Tumor Infiltrating Lymphocytes in Pet Rabbit Mammary Carcinomas: A Study with Relevance to Comparative Pathology
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Samples
2.2. Light Microscopic Evaluation of Tumor Infiltrating Lymphocytes
2.3. Data Obtained from Diagnostic Records
2.4. Statistical Evaluation
3. Results
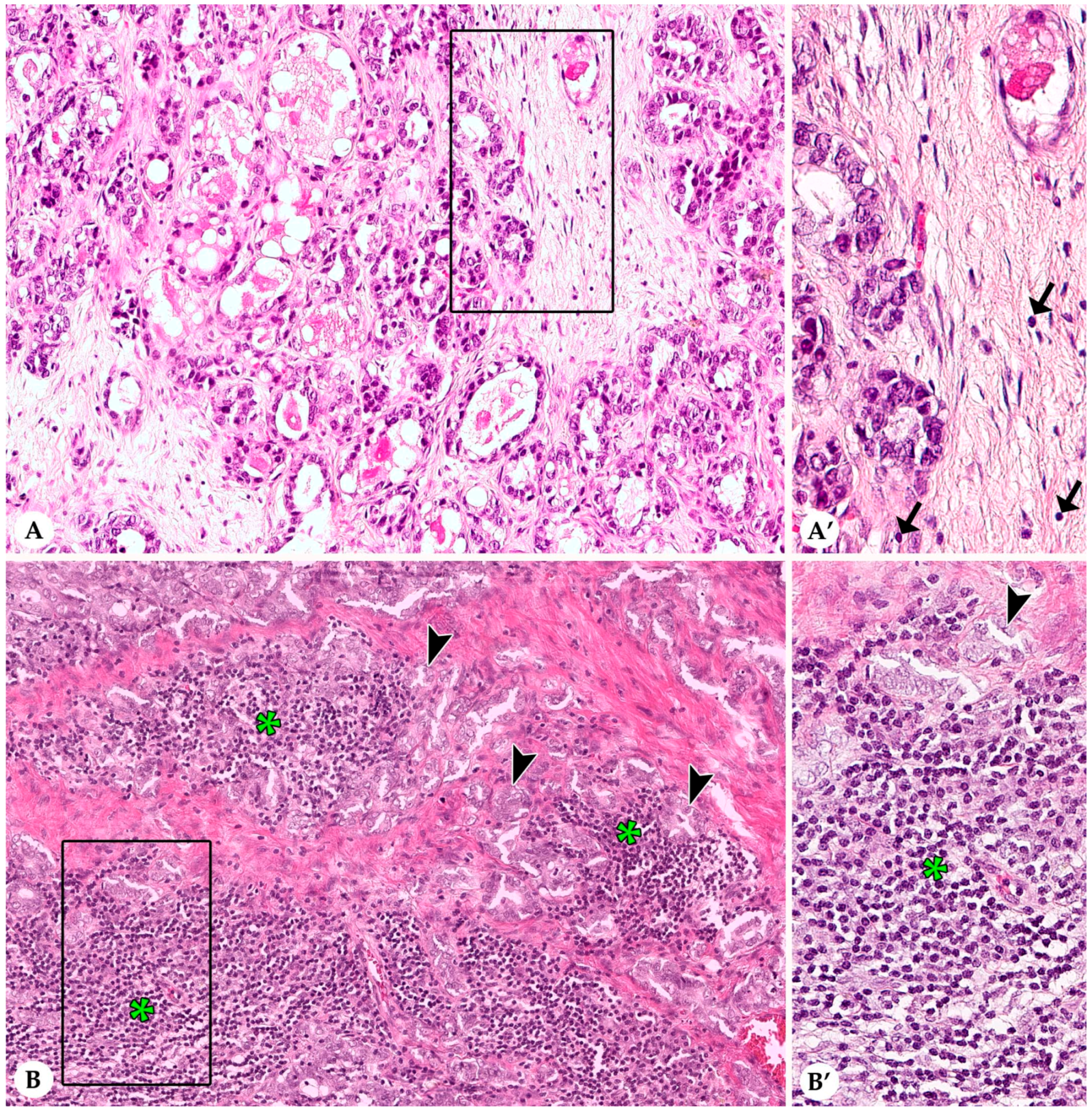
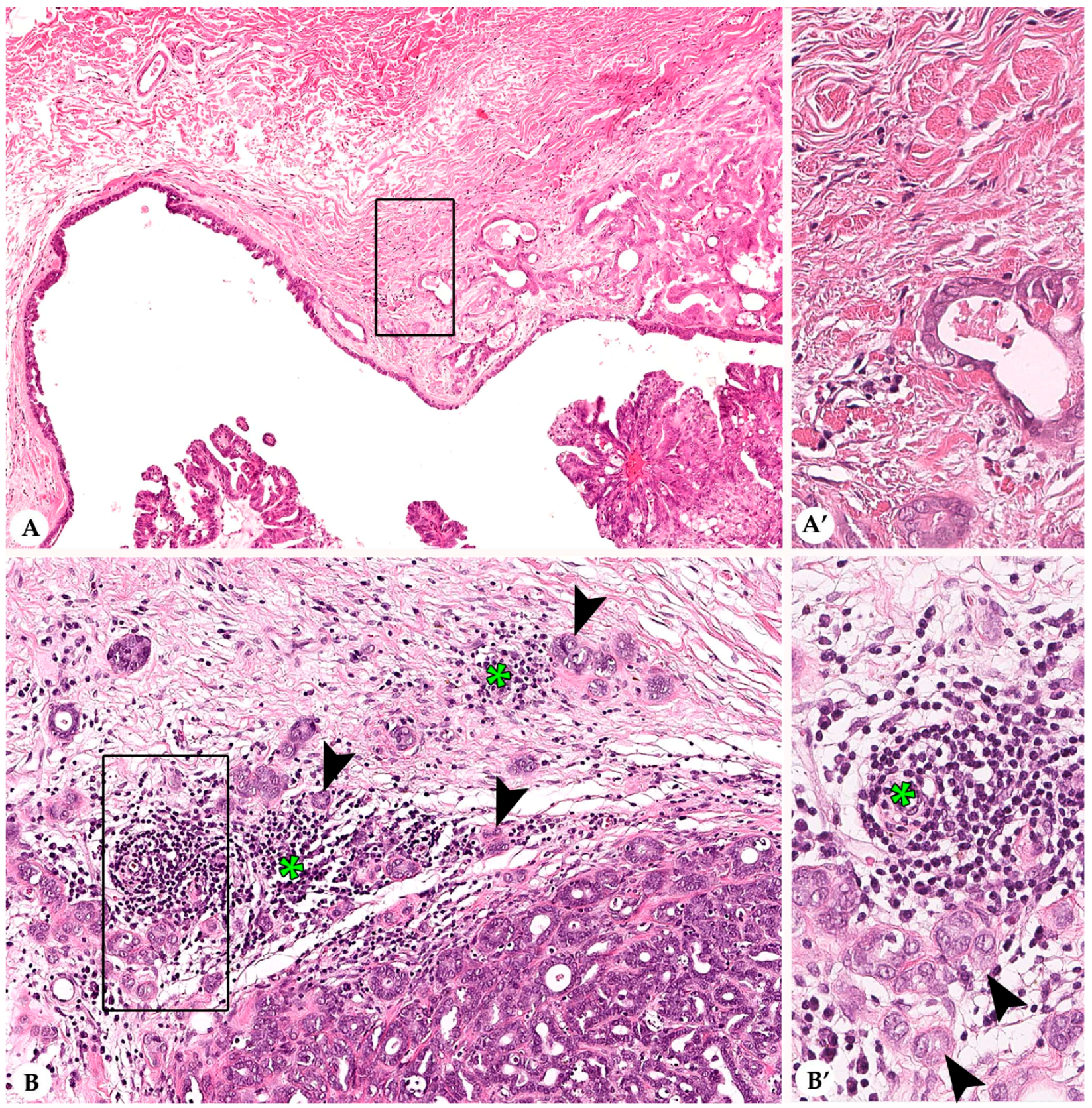
3.1. Stromal Tumor Infiltrating Lymphocytes
3.2. Infiltrating Lymphocytes within Nests of Carcinoma Cells
3.3. Lymphocytes and Plasma Cells in the Adjacent Non-Neoplastic Tissue
3.4. Histological and Immunohistochemical Features
3.5. Statistical Correlations
4. Discussion
4.1. Evaluation of Tumor Infiltrating Lymphocytes in Routinely Fixed, Processed, and Stained Tissue
4.2. Distribution of Stromal and Intra-Tumoral Tumor Infiltrating Lymphocytes
4.3. Tumor Infiltrating Lymphocytes and Histological Features
4.4. Tumor Infiltrating Lymphocytes and Immunohistochemical Features
4.5. Tumor Infiltrating Lymphocytes in Tumors of Domestic Animals
4.6. Tumor Infiltrating Lymphocytes in Human Breast Cancer
4.7. Future Perspectives on the Concept of “One Health, One Medicine”
4.7.1. Standardized Evaluation of Tumor Infiltrating Lymphocytes
4.7.2. Rabbits as Animal Model for Immunoncological Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mage, R.G. Immunology of lagomorphs, Chapter IV. In Handbook of Vertebrate Immunology; Pastoret, P.-P., Griebel, P., Bazin, H., Govaerts, A., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 223–260. [Google Scholar]
- Shiomi, M. Rabbits as model for the study of human diseases, Chapter 7. In Rabbit Biotechnology: Rabbit Genomics, Transgenesis, Cloning and Models; Houdebine, L.-M., Fan, J., Eds.; Springer Science and Business Media, B.V.: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; New York, NY, USA; London, UK, 2009; pp. 49–64. [Google Scholar]
- Peng, X.; Knouse, J.A.; Hernon, K.M. Rabbit models for studying human infectious diseases. Comp. Med. 2015, 65, 499–507. [Google Scholar]
