Eurasian Beaver (Castor fiber) Winter Foraging Preferences in Northern Poland—The Role of Woody Vegetation Composition and Anthropopression Level
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Studies and Data Analysis
2.3. Statistical Analysis
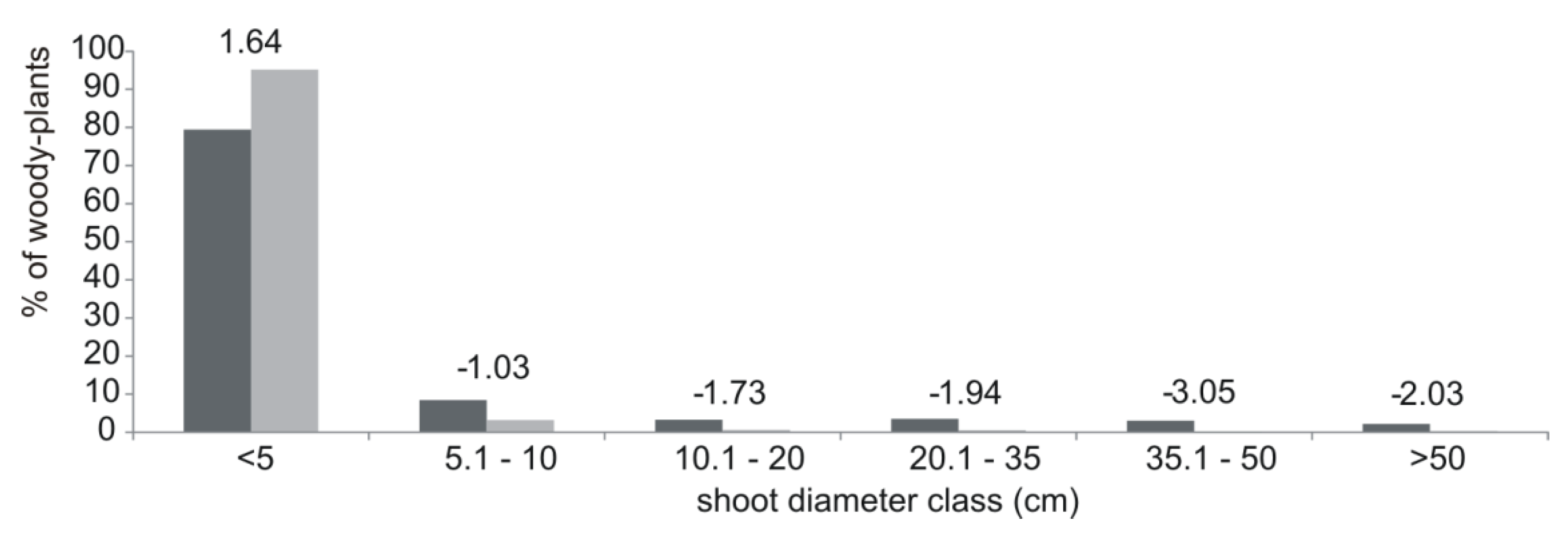
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jenkins, S.H.; Busher, P.E. Castor canadensis. Mamm. Species 1979, 120, 1–8. [Google Scholar] [CrossRef]
- Jenkins, S.H. Problems, progress, and prospects in studies of food selection by beavers. In Proceedings of the Worldwide Furbearer Conference, Frostburg, MD, USA, 3–11 August 1980; Chapman, J.A., Pursley, D., Eds.; Worldwide Furbearer Conference: Baltimore, MD, USA, 1981; pp. 559–579. [Google Scholar]
- Hill, E.P. Beaver. In Wild Mammals of North America. Biology, Management and Economics; Chapman, J.P., Felhamer, G.A., Eds.; The John Hopkins University Press: Baltimore, MD, USA, 1982; pp. 256–281. [Google Scholar]
- Busher, P.E. Family Castoridae (Beavers). In Handbook of Mammals of the World: Lagomorphs and Rodents I; Wilson, D.E., Lacher, T.E., Jr., Mittermeier, R.A., Eds.; Barcelona Lynx Editions: Barcelona, Spain, 2016; Volume 6, pp. 150–168. [Google Scholar]
- Northcott, T. Feeding habits of beaver in Newfoundland. Oikos 1971, 22, 407–410. [Google Scholar] [CrossRef]
- Svendsen, G.E. Seasonal change in feeding patterns of beaver in southern Ohio. J. Wildl. Manag. 1980, 44, 285–290. [Google Scholar] [CrossRef]
- Fryxell, J.M.; Doucet, C.M. Provisioning time and central-place foraging in beavers. Can. J. Zool. 1991, 69, 1308–1313. [Google Scholar] [CrossRef]
- Barnes, D.M.; Mallik, A.U. Effects of beaver, Castor canadensis, herbivory on streamside vegetation in a northern Ontario watershed. Can. Field Nat. 2001, 115, 9–21. [Google Scholar]
- Haarberg, O.; Rosell, F. Selective foraging on woody plant species by the Eurasian beaver (Castor fiber) in Telemark, Norway. J. Zool. 2006, 270, 201–208. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Atkinson, S.R.; Gamez, K.; Pickering, S.P.; Dutton, J.S. Forage preferences of the European beaver Castor fiber: Implications for re-introduction. Conserv. Soc. 2008, 6, 190–194. [Google Scholar]
- Krojerová-Prokešová, J.; Barančeková, M.; Hamšíková, L.; Vorel, A. Feeding habits of reintroduced Eurasian beaver: Spatial and seasonal variation in the use of food resources. J. Zool. 2010, 281, 183–193. [Google Scholar] [CrossRef]
- Schoener, T.W. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 1971, 2, 369–404. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the theory of optimal diets. Am. Nat. 1974, 108, 59–74. [Google Scholar] [CrossRef]
- Pyke, G.H.; Pulliam, H.R.; Charnov, E.L. Optimal foraging: A selective review of theory and tests. Q Rev. Biol. 1977, 52, 137–154. [Google Scholar] [CrossRef]
