Chemical Composition and Microstructural Morphology of Spines and Tests of Three Common Sea Urchins Species of the Sublittoral Zone of the Mediterranean Sea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
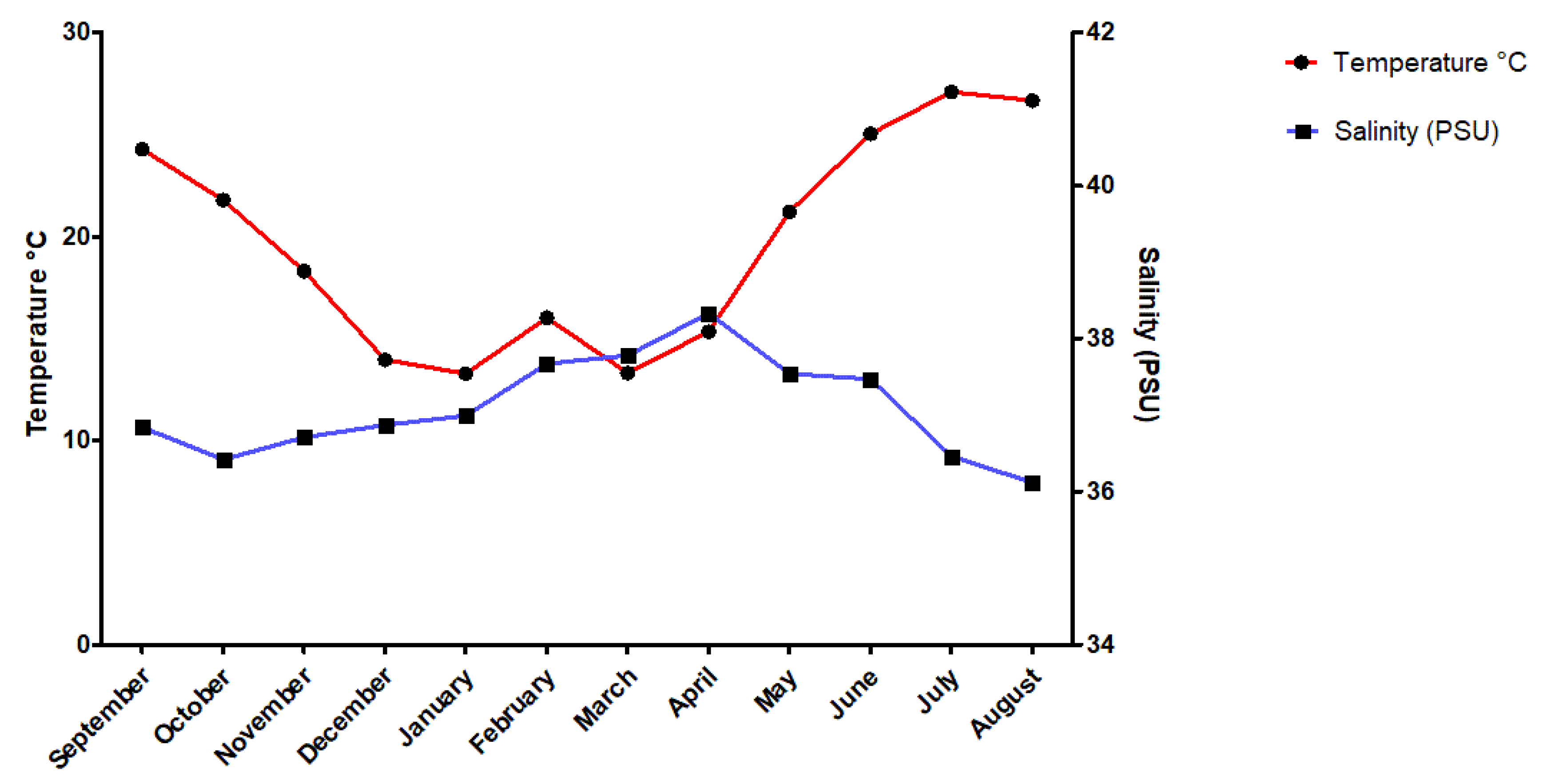
2.1. Sampling Site and Study Period
2.2. Microstructural Morphology and Elemental Analysis
2.3. X-ray Powder Diffraction Analysis
2.4. Statistical Analysis
3. Results
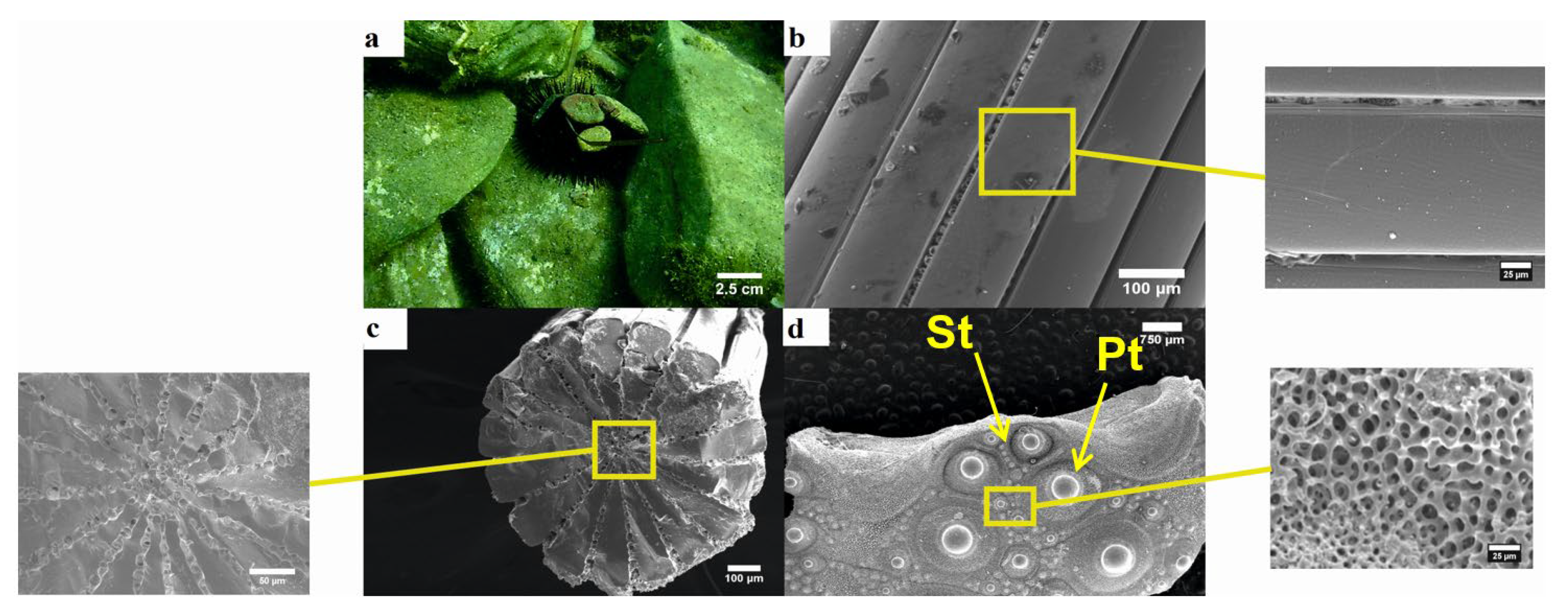
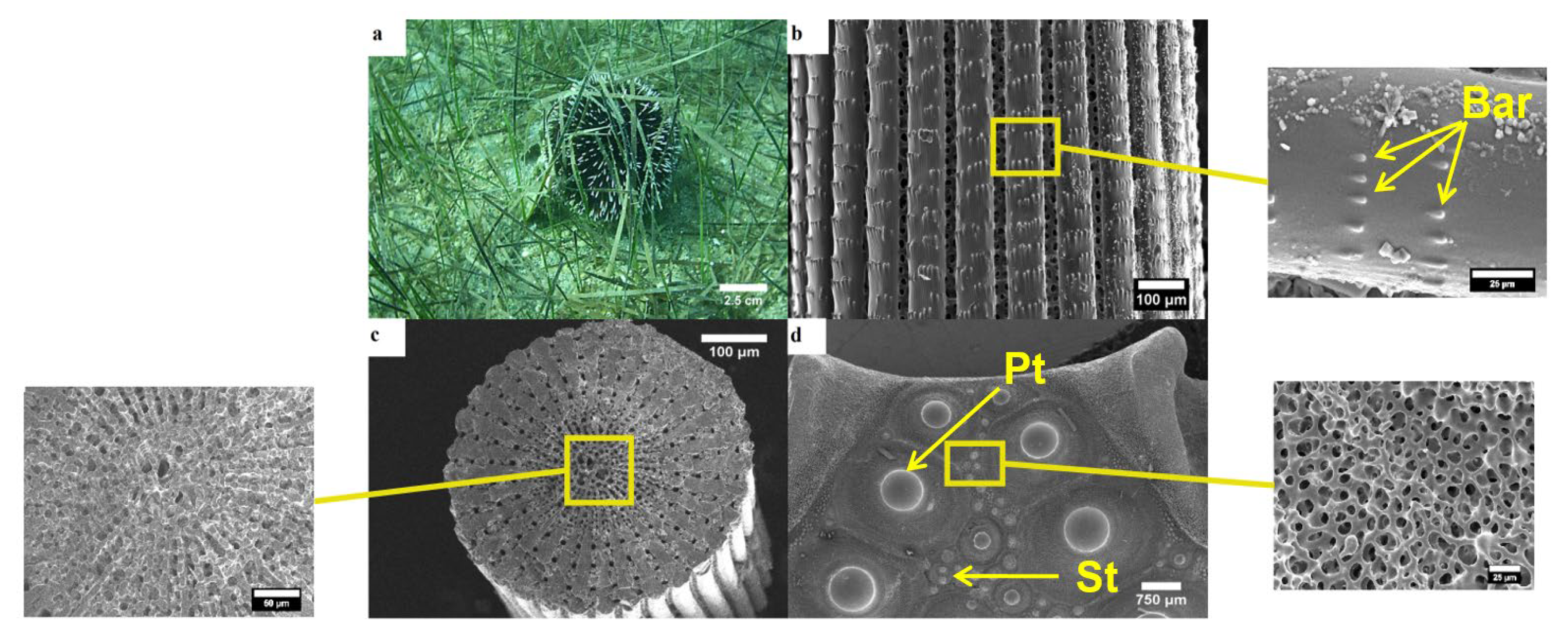
3.1. Morphology of Tests and Spines
3.1.1. Arbacia Lixula
3.1.2. Paracentrotus Lividus
3.1.3. Sphaerechinus Granularis
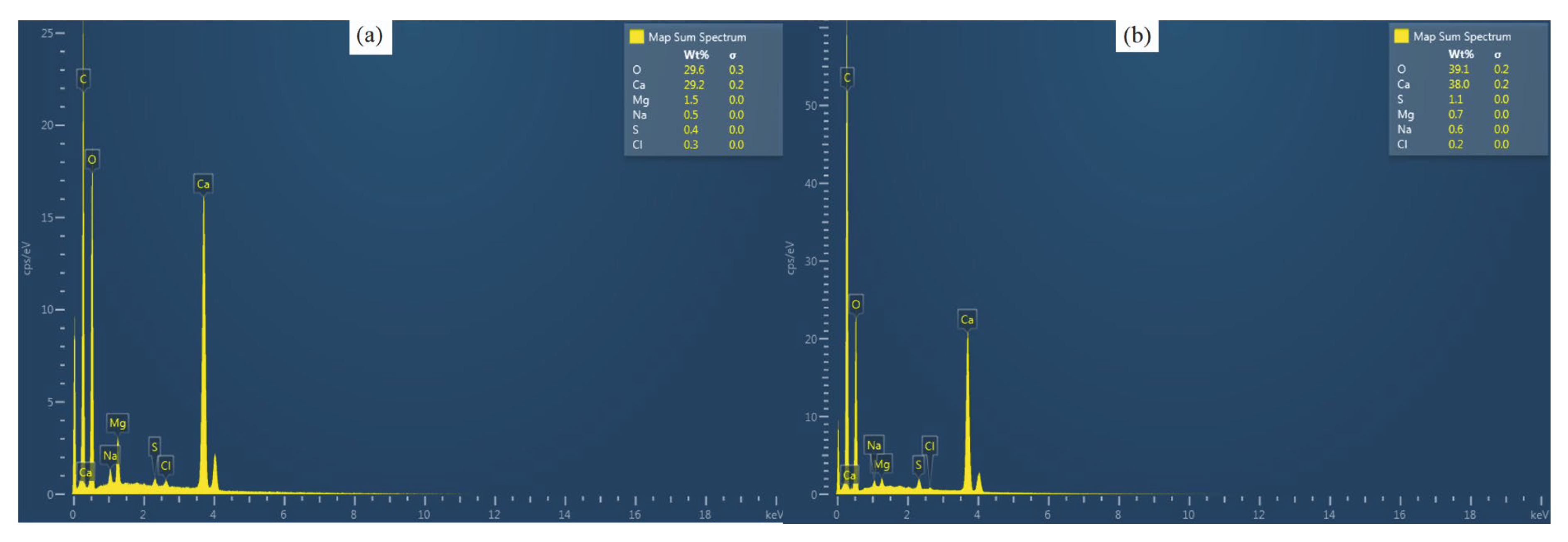
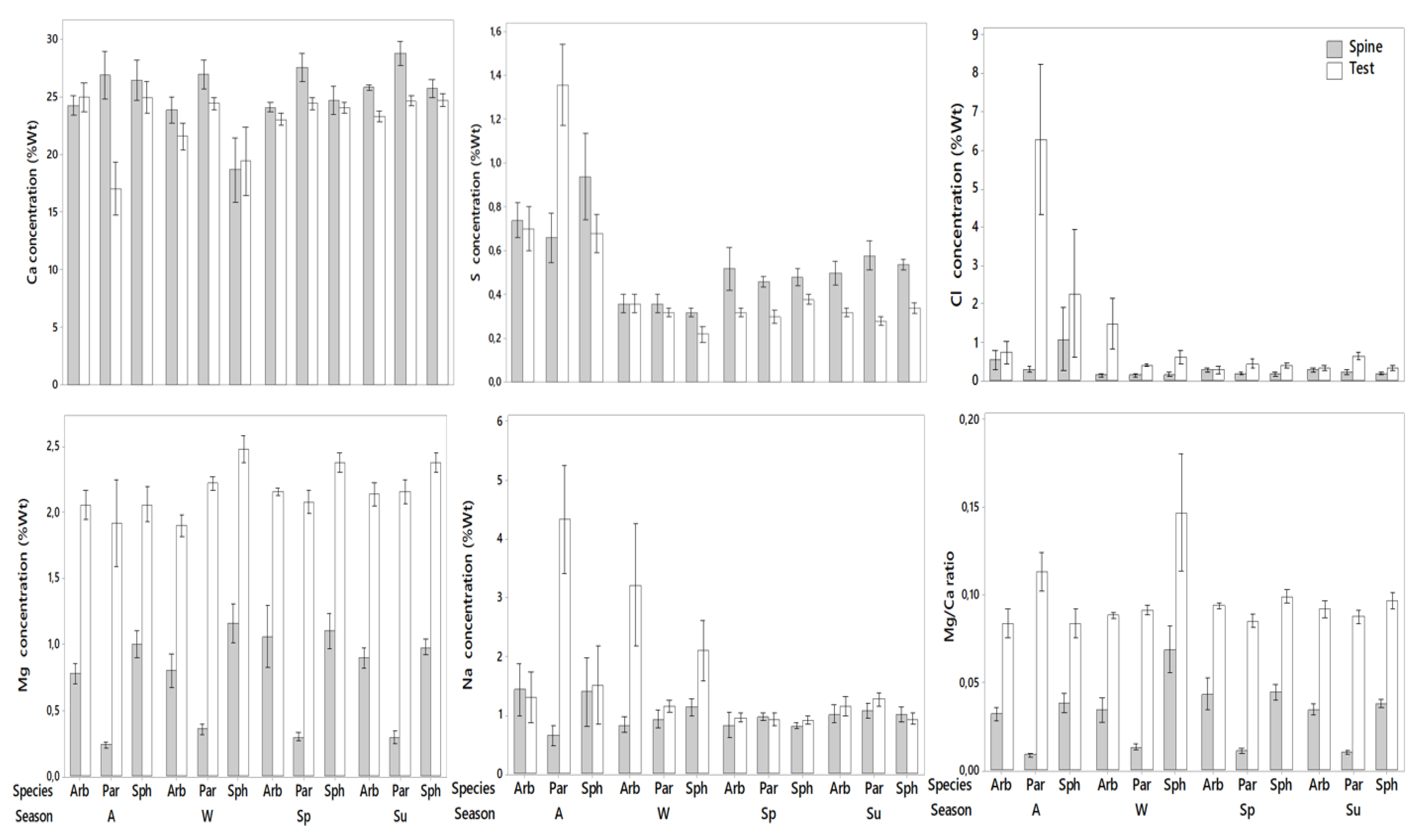
3.2. Elemental Analysis
3.2.1. Calcium
Spines
Test
3.2.2. Magnesium
Spines
Test
3.2.3. Sulfur
Spines
Test
3.2.4. Sodium
Spines
Test
3.2.5. Chlorine
Spines
Test
3.2.6. Mg/Ca Ratio
Spines
Test
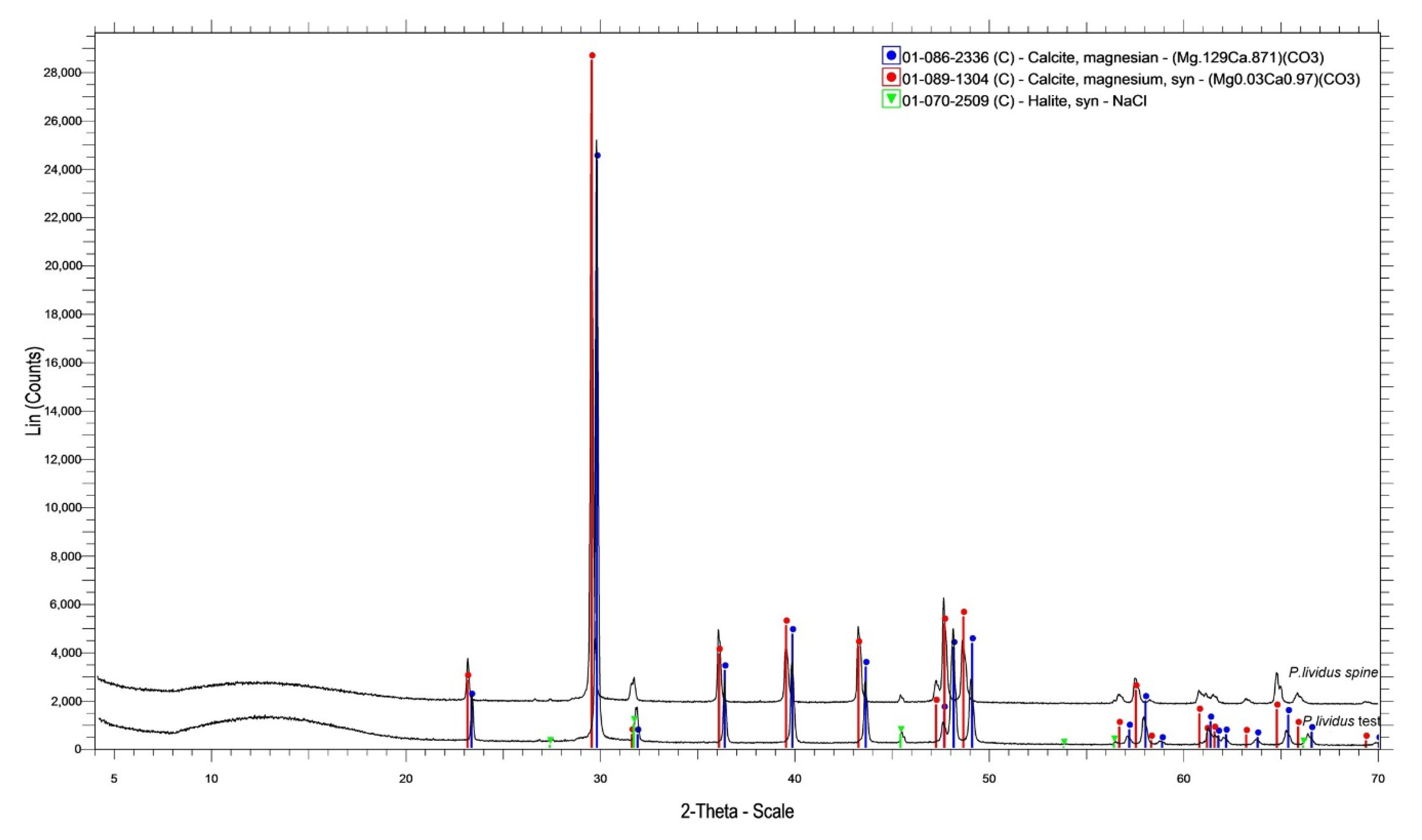
3.3. X-ray Powder Diffraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Seto, J.; Ma, Y.R.; Davis, S.A.; Meldrum, F.; Gourrier, A.; Kim, Y.Y.; Schilde, U.; Sztucki, M.; Burghammer, M.; Maltsev, S.; et al. Structure-property relationships of a biological mesocrystal in the adult sea urchin spine. Proc. Natl. Acad. Sci. USA 2012, 109, 3699–3704. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Kamat, S.; Heuer, A.H. The structure of sea urchin spines, large biogenic single crystals of calcite. J. Mater. Sci. 2000, 35, 5545–5551. [Google Scholar] [CrossRef]
- Lawrence, J.M. On the Relationships between Marine Plants and Sea Urchins. In Oceanography and Marine Biology; CRC press: Boca Raton, FL, USA, 1975. [Google Scholar]
