Anti-Oxidative Effects of Human Adipose Stem Cell Conditioned Medium with Different Basal Medium during Mouse Embryo In Vitro Culture
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Chemicals
2.3. Human Adipose Stem Cell Culture and Characterization
2.4. Preparation of Adipose Stem Cell Conditioned Medium
2.5. Experimental Animals
2.6. In Vitro Fertilization
2.7. Experimental Groups
2.8. Embryo Development Evaluation and Total Cell Counts
2.9. Measurement of ROS and Glutathione (GSH) Levels
2.10. Immunofluorescence Staining
2.11. Statistical Analysis
3. Results
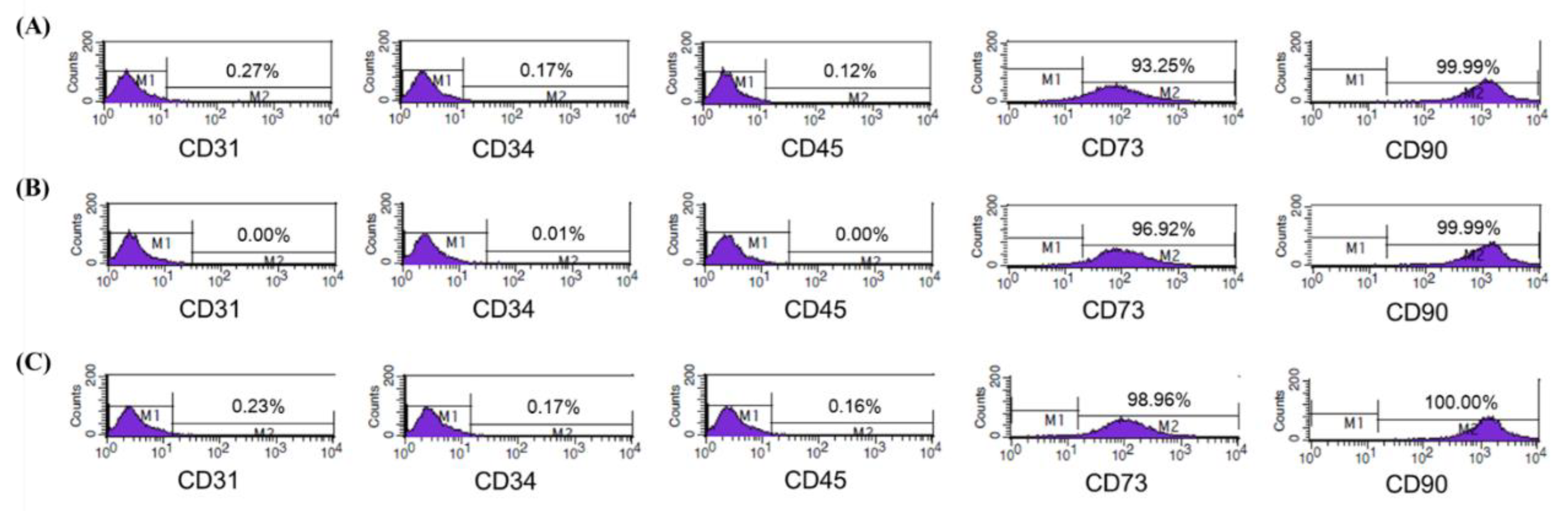
3.1. Characterization of Isolated ASCs
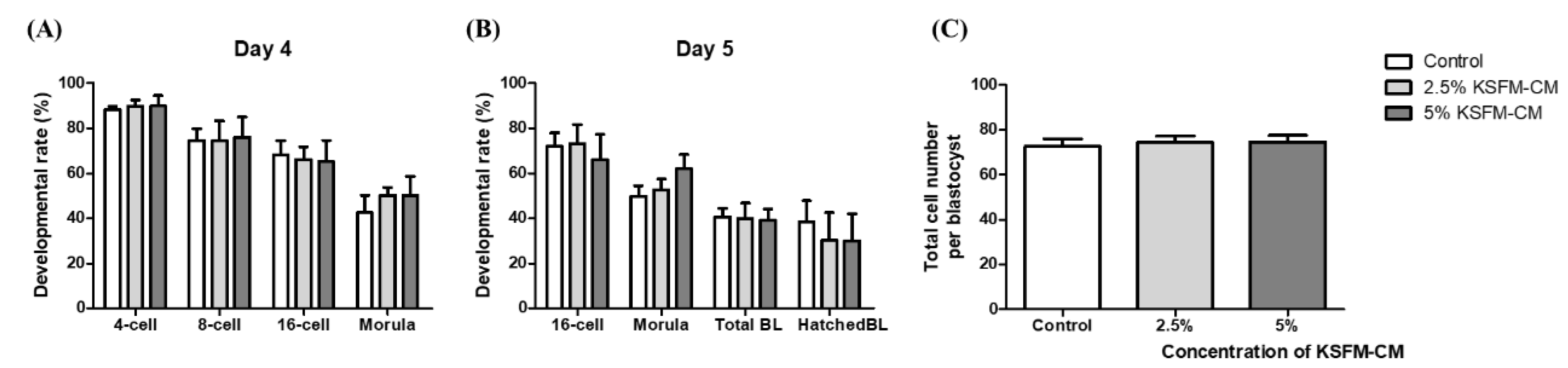
3.2. Effects of KSFM-CM on the Developmental Competence of Embryo
