Investigation of the Prevalence, Virulence Genes, and Antibiogram of Motile Aeromonads Isolated from Nile Tilapia Fish Farms in Egypt and Assessment of their Water Quality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Area and Data Collection
2.3. Sampling
2.4. Assessment of Water Quality Parameters
2.5. Bacteriological Examination
2.6. Molecular Identification of the Recovered Aeromonas Isolates
2.7. Screening of Antimicrobial Susceptibility of Aeromonas and MAR Index
2.8. Histopathological Examination of Naturally Diseased Nile Tilapia
| Genes | Primers Sequence ((5′-3′) | PCR Conditions | Product Sizes/(Bp) | Reference |
|---|---|---|---|---|
| Gyrase B (gyrB) | TCCGGCGGTCTGCACGGCGT TTGTCCGGGTTGTACTCGTC | • Initial denaturation 94 °C/5 min. • 30 cycles of denaturation at 94 °C/30 s • Annealing at 59 °C/30 s • Extension at 72 °C/1 min | 1100 | [20] |
| Aerolysin (aer) | AACCGAACTCTCCAT CGCCTTGTCCTTGTA | • Initial denaturation step at 94 °C/5 min • 30 cycles with denaturation at 94 °C/30 s • Annealing at 54 °C/30 s • Extension at 72 °C/1 min | 301 | [21] |
| Elastase (ahp) | ACACGGTCAAGGAGATCAAC CGCTGGTGTTGGCCAGCAGG | • Initial denaturation: 94 °C/4 min • 35 cycles of denaturation at 94 °C for 30 s • Annealing at 55.5 °C for 30 s • Extension step at 72 °C for 30 s | 540 | [27] |
| Hemolysin (hyl) | CACAGCCAATATGTCGGTGAAG GTCACCTTCTCGCTCAGGC | • Same amplification conditions for elastase gene except for annealing at 60.6 °C for 30 s | 326 | [22] |
| Lipase (lip) | ATCTTCTCCGACTGGTTCGG CCGTGCCAGGACTGGGTCTT | • Same amplification conditions for elastase gene except for annealing at 58.2 °C for 30 s | 383–389 | [27] |
2.9. Statistical Analysis
3. Results
3.1. Characteristics of Fish Farms
3.2. Water Quality Parameters
3.3. Fish Examination
3.4. Bacteriological and Phenotypic Characterization
3.5. Total and Monthly Prevalence of Aeromonas
3.6. Molecular Identification of Aeromonads
3.7. Antibiotic Resistance Patterns of Motile Aeromonads
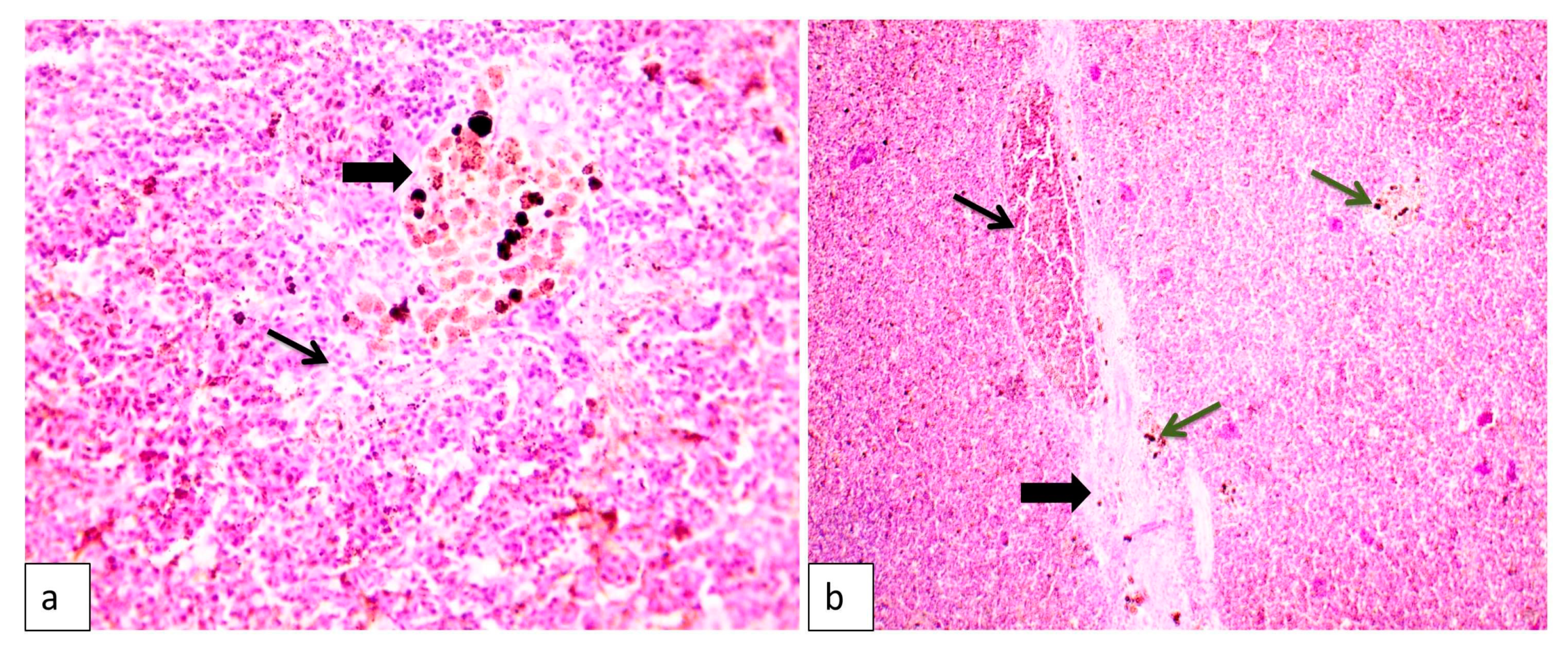
3.8. Tissue Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean. Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Wally, A. The State and Development of Aquaculture in Egypt. Global Agricultural Information Network. USDA Foreign Agriculture Service, 2016. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=The%20State%20and%20Development%20of%20Aquaculture%20in%20Egypt%20_Cairo_Egypt_11-6-2016.pdf (accessed on 19 June 2020).
- Feidi, I. Will the new large-scale aquaculture projects make Egypt self sufficient in fish supplies? Mediterranean Fish. Aquac. Res. 2018, 1, 31–41. [Google Scholar]
- GAFRD. General authority for fish resources development. In Fish Statistics Year Book; Ministry of Agriculture and Land Reclamation: Cairo, Egypt, 2014. [Google Scholar]
