Evaluation of the Impact of Behavioral Opportunities on Four Zoo-Housed Aardvarks (Orycteropus afer)
Simple Summary
Abstract
1. Introduction
- (1)
- How did the amount and rate of use vary depending on the stated enrichment goal?
- (2)
- Which enrichment items better achieved their stated goals based on use throughout the night? What about throughout the study (i.e., was habituation observed)?
- (3)
- Following this logic, we developed the following question post hoc. Which enrichment items better achieved their stated goal based on promoting overall rates of goal behaviors, including behaviors directed towards non-enrichment as well as enrichment items?
- (4)
- Did certain enrichment types or increased enrichment use correspond to positive changes in specific welfare indicators, including FGM concentrations, behavioral diversity, pacing, or social behaviors?
2. Materials and Methods
2.1. Subjects and Housing
2.2. Enrichment Events
2.3. Behavioral Data Collection
2.4. Hormone Sampling
2.5. Statistical Analyses
3. Results
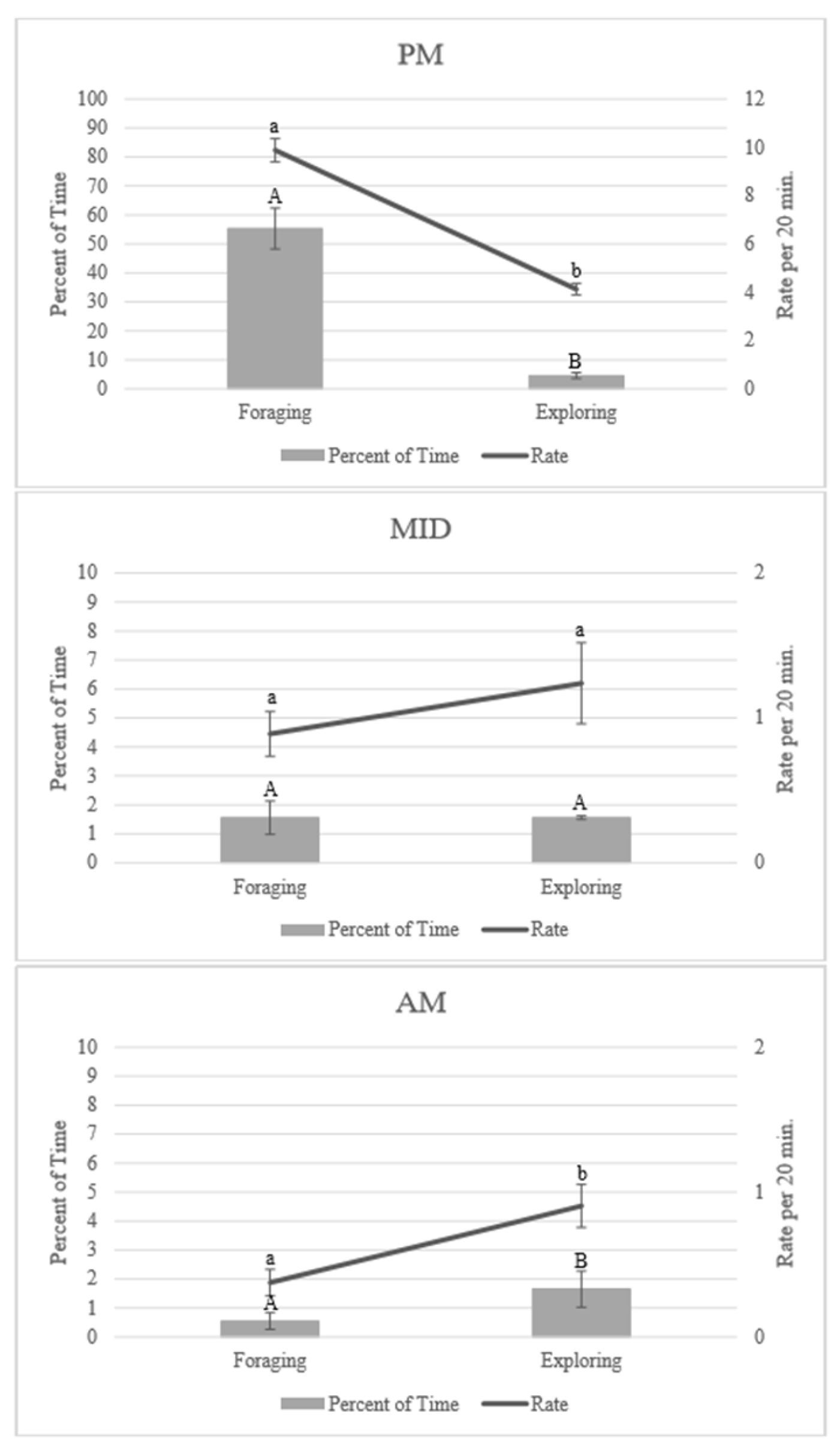
3.1. Enrichment Use: Time of Night
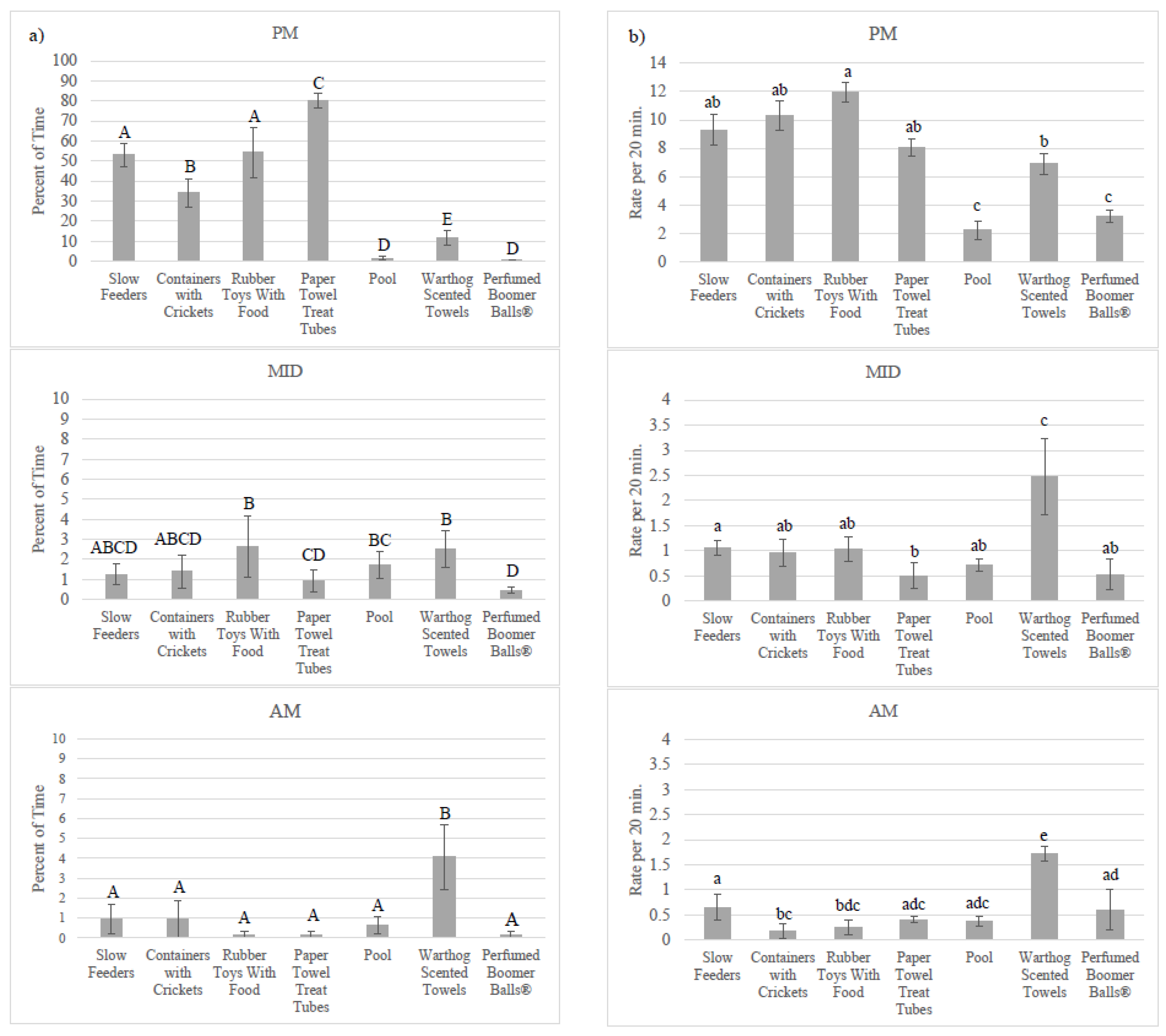
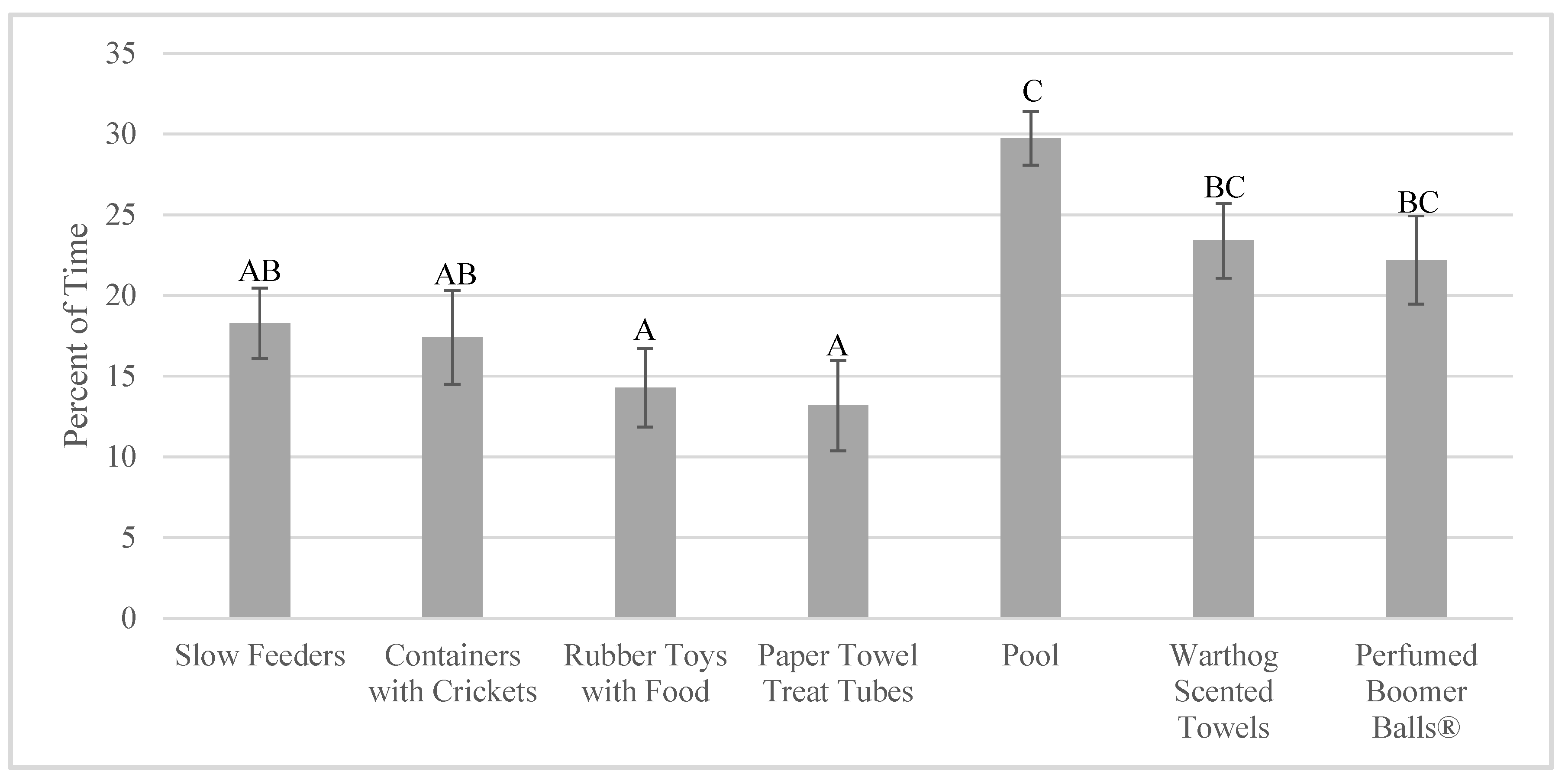
3.2. Enrichment Use: Goal Achievement
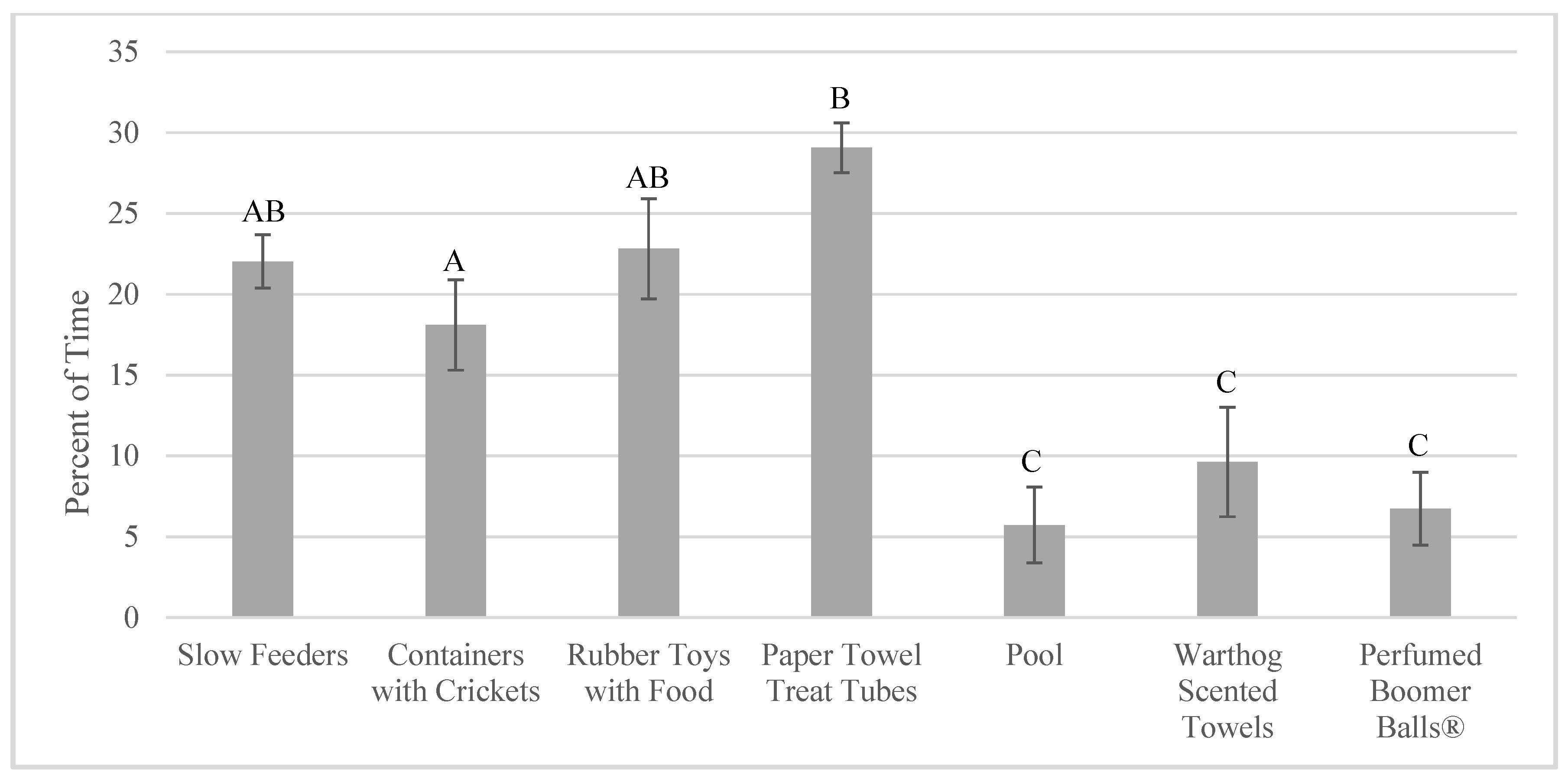
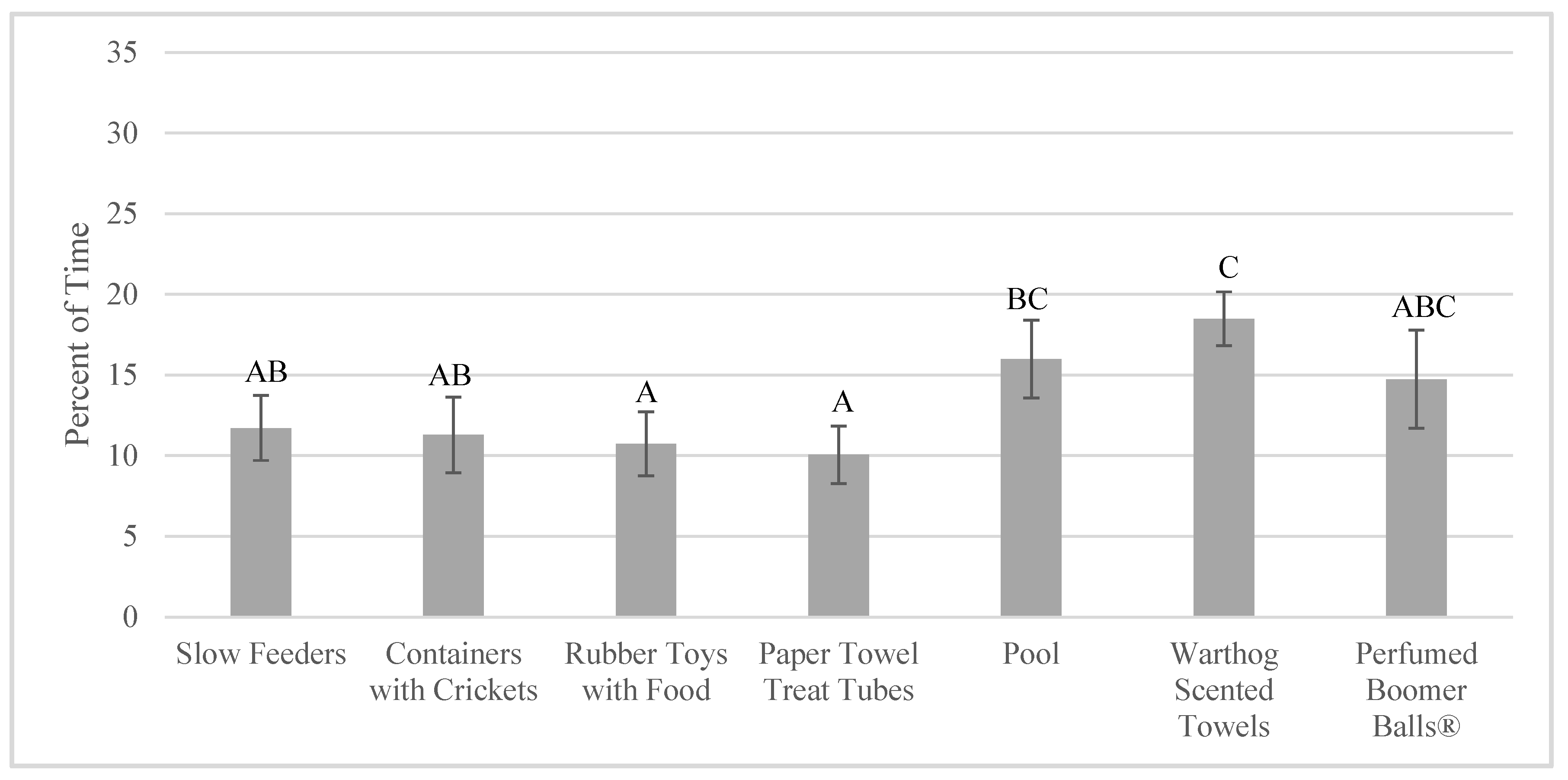
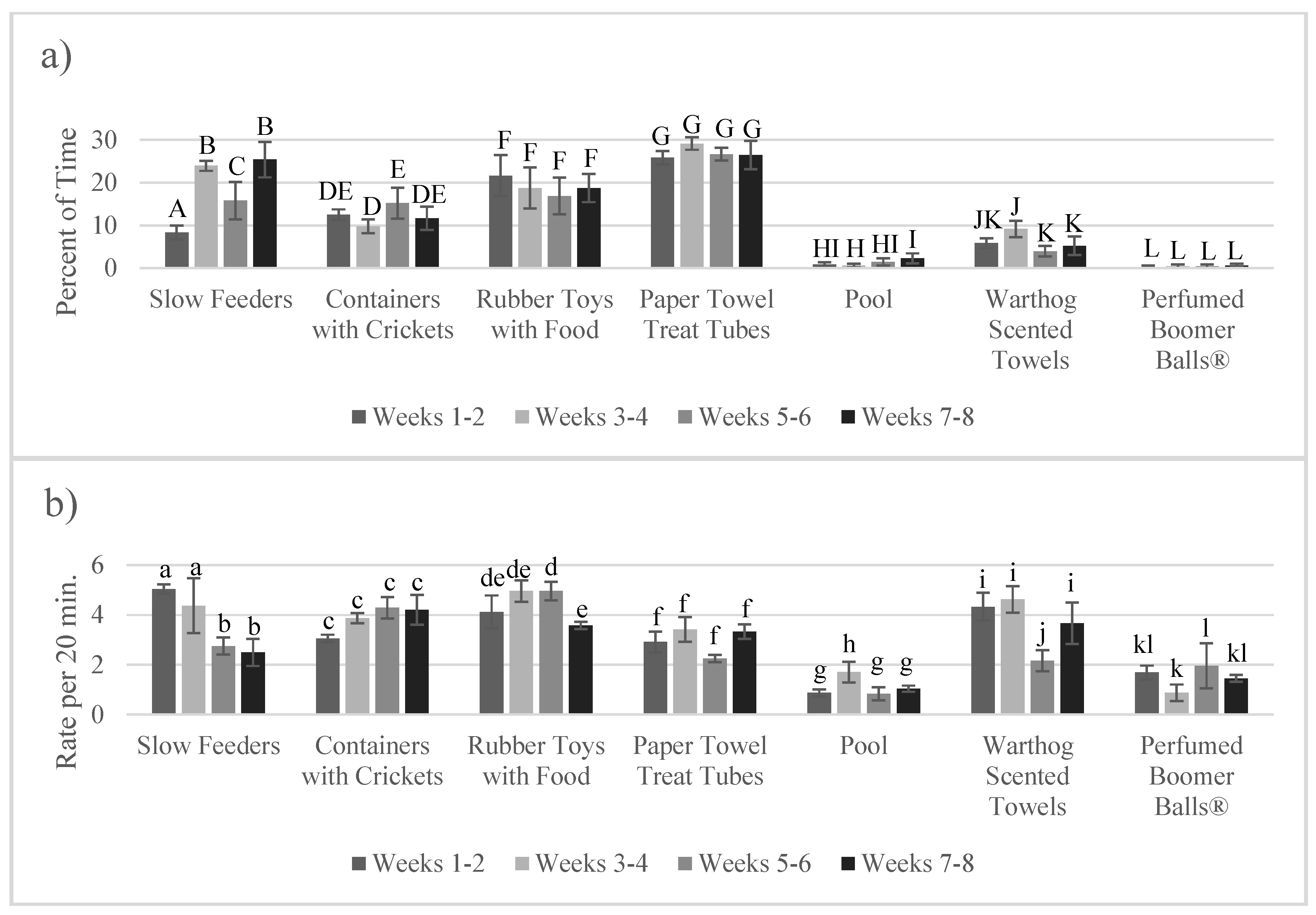
3.3. Enrichment Use and Goal Achievement: Change over Time (Habituation)
3.4. Effects on Indicators of Welfare
4. Discussion
4.1. Enrichment Use throughout the Night
4.2. Achieving the Goal
4.3. Lack of Habituation to Enrichment