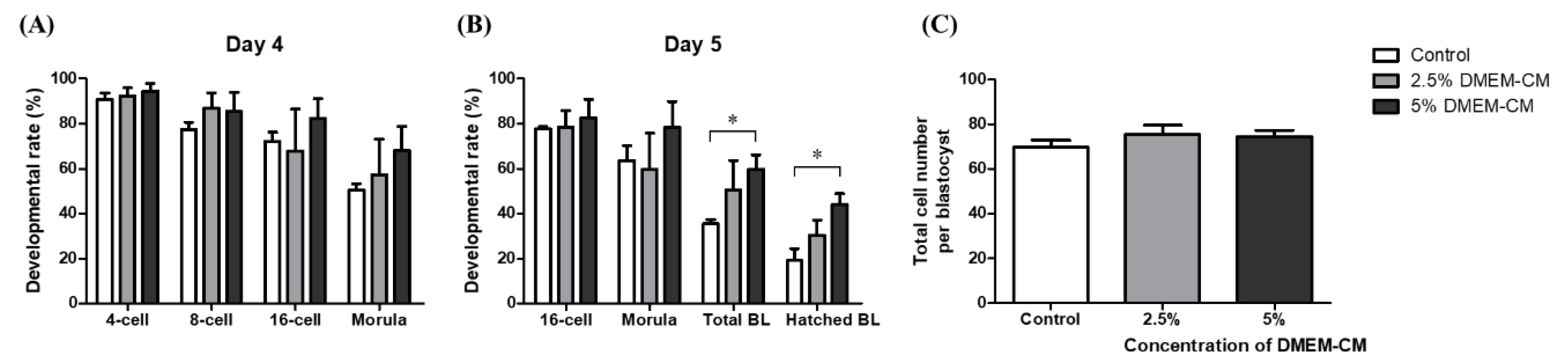
3.3. Effects of DMEM-CM on Developmental Competence of Embryo
3.4. Comparative Effects of KSFM-CM and DMEM-CM on Embryo Development
3.5. Effects of KSFM-CM and DMEM-CM on Oxidative Stress in BL
3.6. Effects of KSFM-CM and DMEM-CM on Apoptosis in BL
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ata, B.; Seli, E. Economics of assisted reproductive technologies. Curr. Opin. Obstet. Gynecol. 2010, 22, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.B.; Troisi, R.; Rexrode, K.M. Women and Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2013; 1581p. [Google Scholar]
- What Is Assisted Reproductive Technology? Available online: https://www.cdc.gov/art/whatis.html (accessed on 4 February 2020).