- Tegner, M.J.; Dayton, P.K. Sea urchin recruitment patterns and implications of commercial fishing. Science 1977, 196, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Boudouresque, C.F.; Harmelin-Vivien, M. Fishing, trophic cascades, and the structure of algal assemblages: Evaluation of an old but untested paradigm. Oikos 1998, 82, 425–439. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Sini, M.; Doumas, O.; Trygonis, V.; Katsanevakis, S. Assessment of grazing effects on phytobenthic community structure at shallow rocky reefs: An experimental field study in the North Aegean Sea. J. Exp. Mar. Biol. Ecol. 2018, 503, 31–40. [Google Scholar] [CrossRef]
- Bonaviri, C.; Vega Fernández, T.; Fanelli, G.; Badalamenti, F.; Gianguzza, P. Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean. Mar. Biol. 2011, 158, 2505–2513. [Google Scholar] [CrossRef]
- Shears, N.T.; Babcock, R.C. Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 2002, 132, 131–142. [Google Scholar] [CrossRef]
- Guidetti, P.; Terlizzi, A.; Boero, F. Effects of the edible sea urchin, Paracentrotus lividus, fishery along the Apulian rocky coast (SE Italy, Mediterranean Sea). Fish. Res. 2004, 66, 287–297. [Google Scholar] [CrossRef]
- Vafidis, D.; Antoniadou, C.; Kyriakouli, K. Reproductive cycle of the edible sea urchin Paracentrotus lividus (E chinodermata: Echinoidae) in the Aegean Sea. Water 2019, 11, 1029. [Google Scholar] [CrossRef]
- Antoniadou, C.; Vafidis, D. Population structure and morphometric relationships of Paracentrotus lividus (Echinodermata: Echinoidae) in the south Aegean Sea. Cah. Biol. Mar. 2009, 50, 293–301. [Google Scholar]
- Bulleri, F. Grazing by sea urchins at the margins of barren patches on Mediterranean rocky reefs. Mar. Biol. 2013, 160, 2493–2501. [Google Scholar] [CrossRef]
- Agneta, D.; Badalmenti, F.; Ceccherelli, G.; Di Trapani, F.; Bonaviri, C.; Gianguzza, P. Role of two co-occurring Mediterranean sea urchins in the formation of barren from Cystoseira canopy. Estuar. Coast. Shelf Sci. 2015, 152, 73–77. [Google Scholar] [CrossRef]