4.4. Impact of Enrichment Use on Welfare Indicators
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AZA. Animal Welfare Committee’s Definition of Animal Welfare. Available online: https://www.aza.org/animal_welfare_committee (accessed on 15 July 2020).
- Whitham, J.C.; Wielebnowski, N. New directions for zoo animal welfare science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- Miller, L.J.; Pisacane, C.B.; Vicino, G.A. Relationship between behavioural diversity and faecal glucocorticoid metabolites: A case study with cheetahs (Acinonyx jubatus). Anim. Welf. 2016, 25, 325–329. [Google Scholar] [CrossRef]
- Hodges, K.; Brown, J.; Heistermann, M. Endocrine monitoring of reproduction and stress. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management, 1st ed.; Kleiman, D.G., Thompson, K.V., Kirk Baer, C., Eds.; The University of Chicago Press: Chicago, IL, USA, 2010; pp. 447–468. [Google Scholar]
- Blanchard, R.J.; McKittrick, C.R.; Blanchard, D.C. Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiol. Behav. 2001, 73, 261–271. [Google Scholar] [CrossRef]
- Shepherdson, D.; Lewis, K.D.; Carlstead, K.; Bauman, J.; Perrin, N. Individual and environmental factors associated with stereotypic behavior and fecal glucocorticoid metabolite levels in zoo housed polar bears. Appl. Anim. Behav. Sci. 2013, 147, 268–277. [Google Scholar] [CrossRef]
- Association of Zoos and Aquariums Behavior Scientific Advisory Group. Definition of Environmental Enrichment. In Proceedings of the Association of Zoos & Aquariums Workshop at Disney’s Animal Kingdom, Lake Buena Vista, FL, USA, April 1999. [Google Scholar]
