Genetic Diversity and Population Structure of Polish Konik Horse Based on Individuals from All the Male Founder Lines and Microsatellite Markers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
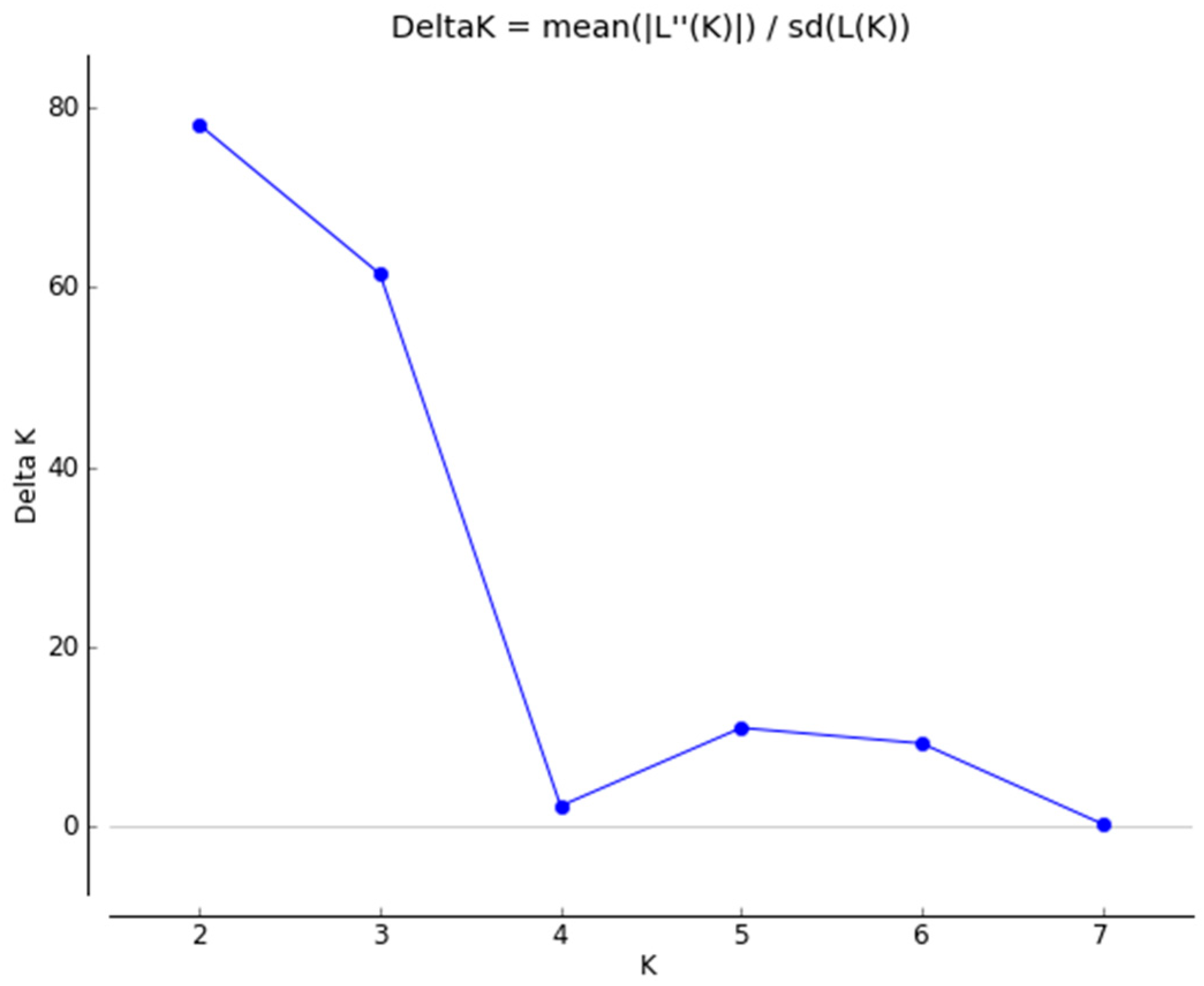
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gralak, B.; Niemczewski, C.; Jaworski, Z. Genetic polymorphism of 12 micorsatellite markers in Polish Primitive Horse. Anim. Sci. Pap. Rep. 2001, 19, 277–283. [Google Scholar]
- Doboszewski, P.; Doktor, D.; Jaworski, Z.; Kalski, R.; Kulakowska, G.; Lojek, J.; Plachocki, D.; Rys, A.; Tylkowska, A. Konik polski horses as a mean of biodiversity maintenance in post-agricultural and forest areas: An overview of Polish experiences. Anim. Sci. Pap. Rep. 2017, 35, 333–347. [Google Scholar]
- Janczarek, I.; Pluta, M.; Paszkowska, A. Pochodzenie, hodowla i użytkowanie koników polskich. Prz. Hod. 2017, 85, 6–10. [Google Scholar]
- Stefaniuk-Szmukier, M.; Ropka-Molik, K.; Piorkowska, K.; Szmatoła, T.; Długosz, B.; Pisarczyk, W.; Bugno-Poniewierska, M. Variation in TBX3 Gene Region in Dun Coat Color Polish Konik Horses. J. Equine Vet. Sci. 2017, 49, 60–62. [Google Scholar] [CrossRef]
- Komosa, M.; Purzyc, H. Konik and Hucul horses: A comparative study of exterior measurements. J. Anim. Sci. 2009, 87, 2245–2254. [Google Scholar] [CrossRef]
- Jodkowska, E.; Kuźniak, A.M.; Pikuła, R.; Smugała, M. Analysis of Polish Primitive Ponies Migration under Registration of the Global Positioning System. GSTF J. Vet. Sci. 2015, 1, 47–68. [Google Scholar] [CrossRef]
- Gurgul, A.; Jasielczuk, I.; Semik-Gurgul, E.; Pawlina-Tyszko, K.; Stefaniuk-Szmukier, M.; Szmatoła, T.; Polak, G.; Tomczyk-Wrona, I.; Bugno-Poniewierska, M. A genome-wide scan for diversifying selection signatures in selected horse breeds. PLoS ONE 2019, 14, 1–24. [Google Scholar] [CrossRef]
- Pluta, M.; Osinski, Z.; Cieśla, A.; Kolstrung, R. Genetic and Phenotypic Characteristics of Polish Konik Horses Maintained in the Reserve and Stable System in Central-Eastern Poland. Acta Sci. Pol. Zootech. 2016, 15, 59–76. [Google Scholar] [CrossRef]
- Pasicka, E. Polish konik horse-characteristics and historical background of native descendants of tarpan. Acta Sci. Pol. Med. Vet. 2013, 12, 25–38. [Google Scholar]
- Siemieniuch, M.J.; Jaworski, Z. 70-lecie hodowli konikow polskich w Popielnie. Hod. Jezdziec. 2020, 1, 28–30. [Google Scholar]
