Supplementation of Spring Pasture with Harvested Fodder Beet Bulb Alters Rumen Fermentation and Increases Risk of Subacute Ruminal Acidosis during Early Lactation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Feed Management
r2 = 0.755, n = 357, p < 0.0001.
2.3. Plant Sub-Sampling and Analyses
2.4. Animal Samples and Analyses
2.5. Statistical Analysis
3. Results
3.1. Feed Measurements
3.2. Estimated Intake and Milk Production
3.3. Milk Fatty Acids
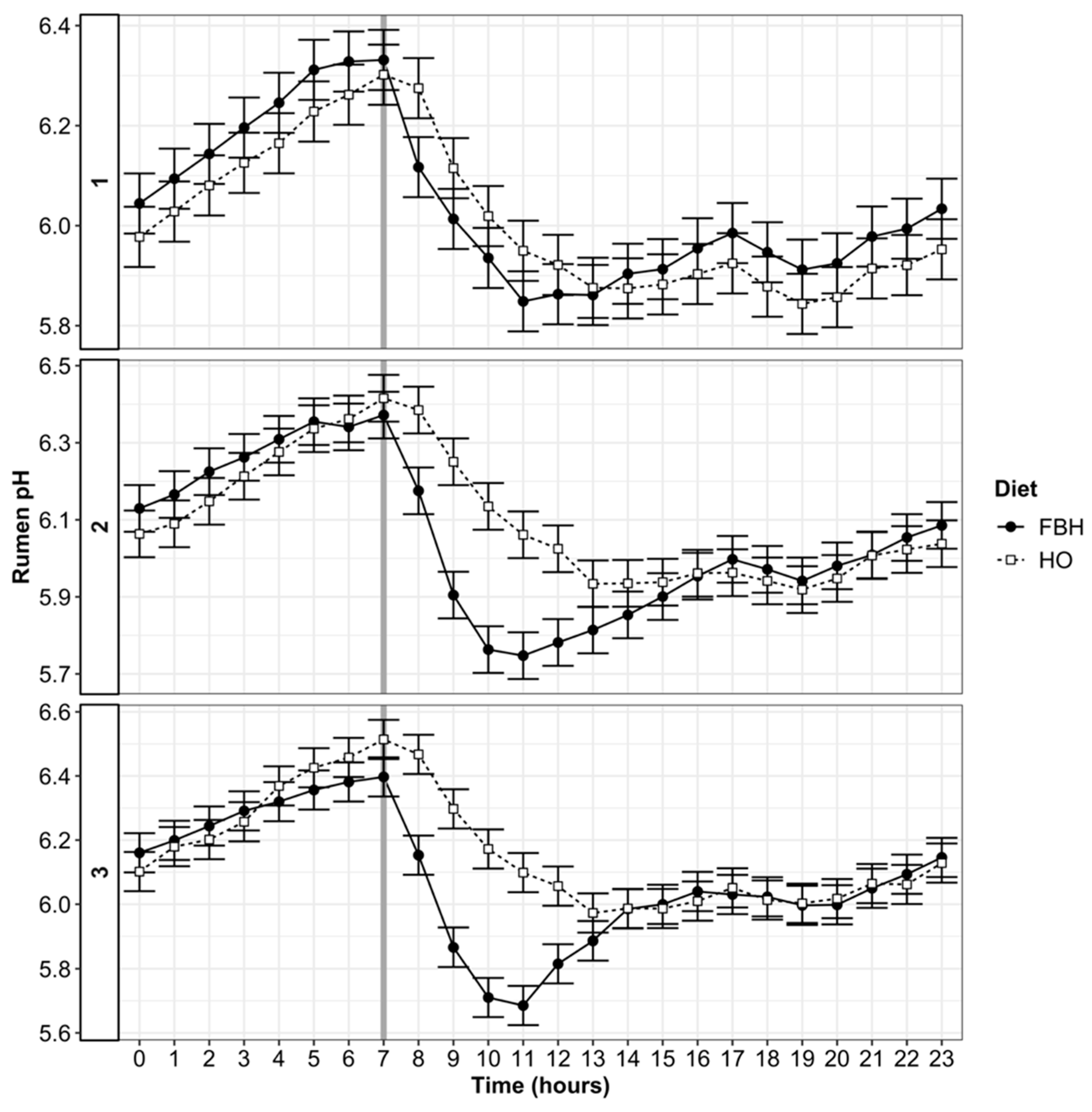
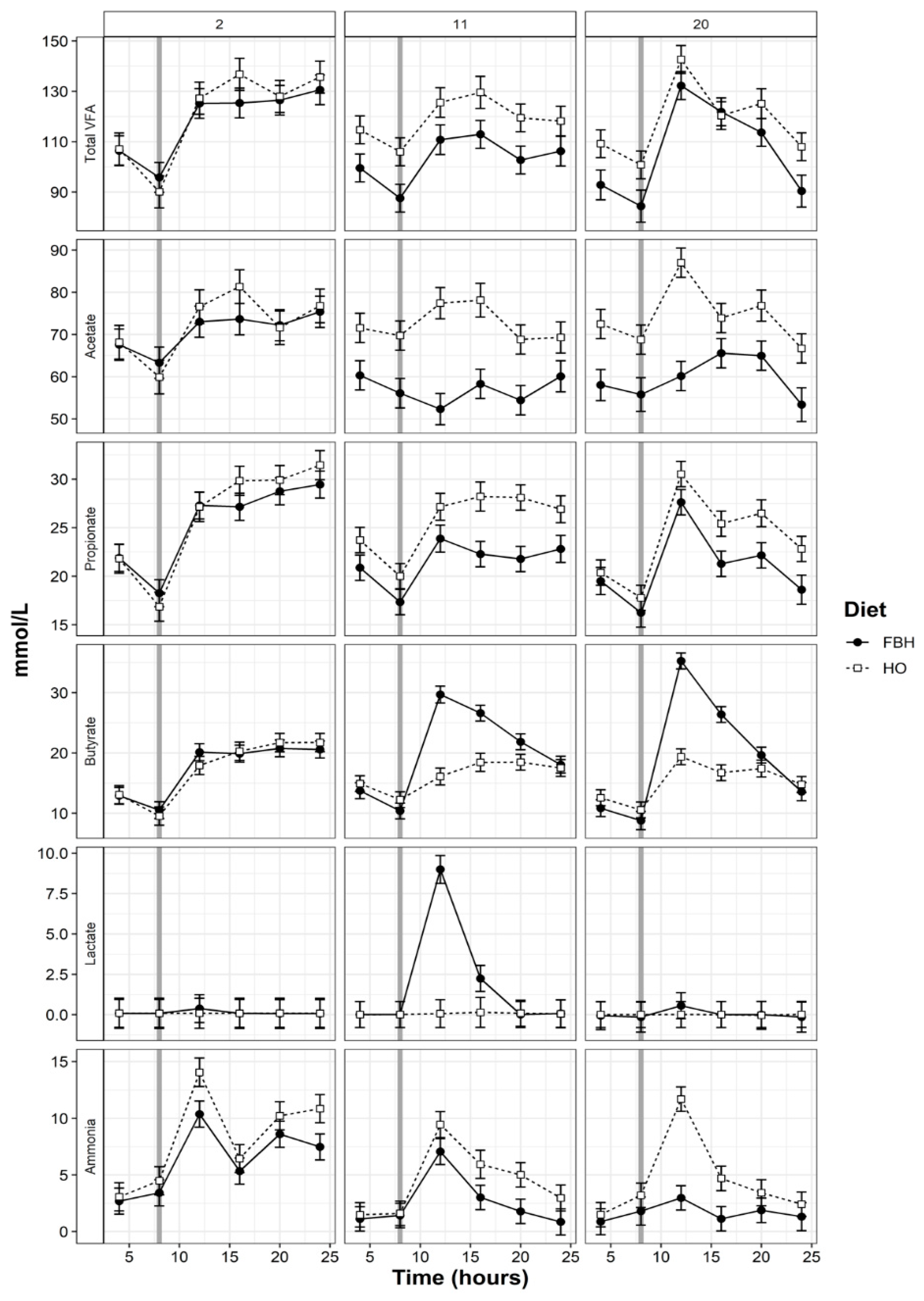
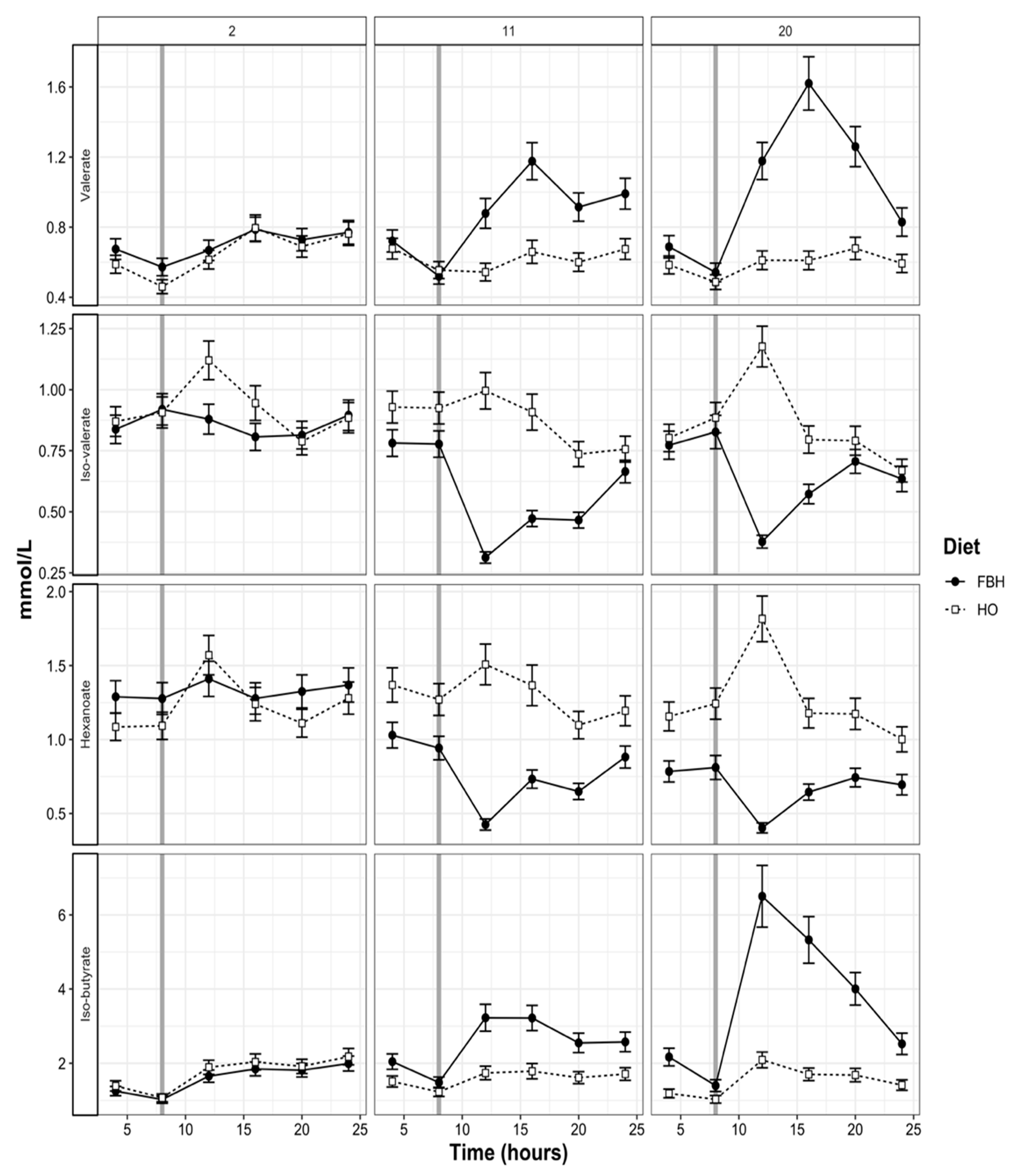
3.4. Rumen pH and VFA Patterns
3.5. Plasma Amino Acids
4. Discussion
4.1. Rumen pH and SARA
4.2. Patterns of Rumen Fermentation
4.3. Rumen Adaptation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, P.; Givens, D.I.; Brunnen, J.M. The chemical composition, digestibility and energy value of fodder-beet roots. Anim. Feed Sci. Technol. 1987, 18, 225–231. [Google Scholar] [CrossRef]
- Chakwizira, E.; Meenken, E.D.; Maley, S.; George, M.; Hubber, R.; Morton, J.; Stafford, A. Effects of potassium, sodium and chloride fertiliser rates on fodder beet yield and quality in Canterbury. Proc. N. Z. Grassl. Assoc. 2013, 75, 261–270. [Google Scholar] [CrossRef]
- Saldias, B.; Gibbs, S.J. Brief Communication: Ad libitum fodder-beet and pasture beef finishing systems–intake, utilisation, grazing behaviour and liveweight gains. Proc. N. Z. Soc. Anim. Prod. 2016, 76, 87–89. [Google Scholar]