- Bloomsmith, M.A.; Brent, L.Y.; Schapiro, S.J. Guidelines for developing and managing an environmental enrichment program for nonhuman primates. Lab. Anim. Sci. 1991, 41, 372–377. [Google Scholar] [PubMed]
- Hoy, J.M.; Murray, P.J.; Tribe, A. Thirty years later: Enrichment practices for captive mammals. Zoo Biol. 2010, 29, 303–316. [Google Scholar] [CrossRef]
- Bacon, H. Behaviour-based husbandry–A holistic approach to the management of abnormal repetitive behavior. Animals 2018, 8, 103. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Mellen, J.; MacPhee, M.S. Philosophy of environmental enrichment: Past, present, and future. Zoo Biol. 2001, 20, 211–226. [Google Scholar] [CrossRef]
- Alligood, C.; Leighty, K. Putting the “E” in SPIDER: Evolving trends in the evaluation of environmental enrichment efficacy in zoological settings. Anim. Behav. Cognit. 2015, 2, 200–217. [Google Scholar] [CrossRef]
- Tarou, L.R.; Bashaw, M.J. Maximizing the effectiveness of environmental enrichment: Suggestions from the experimental analysis of behavior. Appl. Anim. Behav. Sci. 2007, 102, 189–204. [Google Scholar] [CrossRef]
- Blount, J.D.; Taylor, N.J. The relative effectiveness of manipulable feeders and olfactory enrichment for kinkajous. Int. Zoo Yearb. 2000, 37, 381–394. [Google Scholar] [CrossRef]
- Clark, F.E.; Melfi, V.A. Environmental enrichment for a mixed-species nocturnal mammal exhibit. Zoo Biol. 2011, 29, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Waran, N.K.; Young, R.J. Environmental enrichment for Australian mammals. Anim. Welf. 1998, 7, 415–425. [Google Scholar]
