. Sci. J. 1981, 60, 423–433. [Google Scholar]
- Hu, J.Y.; Heste, P.Y.; Makagon, M.M.; Xiong, Y.; Gates, R.S.; Cheng, H.W. Effect of cooled perches on physiological parameters of caged White Leghorn hens exposed to cyclic heat. Poult. Sci. 2019, 98, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Mack, L.A.; Felver-Gant, J.N.; Dennis, R.L.; Cheng, H.W. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013, 92, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council of Animal Care (CCAC). Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing; Canadian Council on Animal Care: Ottawa, ON, Canada, 2009. [Google Scholar]
- Stupart, C.M. Supplementation of Red Seaweed (Chondrus crispus) and Tasco® (Ascophyllum nodosum) in Laying Hen Diets. Master’s Thesis, Dalhousie University, Truro, NS, Canada, 2019. [Google Scholar]
- Martínez, Y.; Ayala, L.; Hurtado, C.; Más, D.; Rodríguez, R. Effects of Dietary Supplementation with Red Algae Powder (Chondrus crispus) on Growth Performance, Carcass Traits, Lymphoid Organ Weights and Intestinal pH in Broilers. Braz. J. Poult. Sci. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Ventura, M.R.; Castanon, J.R.; McNab, J.M. Nutritional value of seaweed (Ulva rigi-da) for poultry. Anim. Feed Sci. Technol. 1994, 49, 87–92. [Google Scholar] [CrossRef]
- Ross, E.G.; Dominy, W.G. The nutritional value of dehydrated, blue-green algae (Spirulina platensis) for poultry. Poult. Sci. J. 1990, 69, 794–800. [Google Scholar] [CrossRef]
- Mookiah, S.; Sieo, C.C.; Kalavathy, R.; Abdullah, N.; Ho, Y.W. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 2014, 94, 341–348. [Google Scholar] [CrossRef] [PubMed]
- El-Deek, A.A.; Brikaa, M.A. Nutritional and biological evaluation of marine seaweed as a feedstuff and as a pellet binder in poultry diet. Int. J. Poult. Sci. 2009, 8, 875–881. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Stadnicka, K.; Kozłowska, I.; Abiuso, C.; Tavaniello, S.; Dankowiakowska, A.; Sławińska, A.; Maiorano, G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal 2016, 10, 1271–1279. [Google Scholar] [CrossRef]
- Nikpiran, H.; Taghavi, M.; Khodadadi, A.; Athari, S.S. Influence of probiotic and prebiotic on broiler chickens performance and immune status. J. Nov. Appl. Sci. 2013, 2, 256–259. [Google Scholar]
- Jaensch, S.M.; Cullen, L.; Shane, R.R. Assessment of liver function in Galahs (Eolophus roseicapillus) after partial hepatectomy: A Comparison of plasma enzyme concentrations, serum bile acid levels, and galactose clearance tests. J. Avian Med. Surg. 2000, 14, 164–171. [Google Scholar] [CrossRef]
- Bona, L.; Staaveren, N.V.; Pokharel, B.B.; Krimpen, M.; Harlander-Matauschek, A. The effect of low protein energy rich diets on plasma hepatic markers, hepatic damage, and discrimination reversal learning in young female chicks. Front. Vet. Sci. 2018, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, B.W.; Harrison, G.J.; Harrison, L.R. Avian Medicine: Principles and Applications; Wingers Publishing Inc.: Lake Worth, FL, USA, 1994; pp. 223–245. [Google Scholar]