- Fleming, A.E.; Edwards, G.R.; Bryant, R.H.; Dalley, D.; Gregorini, P. Milk production and milk fatty acid composition of grazing dairy cows supplemented with fodder beet. N. Z. J. Anim. Sci. Prod. 2018, 78, 6–10. [Google Scholar]
- Dalley, D.; Waugh, D.; Griffin, A.; Higham, C.; De Ruiter, J.M.; Malcolm, B. A comparison of fodder beet and maize silage as supplements to pasture in late lactation to increase milk production and reduce urinary nitrogen excretion. N. Z. J. Agric. Res. 2019, 63, 145–164. [Google Scholar] [CrossRef]
- Pacheco, D.; Muetzel, S.; Lewis, S.; Dalley, D.; Bryant, M.; Waghorn, G.C. Rumen digesta and products of fermentation in cows fed varying proportions of fodder beet (beta vulgaris L.) with fresh pasture or silage or straw. Anim. Prod. Sci. 2020, 60, 524–534. [Google Scholar] [CrossRef]
- Bargo, F.; Muller, L.D.; Kolver, E.S.; Delahoy, J.E. Invited review: Production and digestion of supplemented dairy cows on pasture. J. Dairy Sci. 2003, 86, 1–42. [Google Scholar] [CrossRef]
- Stockdale, C.R. Levels of pasture substitution when concentrates are fed to grazing dairy cows in northern Victoria. Anim. Prod. Sci. 2000, 40, 913–921. [Google Scholar] [CrossRef]
- Dalley, D.E.; Malcolm, B.J.; Chakwizira, E.; de Ruiter, J.M. Range of quality characteristics of New Zealand forages and implications for reducing the nitrogen leaching risk from grazing dairy cows. N. Z. J. Agric. Res. 2017, 60, 319–332. [Google Scholar] [CrossRef]