- Fuller, G.; Kuhar, C.W.; Dennis, P.M.; Lukas, K.E. A survey of husbandry practices for lorisid primates in North American zoos and related facilities. Zoo Biol. 2013, 32, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Van Aarde, R.J.; Willis, C.K.; Skinner, J.D.; Haupt, M.A. Range utilization by the aardvark, Orycteropus afer (Pallas, 1766) in the Karoo, South Africa. J. Arid Environ. 1992, 22, 387–394. [Google Scholar] [CrossRef]
- Shoshani, J.; Goldman, C.A.; Thewissen, J.G.M. Orycteropus afer. Mamm. Species 1988. [Google Scholar] [CrossRef]
- Taylor, W.A.; Lindsey, P.A.; Skinner, J.D. The feeding ecology of the aardvark (Orycteropus afer). J. Arid Environ. 2002, 50, 135–152. [Google Scholar] [CrossRef]
- Allard, S.M.; Fuller, G.A.; Hamilton, J.L. The roles of time and space in zoo animal welfare. In Animal Welfare: Assessment, Challenges and Improvement Strategies; Weaver, J., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 1–54. [Google Scholar]
- Knobbe, A.; Lawless, A. Population Analysis & Breeding and Transfer Plan: Aardvark (Orycteropus afer) AZA Species Survival Plan® Red Program; AZA: Silver Spring, MD, USA, 2019. [Google Scholar]
- White, A.M.; Cameron, E.Z. Communal nesting is unrelated to burrow availability in the common warthog. Anim. Behav. 2009, 77, 87–94. [Google Scholar] [CrossRef]