- Esteves, P.J.; Abrantes, J.; Baldauf, H.-M.; BenMohamed, L.; Chen, Y.; Christensen, N.; González-Gallego, J.; Giacani, L.; Hu, J.; Kaplan, G.; et al. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef]
- Tinkey, P.T.; Uthamanthil, R.K.; Weisbroth, S.H. Rabbit neoplasia. In The Laboratory Rabbit, Guinea Pig, Hamster and Other Rodents; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Academic Press: London, UK; San Diego, CA, USA, 2012; pp. 448–503. [Google Scholar]
- Rossmann, A.; Mandic, R.; Heinis, J.; Höffken, H.; Küssner, O.; Kinscherf, R.; Weihe, E.; Bette, M. Intraperitoneal oxidative stress in rabbits with papillomavirus-associated head and neck cancers induces tumoricidal immune response that is adoptively transferable. Clin. Cancer Res. 2014, 20, 4289–4301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chai, W.; Zeng, J.; Chen, J.; Bi, L.; Niu, L. Irreversible electroporation for the treatment of rabbit VX2 breast cancer. Biomed. Microdevices 2017, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.; Neves, F.; Lemos de Matos, A.; Abrantes, J.; van der Loo, W.; Mage, R.; Esteves, P.J. An overview of the lagomorph immune system and its genetic diversity. Immunogenetics 2016, 68, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Nesburn, A.B.; Bettahi, I.; Dasgupta, G.; Chentoufi, A.A.; Zhang, X.; You, S.; Morishige, N.; Wahlert, A.J.; Brown, D.J.; Jester, J.V.; et al. Functional Foxp3+ CD4+ CD25 (Bright+ ) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J. Virol. 2007, 81, 7647–7661. [Google Scholar] [CrossRef] [PubMed]
- Degner, S.; Schoon, H.-A.; Laik-Schandelmaier, C.; Aupperle-Lellbach, H.; Schöniger, S. Estrogen receptor-α and progesterone receptor expression in mammary proliferative lesions of female pet rabbits. Vet. Pathol. 2018, 55, 838–848. [Google Scholar] [CrossRef]
- Degner, S.; Schoon, H.-A.; Degner, S.; Baudis, M.; Schandelmaier, C.; Aupperle-Lellbach, H.; Schöniger, S. Expression of myoepithelial markers in mammary carcinomas of 119 pet rabbits. Animals 2019, 9, 740. [Google Scholar] [CrossRef]
- Schöniger, S.; Degner, S.; Jasani, B.; Schoon, H.-A. A review on mammary tumors in rabbits: Translation of pathology into medical care. Animals 2019, 9, 762. [Google Scholar] [CrossRef]