- Naziri, D. Financial Services for Sme (Small and Medium-Scale Enterprise) Aquaculture Producers; Egypt Case Study. Draft—Confidential, 25 January 2011, German Agency for Technical Cooperation (GTZ); Natural Resources Institute: Kent, UK, 2011. [Google Scholar]
- Figueras, M.J.; Beaz-Hidalgo, R. Aeromonas infections in humans. In Aeromonas; Caister Academic Press: Norfolk, UK, 2015; pp. 65–108. [Google Scholar]
- Oliveira, S.T.; Gouveia, J.J.; Da Costa, M.M. Molecular characterization of virulence factors in Aeromonas hydrophila obtained from fish. Pesqui. Vet. Bras. 2012, 32, 701–706. [Google Scholar] [CrossRef]
- Beaz-Hidalgo, R.; Figueras, M.J. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. J. Fish. Dis. 2013, 36, 371–388. [Google Scholar] [CrossRef]
- Jutfelt, F.; Sundh, H.; Glette, J.; Mellander, L.; Björnsson, B.T.; Sundell, K. The involvement of Aeromonas salmonicida virulence factors in bacterial translocation across the rainbow trout, Oncorhynchus mykiss (Walbaum), intestine. J. Fish. Dis. 2008, 31, 141–151. [Google Scholar] [CrossRef]
- Abu-Elala, N.; Abdelsalam, M.; Marouf, S.; Setta, A. Comparative analysis of virulence genes, antibiotic resistance and gyrb-based phylogeny of motile aeromonas species isolates from Nile Tilapia and domestic fowl. Lett. Appl. Microbiol. 2015, 5, 429–436. [Google Scholar] [CrossRef]
- Yogananth, N.; Bhakyaraj, R.; Chanthuru, A.; Anbalagan, T.; Nila, K.M. Detection of virulence gene in aeromonas hydrophila isolated from fish samples using pcr technique. Glob. J. Biotech. Biochem. 2009, 4, 51–53. [Google Scholar]
- Hossain, S.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Dahanayake, P.S.; Heo, G.J. Distribution of antimicrobial resistance genes and class 1 integron gene cassette arrays in motile Aeromonas spp. isolated from goldfish (Carassius auratus). Microb. Drug Resist. 2018, 8, 1217–1225. [Google Scholar] [CrossRef]
- Dobiasova, H.; Kutilova, I.; Piačková, V.; Veselý, T.; Cizek, A.; Dolejska, M. Ornamental fish as a source of plasmid-mediated quinolone resistance genes and antibiotic resistance plasmids. Veter. Microbiol. 2014, 171, 413–421. [Google Scholar] [CrossRef]
- Macfadyen, G.; Allah, A.N.; Kenawy, D.A.R.; Ahmed, M.; Hebicha, H.; Diab, A.; Hussein, S.; Abouzied, R.; Naggar, G.E. Value-Chain Analysis of Egyptian Aquaculture; Project Report 2011-54; The WorldFish Center: Penang, Malaysia, 2012; 84p. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer Science and Business Media LLC, Springer: Manhattan, New York City, NY, USA; Available online: https://www.mobt3ath.com/uplode/book/book-23387.pdf (accessed on 15 June 2020).