- Smith, A.B. Stereom Microstructure of the Echinoid Test; Special Papers in Palaeontology: London, UK, 1980; pp. 1–81. [Google Scholar]
- Weber, J.; Greer, R.; Voight, V.; White, E.; Rustum, R. Unusual strength properties of echinoderm calcite related to structure. J. Ultrastruct. Res. 1969, 26, 355–366. [Google Scholar] [CrossRef]
- Weiner, S. Organization of extracellularly mineralized tissues: A comparative study of biological crystal growth. Crc. Crit. Rev. Biochem. 1986, 20, 365–408. [Google Scholar] [CrossRef]
- Dubois, P. The skeleton of postmetamorphic echinoderms in a changing world. Biol. Bull. 2014, 226, 223–236. [Google Scholar] [CrossRef]
- Emerson, C.E.; Reinardy, H.C.; Bates, N.R.; Bodnar, A.G. Ocean acidification impacts spine integrity but not regenerative capacity of spines and tube feet in adult sea urchins. R. Soc. Open Sci. 2017, 4, 170140. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.J.; Mackenzie, F.T.; Bates, N.R. Life on the margin: Implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar. Ecol. Prog. Ser. 2008, 373, 265–273. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Yool, A. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Dupont, S.; Dorey, N.; Thorndyke, M. What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar. Coast. Shelf Sci. 2010, 89, 182–185. [Google Scholar] [CrossRef]
- Smith, A.M.; Clark, D.E.; Lamare, M.D.; Winter, D.J.; Byrne, M. Risk and resilience: Variations in magnesium in echinoid skeletal calcite. Mar. Ecol. Prog. Ser. 2015, 561, 1–16. [Google Scholar] [CrossRef]
- Albéric, M.; Caspi, E.N.; Bennet, M.; Ajili, W.; Nassif, N.; Azaïs, T.; Berner, A.; Fratzl, P.; Zolotoyabko, E.; Bertinetti, L.; et al. Interplay between Calcite, Amorphous Calcium Carbonate, and Intracrystalline Organics in Sea Urchin Skeletal Elements. Cryst. Growth Des. 2018, 18, 2189–2201. [Google Scholar] [CrossRef]