- Cieslak, J.; Wodas, L.; Borowska, A.; Cothran, E.G.; Khanshour, A.M.; Mackowski, M. Characterization of the Polish Primitive Horse (Konik) maternal lines using mitochondrial D-loop sequence variation. PeerJ 2017, 5, e3714. [Google Scholar] [CrossRef]
- Kaminski, S.; Hering, D.M.; Jaworski, Z.; Zabolewicz, T.; Rusc, A.; Kaminski, S.; Hering, D.M.; Jaworski, Z.; Zabolewicz, T.; Rusc, A. Assessment of genomic inbreeding in Polish Konik horses. Pol. J. Vet. Sci. 2017, 20, 603–605. [Google Scholar] [CrossRef]
- Jaworski, Z.; Tomczyk-Wrona, I. Program. Ochrony Zasobów Genetycznych Koni Rasy Konik Polski; Krajowy Ośrodek Koordynacyjny ds. Zasobów Genetycznych Zwierząt, Instytut Zootechniki-PIB w Krakowie: Krakow, Poland, 2009; pp. 1–11. [Google Scholar]
- Tomczyk-Wrona, I. Koniki Polskie-Modelowa populacja ochrony bioróżnorodności. Wiadomości Zootech. 2017, 5, 98–103. [Google Scholar]
- Jaworski, Z.; Siemieniuch, M.; Jastrzębska, E.; Wojciechowska, P. Zróżnicowanie Genetyczne Populacji Koników Polskich. In Proceedings of the LXXXIV Zjazd Naukowy Polskiego Towarzystwa Zootechnicznego w Szczecinie “Osiągniecia i perspektywy zootechniki w aspekcie zrównoważonego rolnictwa i ochrony środowiska”, Szczecin, Poland, 18–20 September 2019; p. 123. [Google Scholar]
- Chodkiewicz, A. Advantages and disadvantages of Polish primitive horse grazing on valuable nature areas—A review. Glob. Ecol. Conserv. 2020, 21, e00879. [Google Scholar] [CrossRef]
- Siemieniuch, M.J.; Jaworski, Z. A 17-year survey of reproductive efficiency in Polish Konik horses. Acta Sci. Pol. Zootech. 2018, 17, 31–40. [Google Scholar] [CrossRef]
- Jaworska, J.; Jaworski, Z.; McDonnell, S.M.; Gorecka-Bruzda, A. Harem stallion changes are not associated with diminished reproductive performance of females in semi-feral Konik polski horses (Equus caballus). Theriogenol. J. 2020, 151, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zabek, T.; Nogaj, A.; Radko, A.; Nogaj, J.; Słota, E. Genetic variation of Polish endangered Biłgoraj horses and two common horse breeds in microsatellite loci. J. Appl. Genet. 2005, 46, 299–305. [Google Scholar] [PubMed]
- Vostry, L.; Vostra-Vydrova, H.; Hofmanova, B.; Vesela, Z.; Majzlik, I. Genetic diversity in Czech Haflinger horses. Poljoprivreda/Agriculture 2015, 21, 163–165. [Google Scholar] [CrossRef]
- Mackowski, M.; Mucha, S.; Cholewinski, G.; Cieslak, J. Genetic diversity in Hucul and Polish primitive horse breeds. Arch. Anim. Breed. 2015, 58, 23–31. [Google Scholar] [CrossRef]