- Neijat, M.; Gakhar, N.; Neufeld, J.; House, J.D. Performance, egg quality, and blood plasma chemistry of laying hens fed hempseed and hempseed oil. Poult. Sci. 2014, 93, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.J.; Gwen, B.F. Clinical Avian Medicine; Harrison’s Bird Foods: Brentwood, TN, USA, 2005; pp. 647–665. [Google Scholar]
- Ikeda, Y.; Fujii, J.; Taniguchi, N.; Meister, A. Expression of an active glycosylated human gamma-glutamyl transpeptidase mutant that lacks a membrane anchor domain. Proc. Natl. Acad. Sci. USA 1995, 92, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Bloomer, S.A.; Brown, K.E.; Buettner, G.R.; Kregel, K.C. Dysregulation of hepatic iron with aging: Implications for heat stress-induced oxidative liver injury. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2008, 294, 1165–1174. [Google Scholar] [CrossRef][Green Version]
- El-Kholy, M.S.; El-Hindawy, M.M.; Alagawany, M.; El-Hack, M.E.; El-Gawad, S.A.; El-Sayed, A.E. Dietary supplementation of chromium can alleviate negative impacts of heat stress on performance, carcass yield, and some blood hematology and chemistry indices of growing Japanese quail. Biol. Trace Elem. Res. 2017, 179, 148–157. [Google Scholar] [CrossRef]
- Weber, G.M.; Machander, M.; Schierle, J.; Aureli, R.; Roos, F.; Pérez-Vendre, A.M. Tolerance of poultry against an overdose of canthaxanthin asmeasured by performance, different blood variables andpost-mortem evaluation. Anim. Feed Sci. Technol. 2013, 186, 91–100. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, E.C.; Na, Y.; Lee, S.R. Effects of dietary supplementation with fermented and nonfermented brown algae by-products on laying performance, egg quality, and blood profile in laying hens. Asian-Australas. J. Anim. Sci. 2018, 31, 1654–1659. [Google Scholar] [CrossRef]
- Evenni, L.S.; Castro, P.W.; Gomes Castro, A.J.; Santos, M.D.S.N.; de Sousa Pinheiro, T.; de Quevedo Florentin, K.; Alves, L.G.; Leite, E.L. Effect of galactofucan sulfate of a brown seaweed on induced hepatotoxicity in rats, sodium pentobarbital-induced sleep, and anti-inflammatory activity. J. Appl. Phycol. 2016, 28, 2005–2017. [Google Scholar]
- Rizk, Y.S.; Ismail, I.I.; Hafsa, S.H.A. Effect of dietary green tea and dried seaweed on productive and physiological performance of laying hens during late phage of production. Egypt Poult. Sci. 2017, 37, 685–706. [Google Scholar] [CrossRef]