- Fuller, G.; Margulis, S.W.; Santymire, R. The effectiveness of indigestible markers for identifying individual animal feces and their prevalence of use in North American zoos. Zoo Biol. 2011, 30, 379–398. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Kuczaj, S.; Lacinak, T.; Fad, O.; Trone, M.; Solangi, M.; Ramos, J. Keeping environmental enrichment enriching. Int. J. Comp. Psychol. 2002, 15, 127–137. [Google Scholar]
- Vick, S.J.; Anderson, J.R.; Young, R. Maracas for Macaca? Evaluation of three potential enrichment objects in two species of zoo-housed macaques. Zoo Biol. 2000, 19, 181–191. [Google Scholar] [CrossRef]
- Wells, D.L.; Egli, J.M. The influence of olfactory enrichment on the behavior of captive black-footed cats, Felis nigripes. Appl. Anim. Behav. Sci. 2004, 85, 107–119. [Google Scholar] [CrossRef]
- Clark, F.; King, A.J. A critical review of zoo-based olfactory enrichment. In Chemical Signals in Vertebrates 11; Hurst, J.L., Beynon, R.J., Roberts, S.C., Wyatt, T.D., Eds.; Springer: New York, NY, USA, 2008; pp. 391–398. [Google Scholar]
- Anderson, C.; Arun, A.S.; Jensen, P. Habituation to environmental enrichment in captive sloth bears—Effect on stereotypies. Zoo Biol. 2010, 29, 705–714. [Google Scholar] [CrossRef]
- Novak, M.A.; Hamel, A.F.; Kelly, B.J.; Dettmer, A.M.; Meyer, J.S. Stress, the HPA axis, and nonhuman primate well-being: A review. Appl. Anim. Behav. Sci. 2013, 143, 135–149. [Google Scholar] [CrossRef]
- Cronin, K.A.; Ross, S.R. Technical contribution: A cautionary note on the use of behavioural diversity (H-Index) in animal welfare science. Anim. Welf. 2019, 28, 157–164. [Google Scholar] [CrossRef]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- Franks, B. What do animals want? Anim. Welf. 2019, 28, 1–10. [Google Scholar] [CrossRef]