- Albéric, M.; Stifler, C.A.; Zou, Z.; Sun, C.; Killian, C.E.; Valencia, S.; Mawass, M.; Bertinetti, L.; Gilbert, P.U.; Politi, Y. Growth and regrowth of adult sea urchin spines involve hydrated and anhydrous amorphous calcium carbonate precursors. J. Struct. Biol. 2018, 1, 100004. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.L.; Sharmankina, V.V.; Zemnukhova, L.A.; Polyakova, N.V. Chemical composition of spines and tests of sea urchins. Biol. Bull. Russ. Acad Sci. 2016, 43, 521–531. [Google Scholar] [CrossRef]
- Kanold, J.M.; Guichard, N.; Immel, F.; Plasseraud, L.; Corneillat, M.; Alcaraz, G.; Brummer, F.; Marin, F. Spine and test skeletal matrices of the Mediterranean sea urchin Arbacia lixula- a comparative characterization of their sugar signature. FEBS J. 2015, 282, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Magdans, U.; Gies, H. Single crystal structure analysis of sea urchin spine calcites: Systematic investigations of the Ca/Mg distribution as a function of habitat of the sea urchin and the sample location in the spine. Eur. J. Miner. 2004, 16, 261–268. [Google Scholar] [CrossRef]
- Bischoff, W.D.; Bishop, F.C.; Mackenzie, F.T. Biogenically produced magnesian calcite: Inhomogeneities in chemical and physical properties: Comparison with synthetic phases. Am. Mineral. 1983, 68, 1183–1188. [Google Scholar]
- Carpenter, S.J.; Lohmann, K.C. Ratios of modern marine calcite: Empirical indicators of ocean chemistry and precipitation rate. Geochim. Cosmochim. Acta 1992, 56, 1837–1849. [Google Scholar] [CrossRef]
- Chave, K.E. Aspects of the Biogeochemistry of Magnesium 1. Calcareous Marine Organisms. J. Geol. 1954, 62, 266–283. [Google Scholar] [CrossRef]