- Clark, A.; Bird, N.K.; Brock, A. Intraductal delivery to the rabbit mammary gland. J. Vis. Exp. 2017, 121, 55209. [Google Scholar] [CrossRef]
- Müller, K.; Schall, H. Kaninchen. In Krankheiten der Heimtiere, 8th ed.; Fehr, M., Sassenburg, L., Zwart, P., Eds.; Schlütersche Verlag: Hannover, Germany, 2014; pp. 1–56. (In German) [Google Scholar]
- Schöniger, S.; Horn, L.C.; Schoon, H.-A. Tumors and tumor-like lesions in the mammary gland of 24 pet rabbits: A histomorphological and immunohistochemical characterization. Vet. Pathol. 2014, 51, 569–580. [Google Scholar] [CrossRef]
- Baum, B.; Hewicker-Trautwein, H. Classification and epidemiology of mammary tumours in pet rabbits (Oryctolagus Cuniculus). J. Comp. Pathol. 2015, 152, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; Van De Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; Van De Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Hanson, H.L.; Donermeyer, D.L.; Ikeda, H.; White, J.M.; Shankaran, V.; Old, L.J.; Shiku, H.; Schreiber, R.D.; Allen, P.M. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity 2000, 13, 265–276. [Google Scholar] [CrossRef]
- Wouters, M.C.A.; Nelson, B.H. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef]
- Ellyard, J.I.; Simson, L.; Parish, C.R. Th2-mediated anti-tumour immunity: Friend or foe? Tissue Antigens 2007, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.G.; Mihm, M.C., Jr.; Bufalino, R.; Zurrida, S.; Collini, P.; Cascinelli, N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996, 77, 1303–1310. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; DeMaria, S.; et al. Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jonas, S.F.; Bataillon, G.; Criscitiello, C.; Salgado, R.; Loi, S.; Viale, G.; Lee, H.J.; Dieci, M.V.; Kim, S.-B.; et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann. Oncol. 2019, 30, 1941–1949. [Google Scholar] [CrossRef]
- Kurozumi, S.; Matsumoto, H.; Kurosumi, M.; Inoue, K.; Fujii, T.; Horiguchi, J.; Shirabe, K.; Oyama, T.; Kuwano, H. Prognostic significance of tumour-infiltrating lymphocytes for oestrogen receptor-negative breast cancer without lymph node metastasis. Oncol. Lett. 2019, 17, 2647–2656. [Google Scholar] [CrossRef]