- Gibbs, J. Fodder beet in the New Zealand dairy industry. In The South Island Dairy Event; Stadium Southland: Invercargil, New Zealand, 2014; pp. 1–8. [Google Scholar]
- Waghorn, G.C.; Law, N.; Bryant, M.; Pacheco, D.; Dalley, D. Digestion and nitrogen excretion by Holstein–Friesian cows in late lactation offered ryegrass-based pasture supplemented with fodder beet. Anim. Prod. Sci. 2019, 59, 1261–1270. [Google Scholar] [CrossRef]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. Acidosis in cattle: A review. J. Anim. Sci. 1998, 76, 275–286. [Google Scholar] [CrossRef]
- Kleen, J.L.; Hooijer, G.A.; Rehage, J.; Noordhuizen, J.P.T.M. Subacute ruminal acidosis (SARA): A review. Transbound. Emerg. Dis. 2003, 50, 406–414. [Google Scholar] [CrossRef]
- Nocek, J.E. Bovine acidosis: Implications on laminitis. J. Dairy Sci. 1997, 80, 1005–1028. [Google Scholar] [CrossRef]
- Steele, M.A.; AlZahal, O.; Hook, S.E.; Croom, J.; McBride, B.W. Ruminal acidosis and the rapid onset of ruminal parakeratosis in a mature dairy cow: A case report. Acta Vet. Scand. 2009, 51, 39. [Google Scholar] [CrossRef]
- Zebeli, Q.; Metzler-Zebeli, B.U.; Ametaj, B.N. Meta-analysis reveals threshold level of rapidly fermentable dietary concentrate that triggers systemic inflammation in cattle. J. Dairy Sci. 2012, 95, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- DeVries, T.J.; Beauchemin, K.A.; Dohme, F.; Schwartzkopf-Genswein, K.S. Repeated