- Castaneda, C.; Juras, R.; Khanshour, A.; Randlaht, I.; Wallner, B.; Rigler, D.; Lindgren, G.; Raudsepp, T.; Cothran, E.G. Population Genetic Analysis of the Estonian Native Horse Suggests Diverse and Distinct Genetics, Ancient Origin and Contribution from Unique Patrilines. Genes 2019, 10, 629. [Google Scholar] [CrossRef]
- Fornal, A.; Radko, A. Ocena polimorfizmu markerów mikrosatelitarnych DNA u koników polskich. Episteme Czas. Nauk. 2012, 14, 287–292. [Google Scholar]
- Solis, A.; Jugo, B.M.; Mériaux, J.C.; Iriondo, M.; Mazón, L.I.; Aguirre, A.I.; Vicario, A.; Estomba, A. Genetic diversity within and among four South European native horse breeds based on microsatellite DNA analysis: Implications for conservation. J. Hered. 2005, 96, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9. 3). Department of Ecology and Evolution, University of Lausanne, Switzerland. J. Hered. 2001, 149, 507–526. [Google Scholar]
- Pritchard, J.K.; Stephens, P.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Earl, D.A.; VonHoldt, B.M. Structure Harvester: A website and program for visualizing Structure output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Paradis, E. Pegas: An R package for population genetics with an integrated-modular approach. Bioinformatics 2010, 26, 419–420. [Google Scholar] [CrossRef]
- Huston, K.A. Statistical Analysis of STR Data. Profiles DNA 1998, 1, 14–15. [Google Scholar]
- Nei, M. Molecular Evolutionary Genetics; New York Columbia University Press: New York, NY, USA, 1987; p. 512. [Google Scholar]
- Ling, Y.; Cheng, Y.; Wang, Y.; Guan, W.; Han, J.; Fu, B.; He, X.; Pu, Y. Genetic diversity of 23 Chinese indigenous horse breeds revealed by microsatellite markers. Biodivers. Sci. 2009, 17, 240–247. [Google Scholar]
- Nei, M.; Roychoudhury, A.K. Sampling variances of heterozygosity and genetic distance. Genetics 1974, 76, 379–390. [Google Scholar]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- May-Davis, S.; Brown, W.Y.; Shorter, K.; Vermeulen, Z.; Butler, R.; Koekkoek, M. A novel non-invasive selection criterion for the preservation of primitive dutch konik horses. Animals 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Wallner, B.; Brem, G.; Müller, M.; Achmann, R. Fixed nucleotide differences on the Y chromosome indicate clear divergence between Equus przewalskii and Equus caballus. Anim. Genet. 2003, 34, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Wallner, B.; Vogl, C.; Shukla, P.; Burgstaller, J.P.; Druml, T.; Brem, G. Identification of Genetic Variation on the Horse Y Chromosome and the Tracing of Male Founder Lineages in Modern Breeds. PLoS ONE 2013, 8, e60015. [Google Scholar] [CrossRef]