- Roberts, R.M. Embryo culture conditions: What embryos like best. Endocrinology 2005, 146, 2140–2141. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Takayama, S.; Swain, J.E. Rethinking in vitro embryo culture: New developments in culture platforms and potential to improve assisted reproductive technologies. Biol. Reprod. 2012, 86, 62. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Ansari, M.M.; Parmar, M.S.; Chandra, V.; Sharma, G.T. Stem cell conditioned media contains important growth factors and improves in vitro buffalo embryo production. Anim. Biotechnol. 2016, 27, 118–125. [Google Scholar] [CrossRef]
- Richter, K.S. The importance of growth factors for preimplantation embryo development and in-vitro culture. Curr. Opin. Obstet. Gynecol. 2008, 20, 292–304. [Google Scholar] [CrossRef]
- Lee, S.H.; Setyawan, E.M.N.; Choi, Y.B.; Ra, J.C.; Kang, S.K.; Lee, B.C.; Kim, G.A. Clinical assessment after human adipose stem cell transplantation into dogs. J. Vet. Sci. 2018, 19, 452–461. [Google Scholar] [CrossRef]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Chu, D.T.; Nguyen Thi Phuong, T.; Tien, N.L.B.; Tran, D.K.; Minh, L.B.; Thanh, V.V.; Gia Anh, P.; Pham, V.H.; Thi Nga, V. Adipose tissue stem cells for therapy: An update on the progress of isolation, culture, storage, and clinical application. J. Clin. Med. 2019, 8, 917. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Bertolini, F.; Lohsiriwat, V.; Petit, J.Y.; Kolonin, M.G. Adipose tissue cells, lipotransfer and cancer: A challenge for scientists, oncologists and surgeons. Biochim. Biophys. Acta 2012, 1826, 209–214. [Google Scholar] [CrossRef]
- Da Costa Goncalves, F.; Grings, M.; Nunes, N.S.; Pinto, F.O.; Garcez, T.N.; Visioli, F.; Leipnitz, G.; Paz, A.H. Antioxidant properties of mesenchymal stem cells against oxidative stress in a murine model of colitis. Biotechnol. Lett. 2017, 39, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Ruder, E.H.; Hartman, T.J.; Blumberg, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Update 2008, 14, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Hwang, K.J.; Kwon, H.C.; Kim, H.S.; Choi, K.W.; Oh, K.S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 1998, 13, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Oyawoye, O.; Abdel Gadir, A.; Garner, A.; Constantinovici, N.; Perrett, C.; Hardiman, P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: Relationship to outcome. Hum. Reprod. 2003, 18, 2270–2274. [Google Scholar] [CrossRef]
- Karja, N.W.; Kikuchi, K.; Fahrudin, M.; Ozawa, M.; Somfai, T.; Ohnuma, K.; Noguchi, J.; Kaneko, H.; Nagai, T. Development to the blastocyst stage, the oxidative state, and the quality of early developmental stage of porcine embryos cultured in alteration of glucose concentrations in vitro under different oxygen tensions. Reprod. Biol. Endocrinol. 2006, 4, 54. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, B.S.; Sung, J.H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin. Biol. Ther. 2009, 9, 879–887. [Google Scholar] [CrossRef]
- Sriramulu, S.; Banerjee, A.; Di Liddo, R.; Jothimani, G.; Gopinath, M.; Murugesan, R.; Marotta, F.; Pathak, S. Concise review on clinical applications of conditioned medium derived from human umbilical cord-mesenchymal stem cells (UC-MSCs). Int. J. Hematol. Oncol. Stem. Cell Res. 2018, 12, 230–234. [Google Scholar]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. conditioned medium from human mesenchymal stromal cells: Towards the clinical translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, Y.W.; Tan, K.P.; Shen, Y.S.; Wang, Y.H.; Chang, C.H. Can mesenchymal stem cells and their conditioned medium assist inflammatory chondrocytes recovery? PLoS ONE 2018, 13, e0205563. [Google Scholar] [CrossRef]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. Biomed. Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Espana, E.M.; He, H.; Smiddy, R.; Parel, J.M.; Liu, C.Y.; Tseng, S.C. Preservation and expansion of the primate keratocyte phenotype by downregulating TGF-beta signaling in a low-calcium, serum-free medium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1918–1927. [Google Scholar] [CrossRef]
- Kim, S.S.; Gwak, S.J.; Han, J.; Park, M.H.; Song, K.W.; Kim, B.S. Regeneration of kidney tissue using in vitro cultured fetal kidney cells. Exp. Mol. Med. 2008, 40, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gurdal, M.; Barut Selver, O.; Baysal, K.; Durak, I. Comparison of culture media indicates a role for autologous serum in enhancing phenotypic preservation of rabbit limbal stem cells in explant culture. Cytotechnology 2018, 70, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar] [PubMed]
- Menezo, Y.; Lichtblau, I.; Elder, K. New insights into human pre-implantation metabolism in vivo and in vitro. J. Assist. Reprod. Genet. 2013, 30, 293–303. [Google Scholar] [CrossRef]
- Simopoulou, M.; Sfakianoudis, K.; Rapani, A.; Giannelou, P.; Anifandis, G.; Bolaris, S.; Pantou, A.; Lambropoulou, M.; Pappas, A.; Deligeoroglou, E.; et al. Considerations regarding embryo culture conditions: From media to epigenetics. In Vivo 2018, 32, 451–460. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- In Vitro Fertilization (IVF). Available online: http://card.medic.kumamoto-u.ac.jp/card/english/sigen/manual/mouseivf.html (accessed on 6 August 2020).