ruminal acidosis challenges in lactating dairy cows at high and low risk for developing acidosis: Feeding, ruminating and lying behavior. J. Dairy Sci. 2009, 92, 5067–5078. [Google Scholar] [CrossRef] [PubMed]
- Dohme, F.; DeVries, T.J.; Beauchemin, K.A. Repeated Ruminal Acidosis Challenges in Lactating Dairy Cows at High and Low Risk for Developing Acidosis: Ruminal pH. J. Dairy Sci. 2008, 91, 3554–3567. [Google Scholar] [CrossRef]
- Bull, L.S.; Bush, L.J.; Friend, J.D.; Harris Jr, B.; Jones, E.W. Incidence of ruminal parakeratosis in calves fed different rations and its relation to volatile fatty acid absorption. J. Dairy Sci. 1965, 48, 2459–2466. [Google Scholar] [CrossRef]
- Allen, M.S. Relationship Between Fermentation Acid Production in the Rumen and the Requirement for Physically Effective Fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Bartley, E.E.; Fina, L.R.; Anthony, H.D. Relationship of rumen gram-negative bacteria and free endotoxin to lactic acidosis in cattle. J Anim Sci 1978, 47, 1329–1337. [Google Scholar] [CrossRef]
- Gozho, G.N.; Plaizier, J.C.; Krause, D.O.; Kennedy, A.D.; Wittenberg, K.M. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 2005, 88, 1399–1403. [Google Scholar] [CrossRef]