- Kurozumi, S.; Inoue, K.; Matsumoto, H.; Fujii, T.; Horiguchi, J.; Oyama, T.; Kurosumi, M.; Shirabe, K. Prognostic utility of tumor-infiltrating lymphocytes in residual tumor after neoadjuvant chemotherapy with trastuzumab for HER2-positive breast cancer. Sci. Rep. 2019, 9, 1583. [Google Scholar] [CrossRef]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the international immuno-oncology biomarker working group on breast cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Peña, L.; De Andrés, P.J.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: Relationship with clinical and histological characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef]
- Zappulli, V.; Pena, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Mammary Tumors. In Surgical Pathology of Tumors of Domestic Animals; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2019; Volume 2, pp. 1–264. [Google Scholar]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. (In German) [Google Scholar]
- Ellis, I.O.; Lee, A.H.E.; Pinder, S.E.; Rakha, E.A. Tumors of the breast. In Diagnostic Histopathology of Tumors, 4th ed.; Fletcher, C.D.M., Ed.; Elsevier, Saunders: Philadelphia, PA, USA, 2013; Volume 2, pp. 1057–1145. [Google Scholar]
- Saltz, J.; Gupta, R.; Hou, L.; Kurc, T.; Singh, P.; Nguyen, V.; Samaras, D.; Shroyer, K.R.; Zhao, T.; Batiste, R.; et al. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell. Rep. 2018, 23, 181–193.e7. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Heindl, A.; Koelble, K.; Yuan, Y. Beyond immune density: Critical role of spatial heterogeneity in estrogen receptor-negative breast cancer. Mod. Pathol. 2015, 28, 766–777. [Google Scholar] [CrossRef] [PubMed]