- Noga, E.J. Fish Disease: Diagnosis and Treatment; John Wiley and Sons: Hoboken, NJ, USA, 2010; Available online: https://www.wiley.com/en-us/Fish+Disease%3A+Diagnosis+and+Treatment%2C+2nd+Edition-p-9780813806976 (accessed on 10 July 2020).
- Buller, N.B. Bacteria from Fish and Other Aquatic Animals: A Practical Identification Manual; Cabi: Wallingford, Oxfordshire, England, 2004. [Google Scholar]
- Holt, J.; Krieg, N.; Sneath, P.; Staley, J. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Yáñez, M.A.; Catalan, V.; Apráiz, D.; Figueras, M.J.; Martínez-Murcia, A. Phylogenetic analysis of members of the genus Aeromonas based on gyrB gene sequences. Int. J. Syst. Evol. Microbiol. 2003, 53, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Rodgers, M. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: A PCR identification. J. Appl. Microbiol. 2004, 97, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, B.; Basha, O.; Khalil, S.; Tanekhy, M. Molecular characterization, antimicrobial susceptibility and salt tolerance of aeromonas hydrophila from fresh, brackish and marine fishes. Alex. J. Veter. Sci. 2016, 48, 46–53. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Second Informational Supplement; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2014. [Google Scholar]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 2010, 4, 601–604. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone: Edinburgh, UK, 2008. [Google Scholar]
- Sen, K. Development of a rapid identification method for Aeromonas species by multiplex-PCR. Can. J. Microbiol. 2005, 51, 957–966. [Google Scholar] [CrossRef]
- Petit, J. Water supply, treatment, and re-cycling in aquaculture. In Aquaculture; Bamabe, G., Ed.; Ellis Horwood: New York, NY, USA, 1990; Volume 1. [Google Scholar]
- Lawson, T.B. Water quality and environmental requirements. In Fundamentals of Aquacultural Engineering; Springer: Boston, MA, USA, 1995; pp. 12–39. [Google Scholar]
- Zweig, R.D.; Morton, J.D.; Stewart, M.M. Source Water Quality for Aquaculture; World Bank: Washington, DC, USA, 1999. [Google Scholar]
- Stone, N.M.; Shelton, J.L.; Haggard, B.E.; Thomforde, H.K. Interpretation of Water Analysis Reports for Fish Culture; Southern Regional Aquaculture Center: Beltsville, MD, USA, 2013. [Google Scholar]
- Boyd, C.E. Water quality for pond aquaculture. Res. Dev. 1998, 43, 1–11. [Google Scholar]
- Meade, J.W. Aquaculture Management; Springer Science and Business Media LLC: Berlin, Germany, 1989. [Google Scholar]
- Swann, L. Water Quality Water Sources Used in Aquaculture. Water Quality Fact Sheet as-486. Aquaculture Extension; Illinois-Indiana Sea Grant Program; Purdue University: West Lafayette, IN, USA, 1993. [Google Scholar]
- Agoz, H.; Abbas, H.; Mahmoud, H. Rice-fish-azolla integrated culture systems. Egypt. J. Aquat. Biol. Fish. 2005, 2, 65–85. [Google Scholar]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Zamri-Saad, M.; Amal, M.; Siti-Zahrah, A.; Zulkafli, A. Control and prevention of Streptococcosis in Cultured Tilapia in Malaysia: A review. Pertanika. J. Trop. Agric. Sci. 2014, 37, 389–410. [Google Scholar]