- Rendo, F.; Iriondo, M.; Manzano, C.; Estonba, A. Effectes of a 10-years conservation programme on the genetic diversity of the Pottoka pony-new clues regarding their origin. J. Anim. Breed Genet. 2012, 129, 234–243. [Google Scholar] [CrossRef]
- Lopes, M.S.; Mendonca, D.; Rojer, H.; Cabral, V.; Bettencourt, S.X.; da Câmara Machado, A. Morphological and genetic characterization of an emerging Azorean horse breed: The Terceira Pony. Front. Genet. 2015, 5, 1–7. [Google Scholar] [CrossRef]


| Paternal Lineage | N | Na | Na Freq. ≥ 5% | Ae | I | No. Private Alleles | He |
|---|---|---|---|---|---|---|---|
| Wicek | 48 | 6.824 | 4.647 | 3.824 | 1.476 | 0.412 | 0.693 |
| Myszak | 12 | 5.059 | 4.000 | 3.306 | 1.302 | 0.000 | 0.657 |
| Glejt I | 35 | 6.294 | 4.588 | 3.875 | 1.453 | 0.059 | 0.700 |
| Goraj | 32 | 6.118 | 4.176 | 3.627 | 1.396 | 0.000 | 0.678 |
| Chochlik | 36 | 6.471 | 4.765 | 3.730 | 1.449 | 0.000 | 0.689 |
| Liliput | 33 | 6.176 | 4.588 | 3.715 | 1.429 | 0.059 | 0.688 |
| FIT | FST | FIS | |
|---|---|---|---|
| AHT4 | −0.0096 | 0.0213 | −0.0316 |
| AHT5 | 0.0162 | 0.0373 | −0.0219 |
| ASB2 | −0.0100 | 0.0113 | −0.0215 |
| HMS2 | −0.0191 | 0.0053 | −0.0245 |
| HMS3 | 0.0231 | 0.0357 | −0.0130 |
| HMS6 | −0.0185 | 0.0269 | −0.0466 |
| HMS7 | 0.0613 | 0.0673 | −0.0065 |
| HTG10 | −0.0061 | 0.0211 | −0.0278 |
| HTG4 | 0.0342 | 0.0099 | 0.0246 |
| HTG6 | 0.0000 | 0.0526 | −0.0555 |
| HTG7 | 0.0105 | 0.0244 | −0.0143 |
| VHL20 | 0.0261 | 0.0438 | −0.0185 |
| ASB17 | 0.0142 | 0.0166 | −0.0024 |
| ASB23 | 0.0449 | 0.0262 | 0.0193 |
| CA425 | 0.1052 | 0.0756 | 0.0319 |
| HMS1 | 0.0305 | 0.0362 | −0.0059 |
| LEX3 | 0.9562 | 0.0079 | 0.9559 |
| Mean | 0.0741 | 0.0305 | −0.0134 |
| Ho | He | PD | PE | PIC | PE1 | PE2 | PID | |
|---|---|---|---|---|---|---|---|---|
| AHT4 | 0.7602 | 0.7482 | 0.8914 | 0.5274 | 0.7088 | 0.3486 | 0.5261 | 0.1028 |
| AHT5 | 0.7908 | 0.7964 | 0.9280 | 0.5821 | 0.7672 | 0.4240 | 0.6020 | 0.0706 |
| ASB2 | 0.8010 | 0.7895 | 0.9303 | 0.6009 | 0.7670 | 0.4333 | 0.6129 | 0.0669 |
| HMS2 | 0.7398 | 0.7234 | 0.8819 | 0.4925 | 0.6857 | 0.3207 | 0.5013 | 0.1142 |
| HMS3 | 0.7653 | 0.7763 | 0.9101 | 0.5363 | 0.7431 | 0.3902 | 0.5694 | 0.0832 |