- French, J.J.; Cresswell, J.; Wong, W.K.; Seymour, K.; Charnley, R.M.; Kirby, J.A. T cell adhesion and cytolysis of pancreatic cancer cells: A role for E-cadherin in immunotherapy? British J. Cancer 2002, 87, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S. Suppression of cancer phenotypes through a multifunctional actin-binding protein, calponin, that attacks cancer cells and simultaneously protects the host from invasion. Cancer Sci. 2005, 96, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jin, J.P. Calponin isoforms CNN1, CNN2 and CNN3: Regulators for actin cytoskeleton functions in smooth muscle and non-muscle cells. Gene 2016, 585, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.K.; Moore, P.F.; Anderson, D.M.; Moore, R.A.; Parry, N.R.; Gough, G.W.; Stanley, M.A. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+ lymphocytes. Virology 2001, 283, 31–39. [Google Scholar] [CrossRef]
- Paździor-Czapula, K.; Rotkiewicz, T.; Otrocka-Domagała, I.; Gesek, M.; Śmiech, A. Morphology and immunophenotype of canine cutaneous histiocytic tumours with particular emphasis on diagnostic application. Vet. Res. Commun. 2015, 39, 7–17. [Google Scholar] [CrossRef]
- Pérez, J.; Day, M.J.; Mozos, E. Immunohistochemical study of the local inflammatory infiltrate in spontaneous canine transmissible venereal tumour at different stages of growth. Vet. Immunol. Immunopathol. 1998, 64, 133–147. [Google Scholar] [CrossRef]
- Estrela-Lima, A.; Araújo, M.S.S.; Costa-Neto, J.M.; Teixeira-Carvalho, A.; Barrouin-Melo, S.M.; Cardoso, S.V.; Martins-Filho, O.A.; Serakides, R.; Cassali, G.D. Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates. BMC Cancer 2010, 10, 256. [Google Scholar] [CrossRef]
- Saeki, K.; Endo, Y.; Uchida, K.; Nishimura, R.; Sasaki, N.; Nakagawa, T. Significance of tumor-infiltrating immune cells in spontaneous canine mammary gland tumor: 140 cases. J. Vet. Med. Sci. 2012, 74, 227–230. [Google Scholar] [CrossRef]
- Kim, J.-H.; Chon, S.-K.; Im, K.-S.; Kim, N.-H.; Sur, J.-H. Correlation of tumor-infiltrating lymphocytes to histopathological features and molecular phenotypes in canine mammary carcinoma: A morphologic and immunohistochemical morphometric study. Can. J. Vet. Res. 2013, 77, 142–149. [Google Scholar] [PubMed]