- Nugraha Setyawan, E.M.; Oh, H.J.; Kim, M.J.; Kim, G.A.; Lee, S.H.; Choi, Y.B.; Ra, K.; Lee, B.C. Despite the donor’s age, human adipose-derived stem cells enhance the maturation and development rates of porcine oocytes in a co-culture system. Theriogenology 2018, 115, 57–64. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, E.Y.; Lee, S.E.; Choi, H.Y.; Moon, J.J.; Park, M.J.; Son, Y.J.; Lee, J.B.; Jeong, C.J.; Lee, D.S.; et al. Effect of human adipose tissue-derived mesenchymal-stem-cell bioactive materials on porcine embryo development. Mol. Reprod. Dev. 2013, 80, 1035–1047. [Google Scholar] [CrossRef]
- Hardy, K.; Spanos, S. Growth factor expression and function in the human and mouse preimplantation embryo. J. Endocrinol. 2002, 172, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Sargent, I.L.; Martin, K.L.; Barlow, D.H. The use of recombinant growth factors to promote human embryo development in serum-free medium. Hum. Reprod. 1998, 13 (Suppl. 4), 239–248. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.H.; Azarnia, M.; Ghasemian, F.; Ghasemi, F.; Ghadarjani, S.; Khojasteh, N.; Moghaddam, M.A.J. Relevance of hepatocyte growth factor and fibroblast growth factor on mouse preimplantation embryo development. J. Reprod. Contracept. 2009, 20, 195–204. [Google Scholar] [CrossRef]
- Paria, B.C.; Dey, S.K. Preimplantation embryo development in vitro: Cooperative interactions among embryos and role of growth factors. Proc. Natl. Acad. Sci. USA 1990, 87, 4756–4760. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Smith, G.D. Advances in embryo culture platforms: Novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum. Reprod. Update 2011, 17, 541–557. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, J.; Liu, J.; Gong, N.; Chen, L. Effects of adipose stem cell-conditioned medium on the migration of vascular endothelial cells, fibroblasts and keratinocytes. Exp. Ther. Med. 2013, 5, 701–706. [Google Scholar] [CrossRef]
- Kapiteijn, K.; Koolwijk, P.; van der Weiden, R.M.; van Nieuw Amerongen, G.; Plaisier, M.; van Hinsbergh, V.W.; Helmerhorst, F.M. Human embryo-conditioned medium stimulates in vitro endometrial angiogenesis. Fertil. Steril. 2006, 85 (Suppl. 1), 1232–1239. [Google Scholar] [CrossRef]
- Lee, S.E.; Kwon, T.R.; Kim, J.H.; Lee, B.C.; Oh, C.T.; Im, M.; Hwang, Y.K.; Paik, S.H.; Han, S.; Kim, J.Y.; et al. Antiphotoaging and antioxidative activities of natural killer cell conditioned medium following UVB irradiation of human dermal fibroblasts and a reconstructed skin model. Int. J. Mol. Med. 2019, 44, 1641–1652. [Google Scholar] [CrossRef]
- Jedrusik, A. Making the first decision: Lessons from the mouse. Reprod. Med. Biol. 2015, 14, 135–150. [Google Scholar] [CrossRef]