- Ngugi, C.C.; Bowman, J.R.; Omolo, B. A New Guide to Fish Farming in Kenya; Aquaculture Collaborative Research Support Program (ACRSP), Oregon State University: Corvallis, OR, USA, 2007; 95p. [Google Scholar]
- Russell, M.; Shuke, R.; Samantha, S. Effects of Conductivity on Survivorship and Weight of Goldfish (Carassius auratus); Juniata College: Huntingdon, PA, USA, 2011. [Google Scholar]
- Jeffries, K.M.; Jackson, L.J.; Ikonomou, M.G.; Habibi, H.R. Presence of natural and anthropogenic organic contaminants and potential fish health impacts along two river gradients in Alberta, Canada. Environ. Toxicol. Chem. 2010, 29, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Elseady, Y.; Zahran, E. Ameliorating effect of β-carotene on antioxidant response and hematological parameters of mercuric chloride toxicity in Nile tilapia (Oreochromis niloticus). Fish. Physiol. Biochem. 2013, 39, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.; Risha, E.; Awadin, W.; Palić, D. Acute exposure to chlorpyrifos induces reversible changes in health parameters of Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2018, 197, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Shayo, S.; Mwita, C.; Hosea, K. Virulence of pseudomonas and aeromonas bacteria recovered from Oreochromis niloticus (perege) from mtera hydropower Dam; Tanzania. Ann. Biol. Res. 2012, 3, 5157–5161. [Google Scholar]
- Aly, S.M. A Review of Fish Diseases in the Egyptian Aquaculture Sector: Working Report; WorldFish: Penang, Malaysia, 2013; pp. 1–41. [Google Scholar]
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Veter. Med. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Harper, C.; Wolf, J.C. Morphologic effects of the stress response in fish. ILAR J. 2009, 50, 387–396. [Google Scholar] [CrossRef]
- Abou El-gheit, E. Some Investigations on the role of water parameters in microbial infections of fishes. Egypt. J. Exp. Biol. 2005, 1, 9–14. [Google Scholar]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens; Springer Science and Business Media LLC: Berlin, Germany, 2016. [Google Scholar]
- Moustafa, M.; Mohamed, L.A.; Mahmoud, M.; Soliman, W.; El-Gendy, M. Bacterial infections affecting marine fishes in Egypt. J. Am. Sci. 2010, 6, 603–612. [Google Scholar]
- Salem, M.; Zahran, E.; Saad, R.; Zaki, V. Prevalence, molecular characterization, virulotyping, and antibiotic resistance of motile aeromonads isolated from Nile tilapia farms at northern Egypt. Mansoura Veter. Med. J. 2020, 21, 56–67. [Google Scholar] [CrossRef]
- Hudson, J.A.; De Lacy, K.M. Incidence of motile aeromonads in New Zealand retail foods. J. Food Prot. 1991, 54, 696–703. [Google Scholar] [CrossRef]
- Yucel, N.; Aslim, B.; Beyatli, Y. Prevalence and resistance to antibiotics for Aeromonas species isolated from retail fish in Turkey. J. Food Qual. 2005, 28, 313–324. [Google Scholar] [CrossRef]
- Stock, I.; Grüger, T.; Wiedemann, B. Natural antibiotic susceptibility of strains of the Enterobacter cloacae complex. Int. J. Antimicrob. Agents 2001, 18, 537–545. [Google Scholar] [CrossRef]