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. A role for T-lymphocytes in human breast cancer and in canine mammary tumors. Biomed. Res. Int. 2014, 2014, 130894. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, M.S.; Brandi, A.; de Oliveira Matos Prado, J.K.; Elias, F.; Dalmolian, F.; de Faria Lainetti, P.; Prado, M.C.M.; Leis-Filho, A.F.; Fonseca-Alves, C.E. Tumor-infiltrating CD4+ and CD8+ lymphocytes and macrophages are associated with prognostic factors in triple-negative canine mammary complex type carcinoma. Res. Vet Sci. 2019, 126, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Z.; Qu, X.; Zhang, Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 179. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Simeone, I.; Hendrickx, W.; Wang, E.; Marincola, F.M.; Viens, P.; Mamessier, E.; Ceccarelli, M.; Birnbaum, D.; et al. The immunologic constant of rejection classification refines the prognostic value of conventional prognostic signatures in breast cancer. Br. J. Cancer 2018, 119, 1383–1391. [Google Scholar] [CrossRef]
- Criscitiello, C.; Bagnardi, V.; Pruneri, G.; Vingiani, A.; Esposito, A.; Rotmensz, N.; Curigliano, G. Prognostic value of tumour-infiltrating lymphocytes in small HER2-positive breast cancer. Eur. J. Cancer 2017, 87, 164–171. [Google Scholar] [CrossRef]
- Graur, D.; Duret, L.; Gouy, M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature 1996, 379, 333–335. [Google Scholar] [CrossRef]
- DeMello, M. Rabbits multiplying like rabbits. The rise in the worldwide popularity of rabbits as pets. In Companion Animals in Everyday Life: Situating Animal-Human Engagement within Cultures; Pregowski, P.M., Ed.; Palgrave MacMillan: London, UK; Springer: New York, NY, USA, 2016; pp. 91–108. [Google Scholar]
- Dow, S. A role for dogs in advancing cancer immunotherapy research. Front. Immunol. 2019, 10, 2935. [Google Scholar] [CrossRef]



| TIL Numbers | Stromal TILs in CT (%) | Stromal TILs at IM (%) | IT TILs in CT (%) | IT TILs at IM (%) |
|---|---|---|---|---|
| Average | ||||
| Range | 0.00–70.00 | 0.00–30.00 | 0.00–5.00 | 0.00–10.00 |
| Mean ± SD | 6.21 ± 11.61 | 3.34 ± 5.23 | 0.24 ± 0.67 | 0.24 ± 1.07 |
| Max. per 20× | ||||
| Range | 1.00–90.00 | 0.00–70.00 | 0.00–25.00 | 0.00–40.00 |