- Petihakis, G.; Triantafyllou, G.; Pollani, A.; Koliou, A.; Theodorou, A. Field data analysis and application of a complex water column biogeochemical model in different areas of a semi-enclosed basin: Towards the development of an ecosystem management tool. Mar. Environ. Res. 2005, 59, 493–518. [Google Scholar] [CrossRef]
- Byrne, M.; Smith, A.M.; West, S.; Collard, M.; Dubois, P.; Graba-Landry, A.; Dworjanyn, S.A. Warming Influences Mg2+ Content, While Warming and Acidification Influence Calcification and Test Strength of a Sea Urchin. Enviromental Sci. Technol. 2014, 48, 12620–12627. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Northern Illinois University: DeKalb, IL, USA, 2010. [Google Scholar]
- Martinez-Pita, I.; Sanchez-Espagna, A.I.; Garcia, F.J. Gonadal growth and reproduction in the sea urchin Sphaerechinus granularis (Lamarck 1816) (Echinodermata: Echinoidea) in Southern Spain. Sci. Mar. 2008, 72, 603–611. [Google Scholar]
- Lauer, C.; Grun, T.B.; Zutterkirch, I.; Jemmali, R.; Nebelsick, J.H.; Nickel, K.G. Morphology and porosity of the spines of the sea urchin Heterocentrotus mamillatus and their implications on the mechanical performance. Zoomorphology 2017, 137, 139–154. [Google Scholar] [CrossRef]
- Wainwright, S.A.; Biggs, W.D.; Currey, J.D.; Gosline, J.M. Mechanical Design in Organisms; Princeton University Press: Princeton, NJ, USA, 1976. [Google Scholar]
- Grossmann, J.N.; Nebelsick, J.H. Comparative morphological and structural analysis of selected cidaroid and camarodont sea urchin spines. Zoomorphology 2013, 132, 301–315. [Google Scholar] [CrossRef]
- Moureaux, C.; Pérez-Huerta, A.; Compère, P.; Zhu, W.; Leloup, T.; Cusask, M.; Dubois, P. Structure, composition and mechanical relations to function in sea urchin spine. J. Struct. Biol. 2010, 170, 41–49. [Google Scholar] [CrossRef]