- Matter, A.F.; El Asely, A.M.; Shaheen, A.A.; El-Gawad, E.A.A.; El-Abd, H.; Abbass, A.A. Phenotypic and molecular characterization of bacterial pathogens isolated from diseased freshwater fishes. Int. J. Fish. Aquat. Stud. 2018, 6, 34–41. [Google Scholar]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; Mohamed, M.E.; Rezk, M.M.; Gharieb, R.M.; Abdel-Maksoud, S.A. Aeromonas hydrophila in Fish and Humans; Prevalence, Virulotyping and Antimicrobial Resistance. Slov. Vet. Res. 2018, 55, 113–124. [Google Scholar]
- Ashiru, A. Isolation and antibiotic profile of Aeromonas species from tilapia fish (Tilapia nilotica) and catfish (Clarias betrachus). Pak. J. Nutr. 2011, 10, 982–986. [Google Scholar] [CrossRef]
- Popovic, N.T.; Teskeredžić, E.; Strunjak-Perović, I.; Čož-Rakovac, R. Aeromonas hydrophila isolated from wild freshwater fish in Croatia. Veter. Res. Commun. 2000, 24, 371–377. [Google Scholar] [CrossRef]
- Castro-Escarpulli, G.; Figueras, M.J.; Aguilera-Arreola, G.; Soler, L.; Fernández-Rendón, E.; Aparicio, G.O.; Guarro, J.; Chacon, M.R. Characterisation of Aeromonas spp. isolated from frozen fish intended for human consumption in Mexico. Int. J. Food Microbiol. 2003, 84, 41–49. [Google Scholar] [CrossRef]
- Ramadan, H.; Ibrahim, N.; Samir, M.; El-Moaty, A.A.; Gad, T. Aeromonas hydrophila from marketed mullet (Mugil cephalus) in Egypt: PCR characterization of β-lactam resistance and virulence genes. J. Appl. Microbiol. 2018, 124, 1629–1637. [Google Scholar] [CrossRef]
- Arslan, S.; Küçüksari, R. Phenotypic and genotypic virulence factors and antimicrobial resistance of motile Aeromonas spp. from fish and ground beef. J. Food Saf. 2015, 35, 551–559. [Google Scholar] [CrossRef]
- Ye, Y.; Fan, T.; Li, H.; Lu, J.; Jiang, H.; Hu, W.; Jiang, Q. Characterization of Aeromonas hydrophila from hemorrhagic diseased freshwater fishes in Anhui Province, China. Int. Food Res. J. 2013, 20, 1449. [Google Scholar]
- Simon, S.S.; Lalitha, K.V.; Joseph, T.C. Virulence properties of Aeromonas spp. from modified-atmosphere- and vacuum-packed milk fish (Chanos chanos Forsskal, 1775). Ann. Microbiol. 2016, 66, 1109–1115. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Ahmad, A. Antibiotic resistance profiling and phenotyping of Aeromonas species isolated from aquatic sources. Saudi J. Biol. Sci. 2015, 24, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Citarasu, T.; Dhas, A.; Velmurugan, S.; Viji, T.; Kumaran, T.; Babu, M.M.; Selvaraj, T. Isolation of aeromonas hydrophila from infected ornamental fish hatchery during massive disease outbreak. Int. J. Curr. Res. 2011, 2, 37–41. [Google Scholar]
- Sarkar, A.; Saha, M.; Roy, P. Detection of 232bp virulent gene of pathogenic aeromonas hydrophila through PCR based technique: (a rapid molecular diagnostic approach). Adv. Microbiol. 2013, 3, 83–87. [Google Scholar] [CrossRef]
- Dar, G.H.; Dar, S.A.; Kamili, A.N.; Chishti, M.Z.; Ahmad, F. Detection and characterization of potentially pathogenic Aeromonas sobria isolated from fish Hypophthalmichthys molitrix (Cypriniformes: Cyprinidae). Microb. Pathog. 2016, 91, 136–140. [Google Scholar] [CrossRef]