| Mean ± SD | 19.20 ± 23.29 | 10.28 ± 13.32 | 0.98 ± 3.00 | 1.00 ± 4.00 |
| Min. per 20× | ||||
| Range | 0.00–60.00 | 0.00–10.00 | 0.00–1.00 | 0.00–2.00 |
| Mean ± SD | 2.09 ± 6.97 | 1.11 ± 1.65 | 0.06 ± 0.23 | 0.07 ± 0.32 |
| Analyzed Parameters | Range | Mean ± SD |
|---|---|---|
| Tubular growth | 5–90% | 56% ± 25% |
| Mitoses per ten 40× HPFs | 0–32 | 6 ± 6 |
| IRS estrogen receptor | 0–2.28 | 0.16 ± 0.42 |
| IRS progesterone receptor | 0–3.66 | 0.56 ± 1.06 |
| H-score estrogen receptor | 0–101.35 | 11.29 ± 26.86 |
| H-score progesterone receptor | 0–149.07 | 25.44 ± 45.19 |
| Calponin positive tumor cells | 0–22% | 8% ± 5% |
| Parameter 1 | Parameter 2 | p-Value, ρ-Value | Cases |
|---|---|---|---|
| Tumor Infiltrating Lymphoc Ytes | |||
| Average stromal TILs in CT | Max. stromal TILs in CT | p = 0.000; ρ = 0.731 | n = 107 |
| Average stromal TILs at IM | p = 0.000; ρ = 0.563 | n = 102 | |
| Max. stromal TILs at IM | p = 0.000; ρ = 0.420 | n = 102 | |
| Max. stromal TILs in CT | Max. stromal TILs at IM | p = 0.000; ρ = 0.546 | n = 102 |
| Average stromal TILs at IM | Max. stromal TILs at IM | p = 0.000; ρ = 0.698 | n = 102 |
| Max. stromal TILs in CT | p = 0.000; ρ = 0.436 | n = 102 | |
| Average IT TILs at IM | p = 0.038; ρ=0.206 | n = 102 | |
| Average IT TILs in CT | Max. IT TILs in CT | p = 0.000; ρ = 0.831 | n = 107 |
| Max. IT TILs at IM | p = 0.000; ρ = 0.734 | n = 102 | |
| Average IT TILs at IM | p = 0.000; ρ = 0.739 | n = 102 | |
| Max. IT TILs in CT | Max. IT TILs at IM | p = 0.000; ρ = 0.936 | n = 102 |
| Average IT TILs at IM | p = 0.000; ρ = 0.899 | n = 102 | |
| Histological Features | |||
| Degree of invasion | Degree of necrosis | p = 0.000; ρ = 0.352 | n = 103 |
| Grading score | Degree of invasion | p = 0.000; ρ = 0.364 | n = 103 |
| Degree of necrosis | p = 0.003; ρ = 0.284 | n = 107 | |
| Tumor grade | Degree of invasion | p = 0.002; ρ = 0.299 | n = 103 |
| Degree of necrosis | p = 0.029; ρ = 0.211 | n = 107 | |
| Tumor Infiltrating Lymphocytes and Histological Features | |||
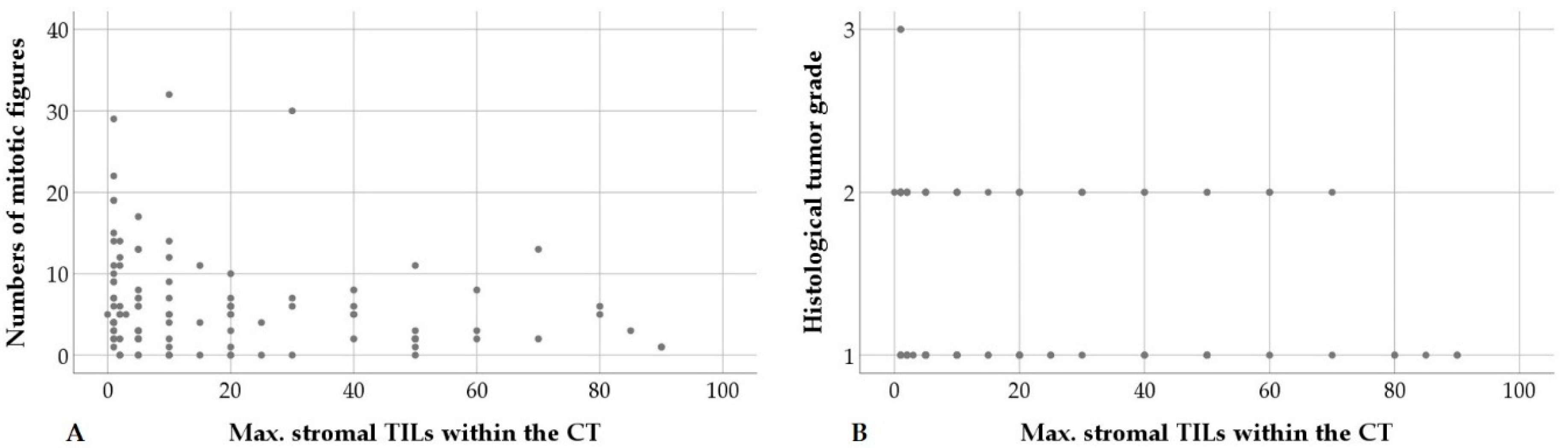
| Max. stromal TILs in CT | Mitotic count | p = 0.042; ρ = −0.197 | n = 107 |