- Richter, S.H.; Hintze, S. From the individual to the population-and back again? Emphasising the role of the individual in animal welfare science. Appl. Anim. Behav. Sci. 2019, 212, 1–8. [Google Scholar] [CrossRef]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 approach to promoting optimal welfare for captive wild animals. Behav. Process. 2018, 156, 83–95. [Google Scholar] [CrossRef] [PubMed]
| Indoor Habitat | Features |
|---|---|
| Upper Habitat | Cement habitat with two wooden nest boxes, one plastic culvert, heavy rubber mats, and a digging area with wood shavings Approximate area: 65 m2 |
| Lower Habitat | Half-cement and half-dirt habitat with two plastic culverts, two plastic dens, and heavy rubber mats Approximate area: 45 m2 |
| Day of the Week | Enrichment Item | Goal of Item |
|---|---|---|
| Sunday | Paper towel treat tubes (including avocado, melon, Mazuri® insectivore diet) | Foraging |
| Monday | Slow feeders with food (including avocado, melon, Mazuri® insectivore diet) | Foraging |
| Tuesday | Pool with water | Exploring |
| Wednesday | Containers with crickets | Foraging |
| Thursday | Warthog-scented towels | Exploring |
| Friday | Rubber toys with food (including avocado, melon, Mazuri® insectivore diet) | Foraging |
| Saturday | Perfumed Boomer balls® | Exploring |
| Behavior | Description |
|---|---|
| Affiliative Interaction * | Focal is engaged in any positive social interaction with another individual such as calm contact, sniffing, or social play. |