- Krams, I.; Vrublevska, J.; Cirule, D.; Kivleniece, I.; Krama, T.; Rantala, M.J.; Sild, E.; Hõrak, P. Heterophil/lymphocyte ratios predict the magnitude of humoral immune response to a novel antigen in great tits (Parus major). Comp. Biochem. Phys. 2012, 161, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S.; Friend, T.H.; Caldwell, D.; Ameiss, K.; Krawczel, P.D. Effect of the seaweed Ascophyllum nodosum on lambs during forced walking and transport. J. Anim. Sci. 2007, 85, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, E.; Jafari, M. Effects of dietary supplementation with red seaweed, Gracilaria pygmaea, on growth, carcass composition and hematology of juvenile rainbow trout, Oncorhynchus mykiss. Aquac. Int. 2015, 25, 1857–1867. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effects of various forms of dietary CLV supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Sci. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Vanderlei, S.O.; Araújo, I.W.; Quinderé, A.L.; Fontes, B.P.; Eloy, Y.R.; Rodrigues, J.A.; Silva, A.A.; Chaves, H.V.; Jorge, R.J.; Menezes, D.B.; et al. The involvement of the HO-1 pathway in the anti-inflammatory action of a sulfated polysaccharide isolated from the red seaweed Gracilaria birdiae. Inflamm. Res. 2011, 60, 1121–1130. [Google Scholar] [CrossRef]
- Vetvicka, V.P.; Oliveira, C. β(1-3)(1-6)-d-glucans modulate immune status in pigs: Potential importance for efficiency of commercial farming. Ann. Transl. Med. 2014, 2, 16. [Google Scholar]
- Gross, W.B.; Siegel, H.S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983, 27, 972–979. [Google Scholar] [CrossRef]
- Mashaly, M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]

| Phase 1 | Phase 2 | Phase 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ingredients | 0% | 3% CC | 0.5% AN | 0% | 3% CC | 0.5% AN | 0% | 3% CC | 0.5% AN |
| Ground Corn | 532.56 | 497.47 | 524.5 | 538.45 | 503.37 | 530.47 | 549.13 | 513.17 | 541.13 |
| Canola Meal | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Wheat | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Soybean Meal | 143.59 | 136.64 | 144.28 | 135.57 | 126.62 | 136.26 | 123.35 | 116.42 | 124.05 |
| Limestone | 45.15 | 44.96 | 45.07 | 46.43 | 46.26 | 46.35 | 47.7 | 47.55 | 47.61 |
| Shell Mix | 22.58 | 22.49 | 22.53 | 23.23 | 23.13 | 23.17 | 23.85 | 23.77 | 23.81 |
| Oyster Shell | 22.58 | 22.49 | 22.53 | 23.21 | 23.13 | 23.17 | 23.85 | 23.77 | 23.81 |
| Animal/Vegetable Fat | 11.82 | 27.47 | 14.7 | 11.93 | 27.57 | 14.81 | 11.42 | 27.09 | 14.31 |
| Dicalcium Phosphate | 11.18 | 11.39 | 11.21 | 10.8 | 11.01 | 10.83 | 10.44 | 10.66 | 10.48 |
| Vitamin/Mineral Premix 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Salt | 3.73 | 0.14 | 3.33 | 3.61 | 0.02 | 3.21 | 3.63 | 0.04 | 3.22 |
| Mehionine Premix 2 | 1.8 | 1.91 | 1.75 | 1.77 | 1.88 | 1.72 | 1.62 | 1.74 | 1.58 |
| Tasco | 0 | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 5 |
| Ground Chondrus crispus | 0 | 30 | 0 | 0 | 30 | 0 | 0 | 30 | 0 |
| Calculated Composition (%) | |||||||||
| Metabolizable Energy (kCal/kg) | 2800 | 2800 | 2800 | 2800 | 2800 | 2800 | 2800 | 2800 | 2800 |
| Protein (%) | 16.04 | 16.04 | 16.04 | 15.71 | 15.71 | 15.71 | 15.22 | 15.22 | 15.22 |