- Orians, G.H.; Pearson, N.E. On the theory of central place foraging. In Analysis of Ecological Systems; Horn, D.J., Stairs, G.R., Mitchell, R.D., Eds.; Ohio State Iniversity Press: Columbus, OH, USA, 1979; pp. 155–177. [Google Scholar]
- Johnston, C.A.; Naiman, R.J. Boundary dynamics at the aquatic-terrestrial interface: The influence of beaver and geomorphology. Landsc. Ecol. 1987, 1, 47–57. [Google Scholar] [CrossRef]
- Basey, J.M.; Jenkins, S.H. Influences of predation risk and energy maximization on food selection by beavers (Castor canadensis). Can. J. Zool. 1995, 73, 2197–2208. [Google Scholar] [CrossRef]
- Donkor, N.T.; Fryxell, J.M. Lowland boreal forests characterization in Algonquin Provincial Park relative to beaver (Castor canadensis) foraging and edaphic factors. Plant. Ecol. 2000, 148, 1–12. [Google Scholar] [CrossRef]
- Hall, J.G. Willow and aspen in the ecology of beaver in Sagehen Creek, California. Ecology 1960, 41, 484–494. [Google Scholar] [CrossRef]
- Belovsky, G.E. Summer diet optimization by beaver. Am. Midl. Nat. 1984, 111, 209–222. [Google Scholar] [CrossRef]
- Parker, H.; Haugen, A.; Kristensen, Ø.; Myrum, E.; Kolsing, R.; Rosell, F. Landscape use and economic value of Eurasian beaver (Castor fiber) on a large forest in southeast Norway. In Proceedings of the first Euro-American Beaver Congress, Volga-Kama National Nature Preserve, Kazan, Russia, 24–28 August 1999; Busher, P., Gorshkov, Y., Eds.; Volga-Kama National Nature Preserve: Kazan, Russia, 2001; pp. 77–95. [Google Scholar]
- Voelker, B.W.; Dooley, J.L., Jr. Impact by North American Beaver (Castor canadensis) on forest plant composition in the wilds, a surface-mined landscape in Southeastern Ohio. Ohio J. Sci. 2008, 108, 9–15. [Google Scholar]
- McGinley, M.A.; Whitham, T.G. Central place foraging by beavers (Castor canadensis): A test of foraging predictions and the impact of selective feeding on the growth form of cottonwoods (Populus fremontii). Oecologia 1985, 66, 558–562. [Google Scholar] [CrossRef]
- Basey, J.M.; Jenkins, S.H.; Busher, P.E. Optimal central-place foraging by beavers: Tree-size selection in relation to defensive chemicals of quaking aspen. Oecologia 1988, 76, 278–282. [Google Scholar] [CrossRef]
- Donkor, N.T.; Fryxell, J.M. Impact of beaver foraging on structure of lowland boreal forests of Algonquin Provincial Park, Ontario. Ecol. Manag. 1999, 118, 83–92. [Google Scholar] [CrossRef]
- Jenkins, S.H. A size–distance relation in food selection by beavers. Ecology 1980, 61, 740–746. [Google Scholar] [CrossRef]
- Gerwing, G.T.; Johnson, C.J.; Alström-Rapaport, C. Factors influencing forage selection by the North American beaver (Castor canadensis). Mamm. Biol. 2013, 78, 79–86. [Google Scholar] [CrossRef]
- Basey, J.M.; Jenkins, S.H.; Miller, G.C. Food selection by beavers in relation to inducible defenses of Populus tremuloides. Oecologia 1990, 59, 57–62. [Google Scholar] [CrossRef]