| Agonistic Interaction * | Focal is engaged in any negative social interaction with another individual such as displacing, being displaced, biting, hitting with front limbs or torso, being withdrawn from, or withdrawing from an attempt at an affiliative interaction. |
| Investigate Enrichment ▲ | Focal is exploring an enrichment item through consuming, touching, smelling, or visual inspection. Focal can be stationary or in motion. |
| Investigate Object | Focal is exploring a non-food, non-enrichment object or airspace in the habitat through touching, smelling, or visual inspection. Focal can be stationary or in motion. |
| Pace ▲ | Focal is in motion but traveling the same route repeatedly. Must have completed the circuit three times before being considered pacing. |
| Dig | Focal is using anterior or posterior limbs to remove the substrate from a specific area. |
| Drink | Focal is ingesting water. |
| Eat | Focal is consuming, touching, smelling, or visually inspecting food items that are not enrichment items. |
| Groom | Focal’s anterior/posterior limbs are in contact with its own body. |
| Locomote | Focal is in motion, traveling from one point of the habitat to another, and the head is up off the ground. |
| Maintenance | Focal is engaged in voiding behaviors. |
| Stationary–Lay | Focal is inactive with the torso against the ground, but the head is up. Focal may be oriented towards an object/event or just scanning the environment. |
| Stationary–Stand/Sit | Focal is inactive with the torso off the ground. This behavior includes when a focal is in a seated position. Focal may be oriented towards an object/event or just scanning the environment. |