| Calcium (%) | 3.73 | 3.73 | 3.73 | 3.82 | 3.82 | 3.82 | 3.91 | 3.91 | 3.91 |
| Available Phosphorus (%) | 0.4 | 0.4 | 0.4 | 0.39 | 0.39 | 0.39 | 0.38 | 0.38 | 0.38 |
| Sodium (%) | 0.17 | 0.17 | 0.17 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Parameters | Brown Strain | White Strain | SEM | p-Value Strain | p-Value Seaweed | ||
|---|---|---|---|---|---|---|---|
| 0% CC | 3% CC | 0% CC | 3% CC | ||||
| Egg production (%) | 85.1 b | 81.3 b | 88.2 a | 87.1 a | 0.16 | 0.002 | NS 1 |
| Feed Intake (g/bird/day) | 95.1 a | 85.9 b | 90.6 ab | 87.1 b | 29.5 | NS | <0.001 |
| Feed/egg | 108.0 a | 96.7 bc | 96.7 b | 95.2 c | 1.7 | 0.02 | 0.02 |
| Body Weight (g) | 2113.3 a | 2083.9 ab | 1798.4 b | 1743.6 b | 36.3 | <0.001 | NS |
| Weight gain (g) | 98.2 ab | −119 b | 135.8 a | − 142.4 b | 42.8 | NS | <0.001 |
| Plasma Parameter | Units | Brown Strain | White Strain | p-Value Strain | p-Value Seaweed | ||
|---|---|---|---|---|---|---|---|
| 0% CC | 3% CC | 0% CC | 3% CC | ||||
| Protein | g/L | 52.9 ± 3.4 b | 52.3 ± 1.8 b | 54.3 ± 0.9 ab | 64.0 ± 3.1 a | 0.02 | NS 1 |
| Albumin | g/L | 18.1 ± 0.7 | 19.3 ± 1.1 | 17.4 ± 0.6 | 18.1 ± 0.4 | NS | NS |
| Globulin | g/L | 34.8 ± 2.7 b | 33.0 ± 0.9 b | 36.9 ± 1.3 ab | 46.0 ± 3.5 a | <0.01 | NS |
| Glucose | mM/L | 14.2 ± 0.3 | 13.7 ± 0.4 | 13.5 ± 0.3 | 13.2 ± 0.3 | NS | NS |
| Chol 2 | mM/L | 1.1 ± 0.2 | 0.9 ± 0.07 | 0.8 ± 0.07 | 0.8 ± 0.08 | NS | NS |
| ALP 3 | U/L | 246.9 ± 47.2 | 206.8 ± 49.2 | 226.5 ± 44.5 | 202.3 ± 27.6 | NS | NS |
| AST 4 | U/L | 194.9 ± 19.1 | 167.8 ± 8.3 | 181.0 ± 4.9 | 227.2 ± 32.7 | NS | NS |
| ALT 5 | U/L | 4.6 ± 2.8 | 3 ± 0.3 | 2.7 ± 0.5 | 2.8 ± 0.3 | NS | NS |
| GGT 6 | U/L | 24.3 ± 3.1 | 32.9 ± 5.7 | 21.7 ± 1.9 | 22.3 ± 2.3 | NS | NS |
| GLDH 7 | U/L | 2.6 ± 0.4 | 2.4 ± 1.2 | 3.2 ± 1.6 | 1.3 ± 0.2 | NS | NS |
| Plasma Parameter | Units | p-Value Strain | p-Value Seaweed | p -Value Heat Stress | Strain × Seaweed | Seaweed × Heat Stress | Strain × Heat Stress | Strain × Seaweed × Heat Stress |
|---|---|---|---|---|---|---|---|---|
| Protein | g/L | <0.01 | NS 1 | <0.01 | NS | NS | 0.02 | NS |
| Albumin | g/L | NS | 0.04 | NS 1 | NS | NS | NS | NS |
| Globulin | g/L | <0.01 | NS | <0.01 | NS | NS | 0.02 | NS |
| Glucose | mM/L | NS | NS | <0.01 | NS | NS | 0.01 | NS |
| Chol | mM/L | NS | NS | <0.01 | 0.01 | NS | <0.01 | NS |
| ALP | U/L | <0.01 | NS | NS | NS | NS | NS | NS |
| AST | U/L | NS | NS | NS | NS | NS | NS | NS |
| ALT | U/L | 0.01 | NS | <0.01 | NS | NS | <0.01 | NS |
| GGT | U/L | 0.02 | 0.02 | 0.04 | NS | NS | NS | NS |
| GLDH | U/L | NS | NS | NS | NS | NS | <0.01 | NS |
| 0% CC | 3% CC | 0.5% AN | ||||
|---|---|---|---|---|---|---|
| Brown Strain | ||||||
| Plasma Parameter | Control | Heat Stress | Control | Heat Stress | Control | Heat Stress |
| Protein | 59.7 ± 1.4 abc | 45.2 ± 1.9 d | 53.5 ± 3.2 abcd | 47.4 ± 3.4 cd | 55.9 ± 2.2 abcd | 47.6 ± 2.5 bcd |