| Grading score | p = 0.035; ρ = −0.204 | n = 107 | |
| Tumor grade | p = 0.027; ρ = −0.213 | n = 107 | |
| Immunohistochemical and Histological Features | |||
| IRS estrogen receptor | IRS progesterone receptor | p = 0.000; ρ = 0.606 | n = 107 |
| Mitotic count | p = 0.012; ρ = −0.243 | n = 107 | |
| IRS progesterone receptor | Mitotic count | p = 0.008; ρ = −0.255 | n = 107 |
| Degree of necrosis | p = 0.009; ρ = −0.250 | n = 107 | |
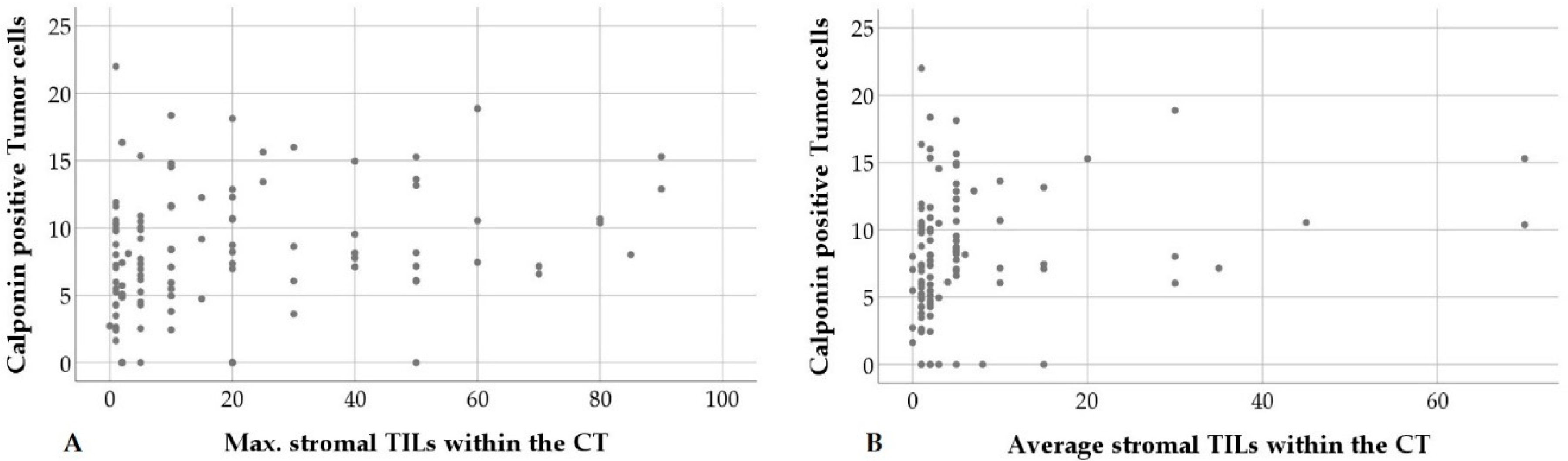
| Calponin pos. tumor cells | Mitotic count | p = 0.035; ρ = −0.204 | n = 107 |
| Calponin Positive Tumor Cells and Tumor Infiltrating lymphocytes | |||
| Calponin positive tumor cells | Max. stromal TILs in CT | p = 0.012; ρ = 0.241 | n = 107 |
| Average stromal TILs in CT | p = 0.026; ρ = 0.215 | n = 107 | |
| Average stromal TILs at IM | p = 0.026; ρ = 0.220 | n = 107 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöniger, S.; Degner, S.; Zhang, Q.; Schandelmaier, C.; Aupperle-Lellbach, H.; Jasani, B.; Schoon, H.-A. Tumor Infiltrating Lymphocytes in Pet Rabbit Mammary Carcinomas: A Study with Relevance to Comparative Pathology. Animals 2020, 10, 1437. https://doi.org/10.3390/ani10081437
Schöniger S, Degner S, Zhang Q, Schandelmaier C, Aupperle-Lellbach H, Jasani B, Schoon H-A. Tumor Infiltrating Lymphocytes in Pet Rabbit Mammary Carcinomas: A Study with Relevance to Comparative Pathology. Animals. 2020; 10(8):1437. https://doi.org/10.3390/ani10081437
Chicago/Turabian StyleSchöniger, Sandra, Sophie Degner, Qian Zhang, Claudia Schandelmaier, Heike Aupperle-Lellbach, Bharat Jasani, and Heinz-Adolf Schoon. 2020. "Tumor Infiltrating Lymphocytes in Pet Rabbit Mammary Carcinomas: A Study with Relevance to Comparative Pathology" Animals 10, no. 8: 1437. https://doi.org/10.3390/ani10081437
APA StyleSchöniger, S., Degner, S., Zhang, Q., Schandelmaier, C., Aupperle-Lellbach, H., Jasani, B., & Schoon, H.-A. (2020). Tumor Infiltrating Lymphocytes in Pet Rabbit Mammary Carcinomas: A Study with Relevance to Comparative Pathology. Animals, 10(8), 1437. https://doi.org/10.3390/ani10081437