- Müller-Schwarze, D.; Schulte, B.A.; Sun, L.; Müller-Schwarze, A.; Müller-Schwarze, C. Red maple (Acer rubrum) inhibits feeding by beaver (Castor canadensis). J. Chem. Ecol. 1994, 20, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Rosell, F.; Czech, A. Responses of foraging Eurasian beavers Castor fiber to predator odours. Wildl. Biol. 2000, 6, 13–21. [Google Scholar] [CrossRef]
- Salandre, J.A.; Beil, R.; Loehr, J.A.; Sundell, J. Foraging decisions of North American beaver (Castor canadensis) are shaped by energy constraints and predation risk. Mammal. Res. 2017, 62, 229–239. [Google Scholar] [CrossRef]
- Parker, J.D.; Caudill, C.C.; Hay, M.E. Beaver herbivory on aquatic plants. Oecologia 2007, 151, 616–625. [Google Scholar] [CrossRef]
- Law, A.; Bunnefeld, N.; Willby, N.J. Beavers and lilies: Selective herbivory and adaptive foraging behaviour. Freshw. Biol. 2014, 59, 224–232. [Google Scholar] [CrossRef]
- Busher, P.E. Food caching behavior of beavers (Castor canadensis): Selection and use of woody species. Am. Midl. Nat. 1996, 135, 343–348. [Google Scholar] [CrossRef]
- Dzięciołowski, R.; Misiukiewicz, W. Winter food caches of beavers Castor fiber in NE Poland. Acta Theriol. 2002, 47, 471–478. [Google Scholar] [CrossRef]
- Janiszewski, P.; Hanzal, V.; Weber, B.; Gugołek, A. Characteristics of riparian trees and shrubs utilized by the European beaver (Castor fiber) in the Jamy Forest District. Ann. Univ. Mariae Curie Sklodowska Zootech 2012, 30, 26–33. [Google Scholar]
- Misiukiewicz, W.; Gruszczyńska, J.; Grzegrzółka, B.; Januszewicz, M. Impact of the European beaver (Castor fiber L.) population on the woody vegetation of Wigry National Park. Sci. Ann. Pol. Soc. Anim. Prod. 2016, 12, 45–46. [Google Scholar] [CrossRef]
- John, F.; Baker, S.; Kostkan, V. Habitat selection of an expanding beaver (Castor fiber) population in central and upper Morava River basin. Eur. J. Wildl. Res. 2010, 56, 663–671. [Google Scholar] [CrossRef]
- Margaletić, J.; Grubešić, M.; Dušak, V.; Konjević, D. Activity of European beavers (Castor fiber L.) in young pedunculate oak (Quercus robur L.) forests. Vet. Arh. 2006, 76, S167–S175. [Google Scholar]
- Fryxell, J.M.; Doucet, C.M. Diet choice and the functional response of beavers. Ecology 1993, 74, 1297–1306. [Google Scholar] [CrossRef]
- McColley, S.D.; Tyers, D.B.; Sowell, B.F. Aspen and willow restoration using beaver on the northern Yellowstone winter range. Restor. Ecol. 2012, 20, 450–455. [Google Scholar] [CrossRef]
- Urban, J.; Suchomel, J.; Dvořak, J. Contribution to the knowledge of woods preferences of European beaver (Castor fiber L. 1758) in bank vegetation on non-forest land in the forest district Soutok (Czech Republic). Acta Univ. Agric. Et Silv. Mendel Brun 2008, 56, 289–294. [Google Scholar] [CrossRef]