- Le Cruguel, S.; Ferre-L’Hotellier, V.; Moriniere, C.; Lemerle, S.; Reynier, P.; Descamps, P.; May-Panloup, P. Early compaction at day 3 may be a useful additional criterion for embryo transfer. J. Assist. Reprod. Genet. 2013, 30, 683–690. [Google Scholar] [CrossRef]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012, 143, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Lee, S.W.; Jeong, H.J.; Yoon, S.H.; Koh, M.W.; Lim, J.H.; Lee, S.G. Clinical outcomes of elective single morula embryo transfer versus elective single blastocyst embryo transfer in IVF-ET. J. Assist. Reprod. Genet. 2012, 29, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Aspects. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A. The fate of the zona pellucida in mice. J. Embryol. Exp. Morphol. 1970, 23, 1–19. [Google Scholar] [PubMed]
- Hammadeh, M.E.; Fischer-Hammadeh, C.; Ali, K.R. Assisted hatching in assisted reproduction: A state of the art. J. Assist. Reprod. Genet. 2011, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.M.; Trounson, A.O.; Lolatgis, N.; Wood, C. Factors affecting the success of human blastocyst development and pregnancy following in vitro fertilization and embryo transfer. Fertil. Steril. 1998, 70, 1022–1029. [Google Scholar] [CrossRef]
- Do, G.Y.; Kim, J.W.; Park, H.J.; Yoon, S.B.; Park, J.Y.; Yang, S.G.; Jung, B.D.; Kwon, Y.S.; Kang, M.J.; Song, B.S.; et al. Native plants (Phellodendron amurense and Humulus japonicus) extracts act as antioxidants to support developmental competence of bovine blastocysts. Asian-Australas. J. Anim. Sci. 2017, 30, 1245–1252. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef]
- Hajian, M.; Hosseini, S.M.; Ostadhosseini, S.; Nasr-Esfahani, M.H. Comparative stepwise pattern of reactive oxygen species production during in vitro development of fertilized and nuclear transferred goat embryos. Int. J. Fertil. Steril. 2017, 11, 93–98. [Google Scholar] [CrossRef]
- Thomas, M.; Jain, S.; Kumar, G.P.; Laloraya, M. A programmed oxyradical burst causes hatching of mouse blastocysts. J. Cell. Sci. 1997, 110 Pt 14, 1597–1602. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Makker, K.; Desai, N.R.; Agarwal, A. Impact of oxidative stress on IVF. Expert Rev. Obstet. Gynecol. 2008, 3, 539–554. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Steinauer, K.K.; Hornung, B.; Irish, J.M.; Lecane, P.; Birrell, G.W.; Peehl, D.M.; Knox, S.J. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002, 9, 252–263. [Google Scholar] [CrossRef]
- Van Soom, A.; Vanroose, G.; de Kruif, A. Blastocyst evaluation by means of differential staining: A practical approach. Reprod. Domest. Anim. 2001, 36, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Li, Y.; Li, R.; Li, Q.; Wu, Y.; Quan, F.; Liu, J.; Guo, Z.; Zhang, Y. Oxamflatin significantly improves nuclear reprogramming, blastocyst quality, and in vitro development of bovine SCNT embryos. PLoS ONE 2011, 6, e23805. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef]
- Gown, A.M.; Willingham, M.C. Improved detection of apoptotic cells in archival paraffin sections: Immunohistochemistry using antibodies to cleaved caspase 3. J. Histochem. Cytochem. 2002, 50, 449–454. [Google Scholar] [CrossRef]
- Coutinho, A.R.; Assumpcao, M.E.; Bordignon, V. Presence of cleaved caspase 3 in swine embryos of different developmental capacities produced by parthenogenetic activation. Mol. Reprod. Dev. 2011, 78, 673–683. [Google Scholar] [CrossRef]