| HMS6 | 0.8163 | 0.7956 | 0.9222 | 0.6297 | 0.7638 | 0.4146 | 0.5929 | 0.0735 |
| HMS7 | 0.6684 | 0.7015 | 0.8654 | 0.3810 | 0.6519 | 0.2914 | 0.4610 | 0.1387 |
| HTG10 | 0.8112 | 0.8012 | 0.9326 | 0.6200 | 0.7747 | 0.4393 | 0.6160 | 0.0661 |
| HTG4 | 0.4541 | 0.4681 | 0.6885 | 0.1504 | 0.4421 | 0.1198 | 0.2785 | 0.3090 |
| HTG6 | 0.1684 | 0.1663 | 0.3011 | 0.0217 | 0.1593 | 0.0138 | 0.0843 | 0.7020 |
| HTG7 | 0.6173 | 0.6195 | 0.7915 | 0.3122 | 0.5522 | 0.1994 | 0.3475 | 0.2121 |
| VHL20 | 0.7806 | 0.7931 | 0.9267 | 0.5636 | 0.7683 | 0.4344 | 0.6121 | 0.0676 |
| ASB17 | 0.7857 | 0.7926 | 0.9197 | 0.5728 | 0.7662 | 0.4311 | 0.6081 | 0.0694 |
| ASB23 | 0.7092 | 0.7371 | 0.8827 | 0.4426 | 0.6954 | 0.3333 | 0.5094 | 0.1108 |
| CA425 | 0.6480 | 0.7122 | 0.8793 | 0.3524 | 0.6813 | 0.3198 | 0.5046 | 0.1137 |
| HMS1 | 0.7296 | 0.7457 | 0.8956 | 0.4755 | 0.7124 | 0.3541 | 0.5358 | 0.0979 |
| LEX3 | 0.0357 | 0.8110 | 0.8230 | 0.0012 | 0.7858 | 0.4552 | 0.6307 | 0.0609 |
| 0.6519 * | 0.7046 * | 0.9999 ** | 0.9999 ** | 0.6721 * | 0.9992 ** | 0.9999 ** | 5.04 × 10−17 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornal, A.; Kowalska, K.; Zabek, T.; Piestrzynska-Kajtoch, A.; Musiał, A.D.; Ropka-Molik, K. Genetic Diversity and Population Structure of Polish Konik Horse Based on Individuals from All the Male Founder Lines and Microsatellite Markers. Animals 2020, 10, 1569. https://doi.org/10.3390/ani10091569
Fornal A, Kowalska K, Zabek T, Piestrzynska-Kajtoch A, Musiał AD, Ropka-Molik K. Genetic Diversity and Population Structure of Polish Konik Horse Based on Individuals from All the Male Founder Lines and Microsatellite Markers. Animals. 2020; 10(9):1569. https://doi.org/10.3390/ani10091569
Chicago/Turabian StyleFornal, Agnieszka, Katarzyna Kowalska, Tomasz Zabek, Agata Piestrzynska-Kajtoch, Adrianna D. Musiał, and Katarzyna Ropka-Molik. 2020. "Genetic Diversity and Population Structure of Polish Konik Horse Based on Individuals from All the Male Founder Lines and Microsatellite Markers" Animals 10, no. 9: 1569. https://doi.org/10.3390/ani10091569
APA StyleFornal, A., Kowalska, K., Zabek, T., Piestrzynska-Kajtoch, A., Musiał, A. D., & Ropka-Molik, K. (2020). Genetic Diversity and Population Structure of Polish Konik Horse Based on Individuals from All the Male Founder Lines and Microsatellite Markers. Animals, 10(9), 1569. https://doi.org/10.3390/ani10091569