- Aguilera-Arreola, M.G.; Hernández-Rodríguez, C.; Zúñiga, G.; Figueras, M.J.; Garduño, R.A.; Castro-Escarpulli, G. Virulence potential and genetic diversity of Aeromonas caviae, Aeromonas veronii, and Aeromonas hydrophila clinical isolates from Mexico and Spain: A comparative study. Can. J. Microbiol. 2007, 53, 877–887. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, C.; Santos, J.A.; García, M.; Otero, A. irulence markers in Aeromonas hydrophila and Aeromonas veronii biovar sobria isolates from freshwater fish and from a diarrhoea case. J. Appl. Microbiol. 2002, 93, 414–419. [Google Scholar] [CrossRef]
- Sha, J.; Kozlova, E.V.; Chopra, A.K. Role of Various enterotoxins in Aeromonas hydrophila-induced gastroenteritis: Generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity. Infect. Immun. 2002, 70, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, X.; Liu, X.; Guo, L.; Hu, T.; Ni, X.; Liu, Y.; Lu, C. Detection of biochemical characters and extracellular proteases in the different isolates of Aeromonas hydrophila. J. Vet. Med. Sci. 2008, 40, 16–19. [Google Scholar]
- Hu, M.; Wang, N.; Pan, Z.; Lu, C.; Liu, Y. Identity and virulence properties of Aeromonas isolates from diseased fish, healthy controls and water environment in China. Lett. Appl. Microbiol. 2012, 55, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.S.; Khedr, M.H.; Zaki, M.S. Occurrence of potentially pathogenic Aeromonas species isolated from raw and ready- to- eat fish marketed in Sharkia Governorate, Egypt. Zagazig Veter. J. 2018, 46, 154–159. [Google Scholar] [CrossRef]
- Abd-Elall, A.; Abd-El-Kader, M.; Atia, A.S. Occurrence, seasonal variations and virulence of Aeromonas hydrophila and Aeromonas caviae in fish farms at East Delta, Egypt. Glob. Vet. 2014, 13, 328–336. [Google Scholar]
- Singh, V.; Rathore, G.; Kapoor, D.; Mishra, B.N.; Lakra, W.S. Detection of aerolysin gene in Aeromonas hydrophila isolated from fish and pond water. Indian J. Microbiol. 2008, 48, 453–458. [Google Scholar] [CrossRef]
- Abd-El-Malek, A.M. Incidence and virulence characteristics of Aeromonas spp. in fish. Veter. World 2017, 10, 34–37. [Google Scholar] [CrossRef]
- Younes, A.; Gaafar, A.; Awad, E.S. Virulence determinants and plasmid profile of Aeromonas hydrophila strains isolated from Oreochromis niloticus. Glob. Vet. 2015, 15, 613–617. [Google Scholar]
- Al-Fatlawy, H.N.K.; Al-Hadrawy, H. Isolation and characterization of A. hydrophila from the Al-Jadryia river in Baghdad (Iraq). Am. J. Educ. Res. 2014, 2, 658–662. [Google Scholar] [CrossRef]
- Hassan, M.A.M.; Noureldin, E.; Mahmoud, M.A.; Fita, N.A. Molecular identification and epizootiology of Aeromonas veronii infection among farmed Oreochromis niloticus in Eastern Province, KSA. Egypt. J. Aquat. Res. 2017, 43, 161–167. [Google Scholar] [CrossRef]
- Salem, M.; Zahran, E.; Rawia, S.; Zaki, H.V. Advanced Study on Motile Aeromonas Septicemia (Mas) in Cultured Nile Tilapia (Oreochromis Niloticus). Ph.D. Thesis, Mansoura University, Mansoura, Egypt, 2020. [Google Scholar]
- El-Barbary, M.I. Some clinical, microbiological and molecular characteristics of Aeromonas hydrophila isolated from various naturally infected fishes. Aquac. Int. 2010, 18, 943–954. [Google Scholar] [CrossRef]

| Key Factors | Farm I | Farm II | Farm III | Farm IV |
|---|---|---|---|---|
| 1- Individual characteristics | ||||