| Albumin | 19.5 ± 0.5 | 18.1 ± 0.4 | 17.5 ± 0.7 | 17.4 ± 1.1 | 19.5 ± 0.9 | 18.6 ± 0.4 |
| Globulin | 40.5 ± 1.1 abc | 27.1 ± 2.1 d | 35.7 ± 3.8 abcd | 30.0 ± 2.5 bcd | 36.4 ± 2.1 abcd | 29.0 ± 2.2 cd |
| Glucose | 14.1 ± 0.3 ab | 11.9 ± 0.6 b | 14.1 ± 0.3 ab | 12.5 ± 0.8 b | 14.4 ± 0.8 ab | 11.8 ± 0.4 ab |
| Chol | 1.4 ± 0.3 c | 3.7 ± 0.4 a | 1.7 ± 0.2 bc | 3.4± 0.5 ab | 2.4 ± 0.3 abc | 3.8 ± 0.6 a |
| ALP | 156.7 ± 31.6 | 190.5 ± 59.8 | 195.4 ± 39.6 | 191.6 ± 66.3 | 171.9 ± 18.1 | 131.7 ± 28.1 |
| AST | 169.2 ± 7.9 | 156.125 ± 10.5 | 153.6 ± 13.7 | 198.1 ± 36.9 | 218.5 ± 30.1 | 194.4 ± 12.2 |
| ALT | 4.0 ± 0.7 bc | 16.0 ± 4.5 a | 3.5 ± 0.9 c | 14.7 ± 2.6 ab | 3.5 ± 1.5 c | 12.2 ± 5.6 abc |
| GGT | 32.5 ± 1.2 | 65.6 ± 30.9 | 42.2 ± 9.5 | 115.5 ± 48.6 | 49.4 ± 7.6 | 133.8 ± 56.3 |
| GLDH | 2.7 ± 2.3 b | 26.6 ± 8.4 a | 1.1 ± 0.6 b | 10.7 ± 7.7 ab | 6.6 ± 3.1 ab | 9.7 ± 5.1 ab |
| White strain | ||||||
| Protein | 59.5 ± 2.1 abc | 58.5 ± 2.1 abcd | 61.5 ± 3.5 ab | 58.4 ± 5.1 abcd | 62.9 ± 2.0 a | 62.4 ± 2.4 a |
| Albumin | 18.0 ± 0.4 | 18.6 ± 0.7 | 18.7 ± 0.5 | 17.2 ± 0.6 | 18.4 ± 0.9 | 20.0 ± 1.1 |
| Globulin | 41.5 ± 2.4 abc | 39.9 ± 1.6 abc | 42.7 ± 3.1 a | 41.1 ± 4.5 abc | 44.5 ± 1.7 a | 42.4 ± 1.8 ab |
| Glucose | 13.7 ± 0.5 ab | 12.5 ± 0.4 ab | 13.7 ± 0.3 ab | 12.7 ± 0.3 ab | 14.0± 0.6 ab | 14.8 ± 0.7 a |
| Chol | 2.8 ± 0.3a bc | 3.5 ± 0.3 ab | 3.4 ± 0.4 ab | 2.2 ± 0.1 abc | 1.6 ± 0.2 bc | 2.5 ± 0.6 abc |
| ALP | 347.6 ± 46.9 | 263.6 ± 47.8 | 218.6 ± 31.4 | 264.7 ± 55.9 | 233.8 ± 9.1 | 219.9 ± 27.6 |
| AST | 190.1 ± 14.9 | 161.4 ± 9.4 | 176.5 ± 11.7 | 225.6 ± 31.9 | 199.6 ± 13.5 | 202.9 ± 27.3 |
| ALT | 6.4 ± 2.9 abc | 3.7 ± 0.8 c | 2.4 ± 0.5 c | 1.6 ± 0.7 c | 5.1 ± 1.5 abc | 6.4 ± 1.3 abc |
| GGT | 26.1 ± 4.5 | 28.0 ± 4.9 | 26.9 ± 6.9 | 20.4± 0.2 | 35.0 ± 1.8 | 139.4 ± 59.5 |
| GLDH | 9.4 ± 7.2 ab | 1.7 ± 0.7 b | 2.8 ± 0.8 b | 2.5 ± 0.5 b | 7.5 ± 2.9 ab | 3.6 ± 1.9 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzouie, S.; M. Rathgeber, B.; M. Stupart, C.; MacIsaac, J.; MacLaren, L.A. Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens. Animals 2020, 10, 1570. https://doi.org/10.3390/ani10091570
Borzouie S, M. Rathgeber B, M. Stupart C, MacIsaac J, MacLaren LA. Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens. Animals. 2020; 10(9):1570. https://doi.org/10.3390/ani10091570
Chicago/Turabian StyleBorzouie, Shima, Bruce M. Rathgeber, Cassie M. Stupart, Janice MacIsaac, and Leslie A. MacLaren. 2020. "Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens" Animals 10, no. 9: 1570. https://doi.org/10.3390/ani10091570
APA StyleBorzouie, S., M. Rathgeber, B., M. Stupart, C., MacIsaac, J., & MacLaren, L. A. (2020). Effects of Dietary Inclusion of Seaweed, Heat Stress and Genetic Strain on Performance, Plasma Biochemical and Hematological Parameters in Laying Hens. Animals, 10(9), 1570. https://doi.org/10.3390/ani10091570