| Social Rest | Focal and another aardvark are within one body length of each other, and both are in a state of inactivity with their torsos against the ground and little to no bodily movement. Head is lowered, and eyes may be closed. |
| Rest | Focal is in a state of inactivity with the torso against the ground; there will be little to no bodily movement. Head is lowered, and eyes may be closed. No other aardvark is resting within one body length of the focal. |
| Other | Focal is engaged in any behavior not defined elsewhere in the ethogram. |
| Not Visible | Focal or his/her behavior is out of sight. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamilton, J.; Fuller, G.; Allard, S. Evaluation of the Impact of Behavioral Opportunities on Four Zoo-Housed Aardvarks (Orycteropus afer). Animals 2020, 10, 1433. https://doi.org/10.3390/ani10081433
Hamilton J, Fuller G, Allard S. Evaluation of the Impact of Behavioral Opportunities on Four Zoo-Housed Aardvarks (Orycteropus afer). Animals. 2020; 10(8):1433. https://doi.org/10.3390/ani10081433
Chicago/Turabian StyleHamilton, Jennifer, Grace Fuller, and Stephanie Allard. 2020. "Evaluation of the Impact of Behavioral Opportunities on Four Zoo-Housed Aardvarks (Orycteropus afer)" Animals 10, no. 8: 1433. https://doi.org/10.3390/ani10081433
APA StyleHamilton, J., Fuller, G., & Allard, S. (2020). Evaluation of the Impact of Behavioral Opportunities on Four Zoo-Housed Aardvarks (Orycteropus afer). Animals, 10(8), 1433. https://doi.org/10.3390/ani10081433