| • Age | 44 | 52 | 51 | 58 |
| • Gender | Male | Male | Male | Male |
| • Education | Lower secondary | High education | High education | Upper secondary |
| 2- Farm characteristics | ||||
| • Type of production | Semi-intensive | Semi-intensive | Semi-intensive | Semi-intensive |
| • Size of the farm (m2) | 12,600 | 8400 | 14,700 | 13,650 |
| • Number of ponds/farms | 5 | 4 | 5 | 5 |
| • Fish stocking density/pond | 10,000 | 14,000 | 18,000 | 18,500 |
| • Water source | Agriculture drainage | Agriculture drainage | Agriculture drainage | Agriculture + sewage water |
| • Fish stocking management | Feed supplements, water exchange | Feed supplements, water exchange | Feed supplements, water exchange and night aeration | Feed supplements, water exchange |
| • Fish production (ton)/feddan | 4.5 | 5 | 5 | 3 |
| • Marketing weight-harvest size (g) | 300–375 | 250–300 | 200–240 | 300–350 |
| • Use of untreated poultry manure | Yes | Yes | Yes | Yes |
| • Average previous mortality rate last 2 years/pond/day | 200 | 400 | 350 | 500 |
| • Current mortality rate/pond/day | 150 | 300 | 250 | 350 |
| • Therapeutic and prophylactic treatment | Humic acid, CaOH powder, probiotics and feed additives | Ca carbonate and manganese | Tetracycline + Probiotics | Chloramphenicol + humic acid and probiotics |
| • Disinfectants used | QAC | QAC | QAC | Ca hypochlorite |
| • Veterinary supervision (yes/no) | Occasionally | No | No | Yes |
| Parameter | Farm I | Farm II | Farm III | Farm IV | Standard Limits [Reference] | ||||
|---|---|---|---|---|---|---|---|---|---|
| March | August | March | August | March | August | March | August | ||
| Temp. (°C) | 23.5 | 28.9 | 21.2 | 34.1 | 23.4 | 30.8 | 24.7 | 33.8 | 28–30 [28] |
| Ph | 7.2 | 8.6 | 7.9 | 8.8 | 7.5 | 8.9 | 8 | 8.5 | 6.5–9 [29] |
| DO (mg/L) | 3.4 | 4 | 4.5 | 3.8 | 4.7 | 3.1 | 4 | 6–14 [30] | |
| EC (mho/cm) | 1588 | 2865 | 1365 | 3771 | 2547 | 3524 | 2620 | 3968 | 60–2000 µS/cm [31] |
| TDS (mg/L) | 796 | 1524 | 674.5 | 1886 | 1281.3 | 1658.4 | 1342.1 | 1978.6 | ≤80 [32] |
| Ammonia (mg/L) | 1.01 | 1.42 | 0.55 | 1.62 | 1.3 | 2.6 | 1.8 | 3.4 | 0.6–2.0 [33] |
| NH3–N (mg/L) | 0.36 | 1.17 | 0.42 | 1.05 | 0.49 | 0.98 | 0.6 | 1.23 | <0.05–1.0 [29] |
| Nitrate (mg/L) | 0 | 0 | 0 | 0.3 | 0.2 | 0.5 | 0 | 0.4 | <3 [33] |
| Nitrite (mg/L) | 0.1 | 0.31 | 0.1 | 0.34 | 0.21 | 0.69 | 0.07 | 0.23 | <0.5 [34] |
| Phosphorus (mg/L) | 1.17 | 1.46 | 0.69 | 0.92 | 0.58 | 0.78 | 1.87 | 1.9 | 0.05–0.5 [31] |
| Farms | Water | Fish | Total Examined Samples | |||
|---|---|---|---|---|---|---|
| No. of Positive | % | No. of Positive | % | No. of Positive | % | |
| I | 0 | 0 | 15 | 25 | 15 | 24.2 |
| II | 0 | 0 | 20 | 33.3 | 20 | 32.3 |
| III | 0 | 0 | 17 | 28.3 | 17 | 27.4 |
| IV | 1 | 50 | 28 | 46.7 | 29 | 46.8 |
| Total | 1 | 12.5 | 80 | 33.3 | 82 | 33.1 |
| Months | Farm I | Farm II | Farm III | Farm IV |
|---|---|---|---|---|
| March | 10 | 0 | 10 | 20 |
| April | 10 | 20 | 30 | 40 |
| May | 20 | 20 | 10 | 40 |
| June | 30 | 50 | 20 | 40 |
| July | 40 | 50 | 60 | 60 |
| August | 40 | 40 | 40 | 80 |
| Total | 25 | 33.3 | 28.3 | 46.7 |
| Genes | No. of Positive | (%) |
|---|---|---|