- Hermans, J.; Borremans, C.; Willenz, P.; Andre´, L.; Dubois, P. Temperature, salinity and growth rate dependences of Mg/Ca and Sr/Ca ratios of the skeleton of the sea urchin Paracentrotus lividus (Lamarck): An experimental approach. Mar. Biol. 2010, 157, 1293–1300. [Google Scholar] [CrossRef]
- Kołbuk, D.; Dubois, P.; Stolarski, J.; Gorzelak, P. Effects of seawater chemistry (Mg2+/Ca2+ ratio) and diet on the skeletal Mg/Ca ratio in the common sea urchin Paracentrotus lividus. Mar. Env. Res. 2019, 145, 22–26. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Ecology of Paracentrotus lividus. In Edible Sea Urchins: Biology and Ecology; Lawrene, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Ma, Y.; Aichmayer, B.; Paris, O.; Fratzl, P.; Meibom, A.; Metzler, R.A.; Politi, Y.; Adaddi, L.; Gilbert, P.U.; Weiner, S. The grinding tip of the sea urchin tooth exhibits exquisite control over calcite crystal orientation and Mg distribution. Proc. Natl. Acad. Sci. USA 2009, 106, 6048–6053. [Google Scholar] [CrossRef]
- Walter, L.M.; Morse, J.W. Mg-calcite stabilities: A re-evaluation. Geochim. Cosmochim. Acta 1984, 48, 1059–1069. [Google Scholar] [CrossRef]
- Bischoff, W.D.; Mackenzie, F.T.; Bishop, F.C. Stabilities of synthetic Mg-calcites in aqueous solution: Comparison with biogenic materials. Geochim. Cosmochim. Acta 1987, 51, 1413–1423. [Google Scholar] [CrossRef]
- Morse, J.W.; Andersson, A.J.; Mackenzie, F.T. Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and “ocean acidification”: Role of high Mg-calcites. Geochim. Cosmochim. Acta 2006, 70, 5814–5830. [Google Scholar] [CrossRef]
- Asnaghi, V.; Chiantore, M.; Mangialajo, L.; Gazeau, F.; Francour, P.; Alliouane, S.; Gattuso, J.P. Cascading effects of ocean acidification in a rocky subtidal community. PLoS ONE 2013, 8, 61978. [Google Scholar] [CrossRef] [PubMed]