- Danilov, P.I.; Kanishev, V.Y. The state of populations and ecological characteristics of European (Castor fiber L.) and Canadian (Castor canadensis Kuhl.) beavers in the northwestern USSR. Acta Zool. Fenn 1983, 174, 95–97. [Google Scholar]
- Nolet, B.A.; Hoekstra, A.; Ottenheim, M.M. Selective foraging on woody species by the beaver Castor fiber, and its impact on a riparian willow forest. Biol. Conserv. 1994, 70, 117–128. [Google Scholar] [CrossRef]
- Hartman, G. Habitat selection by European beaver (Castor fiber) colonizing a boreal landscape. J. Zool. 1996, 240, 317–325. [Google Scholar] [CrossRef]
- Pachinger, K.; Hulik, T. Beavers in an urban landscape. The recent activity of beavers, Castor fiber, in the Greater Bratislava Area. In Beaver Protection, Management and Utilization in Europe and North America; Busher, P., Dzięciołowski, R.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 53–60. [Google Scholar]
- Baker, S.; Fraser, D.; Kostkan, V. A modified method for appraising the suitability of urban sites in Great Britain, for use by the Eurasian (European) beaver (Castor fiber). J. Pract. Ecol. Conserv. 2006, 5, 22–37. [Google Scholar]
- Webb, A.; French, D.D.; Flitsch, A.C.C. Identification and Assessment of Possible Beaver Sites in Scotland. Survey and Monitoring Report; Report No.: 94, Contract No.: RASD/010/96/IBB; Scotish Natural Heritage: Scotland, UK, 1996. [Google Scholar]
- South, A.B.; Rushton, S.P.; Macdonald, D.W.; Fuller, R. Reintroduction of the European Beaver (Castor fiber) to Norfolk, UK: A preliminary modelling analysis. J. Zool. 2001, 254, 473–479. [Google Scholar] [CrossRef]
- Swinnen, K.R.R.; Strubbe, D.; Matthysen, E.; Leirs, H. Reintroduced Eurasian beavers (Castor fiber): Colonization and range expansion across human-dominated landscapes. Biodivers. Conserv. 2017, 26, 1863–1876. [Google Scholar] [CrossRef]
- Fustec, J.; Lode, T.; Le Jacques, D.; Cormier, J.P. Colonization, riparian habitat selection and home range size in a reintroduced population of European beavers in the Loire. Freshw. Biol. 2001, 46, 1361–1371. [Google Scholar] [CrossRef]
- Swinnen, K.R.R.; Hughes, N.K.; Leirs, H. Beaver (Castor fiber) activity patterns in a predator-free landscape. What is keeping them in the dark? Mamm. Biol. 2015, 80, 477–483. [Google Scholar] [CrossRef]
- Czyżowski, P.; Karpiński, M.; Drozd, L. Forage preferences of the European Beaver (Castor fiber L.) on urban and protected areas. Sylwan 2009, 153, 425–432. [Google Scholar]
- Jackowiak, M. Occurence of Eurasian beaver (Castor fiber) and Its Impact on Urban and Natural Environment in Piła Region. Bachelor’s Thesis, Warsaw University of Life Sciences, Warsaw, Poland, 10 July 2015. [Google Scholar]
- Jacobs, J. Quantitative measurement of food selection: A modification of the forage ratio and Ivlev’s electivity index. Oecologia 1974, 21, 157–173. [Google Scholar] [CrossRef]
- Statistica, V. 13.1; StatSoft Inc.: Tulsa, OK, USA, 2016.