| Gyrase B (gyrB) | 65/80 | (81.25) |
| Aerolysin (aer) | 42/80 | (52.5) |
| Elastase (ahp) | 21/80 | (26.25) |
| Hemolysin (hyl) | 28/80 | (35) |
| Lipase (lip) | 3/80 | (3.75) |
| Antimicrobial Resistance Pattern | The Multiple Antibiotic Resistance (MAR) Index | ||||||
|---|---|---|---|---|---|---|---|
| Antibiotics | Number of Resistant Aeromonas Isolates (%) | Isolate Number | Antibiotic Resistance Profile * | MAR ** | Isolate Number | Antibiotic Resistance Profile * | MAR ** |
| Chloramphenicol (C) | 16 (80%) | 1 | C, CIP, TE, K, SXT, CN, AZM | 0.64 | 35 | C, TE, K, SXT, CN, AZM, IPM | 0.64 |
| Ciprofloxacin (CIP) | 14 (70%) | 2 | C, CIP, TE, CXT, K, SXT, CN, AZM, S | 0.82 | 36 | C, CIP, TE, K, SXT, AZM | 0.55 |
| Tetracycline (TE) | 13 (65%) | 12 | C, CIP, TE, K, SXT, AZM, S | 0.64 | 37 | CIP, TE, AM, K, SXT, CN | 0.55 |
| Amoxicillin (AM) | 4 (20%) | 13 | C, CIP, TE, CXT, K, SXT, CN, AZM, S | 0.82 | 39 | C, AM, CXT, K, SXT, CN, AZM | 0.64 |
| Cefotaxime (CXT) | 6 (30%) | 23 | C, CIP, TE, K, CN, AZM, S, IPM | 0.73 | 40 | C, SXT, CN, S | 0.36 |
| Kanamycin (K) | 16 (80%) | 25 | CIP, TE, K, SXT, CN, AZM | 0.55 | 43 | C, CIP, K, SXT | 0.36 |
| Trimethoprim/sulphamethoxazole (SXT) | 17 (75%) | 28 | C, CIP, TE, CXT, K, SXT, CN, AZM, S | 0.82 | 44 | C, CIP, AZM | 0.27 |
| Gentamycin (CN) | 14 (70%) | 29 | CIP, AM, CXT, K, SXT, CN, AZM, S | 0.73 | 45 | C, TE, K, SXT, AZM | 0.45 |
| Azithromycin (AZM) | 16 (80%) | 33 | C, CIP, CXT, K, SXT, CN, AZM, S | 0.73 | 48 | C, TE, SXT | 0.27 |
| Streptomycin (S) | 8 (40%) | 34 | C, CIP, TE, K, SXT, CN, AZM, S | 0.73 | |||
| Imipenem (IPM) | 2 (10%) | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gohary, F.A.; Zahran, E.; Abd El-Gawad, E.A.; El-Gohary, A.H.; M. Abdelhamid, F.; El-Mleeh, A.; Elmahallawy, E.K.; Elsayed, M.M. Investigation of the Prevalence, Virulence Genes, and Antibiogram of Motile Aeromonads Isolated from Nile Tilapia Fish Farms in Egypt and Assessment of their Water Quality. Animals 2020, 10, 1432. https://doi.org/10.3390/ani10081432
El-Gohary FA, Zahran E, Abd El-Gawad EA, El-Gohary AH, M. Abdelhamid F, El-Mleeh A, Elmahallawy EK, Elsayed MM. Investigation of the Prevalence, Virulence Genes, and Antibiogram of Motile Aeromonads Isolated from Nile Tilapia Fish Farms in Egypt and Assessment of their Water Quality. Animals. 2020; 10(8):1432. https://doi.org/10.3390/ani10081432
Chicago/Turabian StyleEl-Gohary, Fatma A., Eman Zahran, Eman A. Abd El-Gawad, Adel H. El-Gohary, Fatma M. Abdelhamid, Amany El-Mleeh, Ehab Kotb Elmahallawy, and Mona Mohieldin Elsayed. 2020. "Investigation of the Prevalence, Virulence Genes, and Antibiogram of Motile Aeromonads Isolated from Nile Tilapia Fish Farms in Egypt and Assessment of their Water Quality" Animals 10, no. 8: 1432. https://doi.org/10.3390/ani10081432
APA StyleEl-Gohary, F. A., Zahran, E., Abd El-Gawad, E. A., El-Gohary, A. H., M. Abdelhamid, F., El-Mleeh, A., Elmahallawy, E. K., & Elsayed, M. M. (2020). Investigation of the Prevalence, Virulence Genes, and Antibiogram of Motile Aeromonads Isolated from Nile Tilapia Fish Farms in Egypt and Assessment of their Water Quality. Animals, 10(8), 1432. https://doi.org/10.3390/ani10081432