- Hall-Spencer, J.M.; Rodolfo-Metalpa, R.; Martin, S.; Ransome, E.; Fine, M.; Turner, S.M.; Rowley, S.J.; Tedesco, D.; Buia, M.C. Volcanic carbon dioxide vents reveal ecosystem effects of ocean acidification. Nature 2008, 454, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Calosi, P.; Rastrick, S.P.S.; Graziano, M.; Thomas, S.C.; Baggini, C.; Carter, H.A.; Hall-Spencer, J.M. Distribution of sea urchins living near shallow water CO2 vents is dependent upon speciesacid−base and ion-regulatory abilities. Mar. Pollut. Bull. 2013, 73, 470–484. [Google Scholar] [CrossRef] [PubMed]

| Chemical Elements | p | F or H | |
|---|---|---|---|
| Ca | Test | 0.5861 | H = 1.069 |
| Spine | 0.001 | H = 13.767 | |
| Mg | Test | 0.0168 | H = 8.19 |
| Spine | 0.0001 | H = 39.674 | |
| S | Test | 0.172 | H = 3.511 |
| Spine | 0.3389 | F = 1.103 | |
| Na | Test | 0.3323 | H = 2.204 |
| Spine | 0.5761 | F = 0.5568 | |
| Cl | Test | 0.0952 | H = 4.705 |
| Spine | 0.1356 | H = 3.996 | |
| Mg/Ca | Test | 0.047 | H = 6.08 |
| Spine | 0.0001 | H = 40.538 | |
| Chemical Elements | A. lixula | P. lividus | S. granularis | ||||
|---|---|---|---|---|---|---|---|
| p | F or H | p | F or H | p | F or H | ||
| Ca | Test | 0.7543 | F = 0.4009 | 0.001 | F = 9.036 | 0.479 | F = 3.280 |
| Spine | 0.07 | F = 2.84 | 0.19 | F = 1.738 | 0.038 | F = 3.553 | |
| Mg | Test | 0.2766 | F = 1.409 | 0.207 | F = 1.7 | 0.0053 | F = 6.206 |
| Spine | 0.479 | F = 0.865 | 0.11 | F = 2.269 | 0.1286 | F = 2.193 | |
| S | Test | 0.379 | H = 3.081 | 0.145 | H = 5.389 | 0.044 | H = 8.056 |
| Spine | 0.013 | F = 4.847 | 0.13 | F = 2.15 | 0.01 | F = 11.311 | |
| Na | Test | 0.03 | F = 3.546 | 0.0003 | F = 11.368 | 0.618 | F = 2.996 |
| Spine | 0.772 | H = 1.119 | 0.4678 | H = 2.542 | 0.6366 | F = 0.58 | |
| Cl | Test | 0.103 | F = 2.424 | 0.0001 | F = 16.404 | 0.4529 | H = 2.621 |
| Spine | 0.2518 | F = 1.503 | 0.3357 | H = 3.387 | 0.93 | H = 0.4222 | |