- Doucet, C.M.; Adams, I.T.; Fryxell, J.M. Beaver dam and cache composition: Are woody species used differently? Ecoscience 1994, 1, 268–270. [Google Scholar] [CrossRef]
- Barnes, D.M.; Mallik, A.U. Use of woody plants in construction of beaver dams in northern Ontario. Can. J. Zool. 1996, 74, 1781–1786. [Google Scholar] [CrossRef]
- Dieter, C.D.; McCabe, T.R. Habitat use by beaver along the Big Sioux River in Eastern South Dakota. In Proceedings of the Practical Approaches to Riparian Resource Management: An Educational Workshop, Billings, MN, USA, 8–11 May 1989; Gresswell, R.E., Barton, B.A., Kershner, J.L., Eds.; Bureau of Land Management: Billings, MN, USA, 1989; pp. 135–140. [Google Scholar]
- Brzyski, J.R.; Schulte, B.A. Beaver (Castor canadensis) impacts on herbaceous and woody vegetation in Southeastern Georgia. Am. Midl. Nat. 2009, 162, 74–86. [Google Scholar] [CrossRef]
- Jenkins, S.H. Seasonal and year-to-year differences and food selection by beavers. Oecologia 1979, 144, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Gallant, D.; Bérubé, C.H.; Tremblay, E.; Vasseur, L. An extensive study of the foraging ecology of beavers (Castor canadensis) in relation to habitat quality. Can. J. Zool. 2004, 82, 922–933. [Google Scholar] [CrossRef]
- Engelhart, A.; Müller-Schwarze, D. Responses of beaver (Castor canadensis Kuhl) to predator chemicals. J. Chem. Ecol. 1995, 21, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Loeb, R.E.; King, S.; Helton, J. Human pathways are barriers to beavers damaging trees and saplings in urban forests. Urban. Urban. Gree. 2014, 13, 290–294. [Google Scholar] [CrossRef]

| Variable | Number of Points Assigned | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Average distance from roads (m) | >301 | 151–300 | 0–150 |
| Number of vehicles (N/h) | <20 | 21–100 | >100 |
| Number of pedestrians and bicycles (N/h) | <50 | 51–100 | >101 |
| Distance from the town centre (km) | >2.5 | 1–2.5 | <1 |
| Variable | Transect | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | |
| (Peripherally Located) | (Semi-Centrally Located) | (Centrally Located) | ||||
| Average distance from roads (m) | 1 | 2 | 1 | 2 | 2 | 3 |
| Number of vehicles (N/day) | 1 | 1 | 1 | 3 | 2 | 3 |
| Number of pedestrians and Bicycles (N/day) | 1 | 1 | 2 | 2 | 3 | 3 |
| Distance from town centre (km) | 1 | 1 | 2 | 2 | 3 | 3 |
| Total | 4 | 5 | 6 | 9 | 10 | 12 |
| Parameter | Transect | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | ||
| Number of woody plants | Total | 151 | 274 | 661 | 437 | 287 | 125 |