- Koustas, G.; Sjoblom, C. Minute changes to the culture environment of mouse pre-implantation embryos affect the health of the conceptus. Asian Pac. J. Reprod. 2016, 5, 287–294. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility—A clinician’s perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]



| DMEM | ||
|---|---|---|
| Component | (g/L) | |
| Inorganic Salts | CaCl2 | 0.2 |
| Fe(NO3)3 • 9H2O | 0.0001 | |
| MgSO4 | 0.09767 | |
| KCl | 0.4 | |
| NaHCO3 | 3.7 | |
| NaCl | 6.4 | |
| NaH2PO4 | 0.109 | |
| Amino Acids | L-Arginine • HCl | 0.084 |
| L-Cystine • 2HCl | 0.0626 | |
| L-Glutamine | 0.584 | |
| Glycine | 0.03 | |
| L-Histidine • HCl • H2O | 0.042 | |
| L-Isoleucine | 0.105 | |
| L-Leucine | 0.105 | |
| L-Lysine • HCl | 0.146 | |
| L-Methionine | 0.03 | |
| L-Phenylalanine | 0.066 | |
| L-Serine | 0.042 | |
| L-Threonine | 0.095 | |
| L-Tryptophan | 0.016 | |
| L-Tyrosine • 2Na • 2H2O | 0.10379 | |
| L-Valine | 0.094 | |
| Vitamins | Choline Chloride | 0.004 |
| Folic Acid | 0.004 | |
| myo-Inositol | 0.0072 | |
| Niacinamide | 0.004 | |
| D-Pantothenic Acid • ½Ca | 0.004 | |
| Pyridoxine • HCl | 0.00404 | |
| Riboflavin | 0.0004 | |
| Thiamine • HCl | 0.004 | |
| Other | D-Glucose | 4.5 |
| Phenol Red • Na | 0.0159 | |
| Pyruvic Acid • Na | 0.11 | |
| CSCM-NX | |
|---|---|
| Inorganic Salts | Calcium Chloride |
| Magnesium Sulfate | |
| Potassium Chloride | |
| Potassium Phosphate | |
| Sodium Chloride | |
| Amino Acids | Alanine |
| Arginine | |
| Asparagin | |
| Aspartic acid | |
| Cystine | |
| Glutamic acid | |
| Glycine | |
| Histidine | |
| Isoleucine | |
| Leucine | |
| Lysine | |
| Methionine | |
| Phenylalanine | |
| Proline | |
| Serine | |
| Threonine | |
| Tryptophan | |
| Tyrosine | |
| Valine | |
| Energy Substrates | Dextrose |
| Sodium Lactate | |
| Sodium Pyruvate | |
| Antioxidants | EDTA |
| Sodium Citrate | |
| Dipeptide | Alanyl-glutamine |
| Buffer | Sodium Bicarbonate |
| Antibiotic | Gentamicin |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ra, K.; Oh, H.J.; Kim, E.Y.; Kang, S.K.; Ra, J.C.; Kim, E.H.; Lee, B.C. Anti-Oxidative Effects of Human Adipose Stem Cell Conditioned Medium with Different Basal Medium during Mouse Embryo In Vitro Culture. Animals 2020, 10, 1414. https://doi.org/10.3390/ani10081414
Ra K, Oh HJ, Kim EY, Kang SK, Ra JC, Kim EH, Lee BC. Anti-Oxidative Effects of Human Adipose Stem Cell Conditioned Medium with Different Basal Medium during Mouse Embryo In Vitro Culture. Animals. 2020; 10(8):1414. https://doi.org/10.3390/ani10081414
Chicago/Turabian StyleRa, Kihae, Hyun Ju Oh, Eun Young Kim, Sung Keun Kang, Jeong Chan Ra, Eui Hyun Kim, and Byeong Chun Lee. 2020. "Anti-Oxidative Effects of Human Adipose Stem Cell Conditioned Medium with Different Basal Medium during Mouse Embryo In Vitro Culture" Animals 10, no. 8: 1414. https://doi.org/10.3390/ani10081414
APA StyleRa, K., Oh, H. J., Kim, E. Y., Kang, S. K., Ra, J. C., Kim, E. H., & Lee, B. C. (2020). Anti-Oxidative Effects of Human Adipose Stem Cell Conditioned Medium with Different Basal Medium during Mouse Embryo In Vitro Culture. Animals, 10(8), 1414. https://doi.org/10.3390/ani10081414