| Mg/Ca | Test | 0.7294 | F = 0.4373 | 0.0002 | F = 12.576 | 0.1653 | F = 1.931 |
| Spine | 0.5873 | F = 0.6622 | 0.419 | F = 0.9983 | 0.0587 | F = 3.055 | |
| Sample | Chemical Composition of Crystalline Phase | |
|---|---|---|
| A. lixula | Test | Magnesian calcite (Mg0.06Ca0.94)CO3, NaCl |
| spine | Magnesian calcite (Mg0.064Ca0.936)CO3, NaCl | |
| P. lividus | Test | Magnesian calcite (Mg0.129Ca0.871)CO3, NaCl |
| spine | Magnesian calcite (Mg0.03Ca0.97)CO3, NaCl | |
| S. granularis | Test | Magnesian calcite (Mg0.06Ca0.94)CO3, NaCl |
| spine | Magnesian calcite (Mg0.06Ca0.94)CO3, NaCl | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varkoulis, A.; Voulgaris, K.; Zaoutsos, S.; Stratakis, A.; Vafidis, D. Chemical Composition and Microstructural Morphology of Spines and Tests of Three Common Sea Urchins Species of the Sublittoral Zone of the Mediterranean Sea. Animals 2020, 10, 1351. https://doi.org/10.3390/ani10081351
Varkoulis A, Voulgaris K, Zaoutsos S, Stratakis A, Vafidis D. Chemical Composition and Microstructural Morphology of Spines and Tests of Three Common Sea Urchins Species of the Sublittoral Zone of the Mediterranean Sea. Animals. 2020; 10(8):1351. https://doi.org/10.3390/ani10081351
Chicago/Turabian StyleVarkoulis, Anastasios, Konstantinos Voulgaris, Stefanos Zaoutsos, Antonios Stratakis, and Dimitrios Vafidis. 2020. "Chemical Composition and Microstructural Morphology of Spines and Tests of Three Common Sea Urchins Species of the Sublittoral Zone of the Mediterranean Sea" Animals 10, no. 8: 1351. https://doi.org/10.3390/ani10081351
APA StyleVarkoulis, A., Voulgaris, K., Zaoutsos, S., Stratakis, A., & Vafidis, D. (2020). Chemical Composition and Microstructural Morphology of Spines and Tests of Three Common Sea Urchins Species of the Sublittoral Zone of the Mediterranean Sea. Animals, 10(8), 1351. https://doi.org/10.3390/ani10081351