| Browsed | 22 | 75 | 576 | 373 | 214 | 82 | |
| Unbrowsed | 129 | 199 | 85 | 64 | 69 | 43 | |
| Percentage (%) of plants located within 10 m from the riverbank | Total | 38.4 | 47.1 | 95.6 | 93.1 | 86.1 | 68.0 |
| Browsed | 63.6 | 92.0 | 100.0 | 98.9 | 96.3 | 100.0 | |
| Number of species | Total | 7 | 11 | 11 | 10 | 13 | 8 |
| Browsed | 6 | 8 | 7 | 8 | 8 | 1 | |
| Unbrowsed | 1 | 3 | 4 | 2 | 5 | 7 | |
| Mean (±SD) diameter of woody plants (cm) | Total | 19.1 (±20.3) | 8.4 (±11.8) | 2.3 (±4.4) | 4.9 (±12) | 7.2 (±14.6) | 9.1 (±16.2) |
| Browsed | 10.7 | 5.9 | 1.8 | 1.9 | 2.3 | 1.5 | |
| Unbrowsed | 20.3 | 8.6 | 6.2 | 22.2 | 22.3 | 23.5 | |
| Mean variation | Total | 411.4 | 139.5 | 19.4 | 143.2 | 213.2 | 264.2 |
| Shoot Diameter (cm) | Alnus | Acer | Crataegus | Populus | Pinus | Prunus | Quercus | Salix | Sambucus | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Indice Value | ||||||||||
| <2.5 | 0.01 | 0.44 | −1.72 | −2.59 | nd | −0.40 | nd | 0.72 | −0.67 | −1.66 |
| 2.5–10 | −0.94 | 0.10 | −1.30 | −1.43 | −1.60 | −0.63 | −0.92 | −0.04 | −1.22 | −1.77 |
| >10 | −2.96 | −0.74 | −135 | −1.60 | nd | nd | nd | nd | nd | −1.15 |
| Factor | Degrees of Freedom | Coefficients | Standard Error | Wald Statistics | p-Value |
|---|---|---|---|---|---|
| Intercept | 1 | 0.3094 | 0.1095 | 4.0433 | 0.044 |
| Diameter | 1 | 0.0654 | 0.0074 | 38.8367 | <0.001 |
| Plant genus | 9 | 3.6853 | 0.8257 | 307.8458 | <0.001 |
| Anthropopression Level | 5 | −1.7431 | 2.3534 | 35.3088 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackowiak, M.; Busher, P.; Krauze-Gryz, D. Eurasian Beaver (Castor fiber) Winter Foraging Preferences in Northern Poland—The Role of Woody Vegetation Composition and Anthropopression Level. Animals 2020, 10, 1376. https://doi.org/10.3390/ani10081376
Jackowiak M, Busher P, Krauze-Gryz D. Eurasian Beaver (Castor fiber) Winter Foraging Preferences in Northern Poland—The Role of Woody Vegetation Composition and Anthropopression Level. Animals. 2020; 10(8):1376. https://doi.org/10.3390/ani10081376
Chicago/Turabian StyleJackowiak, Mateusz, Peter Busher, and Dagny Krauze-Gryz. 2020. "Eurasian Beaver (Castor fiber) Winter Foraging Preferences in Northern Poland—The Role of Woody Vegetation Composition and Anthropopression Level" Animals 10, no. 8: 1376. https://doi.org/10.3390/ani10081376
APA StyleJackowiak, M., Busher, P., & Krauze-Gryz, D. (2020). Eurasian Beaver (Castor fiber) Winter Foraging Preferences in Northern Poland—The Role of Woody Vegetation Composition and Anthropopression Level. Animals, 10(8), 1376. https://doi.org/10.3390/ani10081376