- Zebeli, Q.; Metzler-Zebeli, B.U. Interplay between rumen digestive disorders and diet-induced inflammation in dairy cattle. Res. Vet. Sci. 2012, 93, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, L.; Doherty, M.L.; Mulligan, F.J. Subacute ruminal acidosis (SARA) in grazing Irish dairy cows. Vet. J. 2008, 176, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.L.; Cannizzo, C. Incidence, prevalence and impact of SARA in dairy herds. Anim. Feed Sci. Technol. 2012, 172, 4–8. [Google Scholar] [CrossRef]
- Fulton, W.R.; Klopfenstein, T.J.; Britton, R.A. Adaptation to high concentrate diets by beef cattle. I. Adaptation to corn and wheat diets. J. Anim. Sci. 1979, 49, 775–784. [Google Scholar] [CrossRef]
- Danscher, A.M.; Li, S.; Andersen, P.H.; Khafipour, E.; Kristensen, N.B.; Plaizier, J.C. Indicators of induced subacute ruminal acidosis (SARA) in Danish Holstein cows. Acta Vet. Scand. 2015, 57, 39. [Google Scholar] [CrossRef]
- Penner, G.B.; Taniguchi, M.; Guan, L.L.; Beauchemin, K.A.; Oba, M. Effect of dietary forage to concentrate ratio on volatile fatty acid absorption and the expression of genes related to volatile fatty acid absorption and metabolism in ruminal tissue. J. Dairy Sci. 2009, 92, 2767–2781. [Google Scholar] [CrossRef]
- Duffield, T.; Plaizier, J.C.; Fairfield, A.; Bagg, R.; Vessie, G.; Dick, P.; Wilson, J.; Aramini, J.; McBride, B. Comparison of techniques for measurement of rumen pH in lactating dairy cows. J. Dairy Sci 2004, 87, 59–66. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef]
- DairyNZ. Fodder Beet Feeding Practices and Industry Issues; DairyNZ: Hamilton, New Zealand, 2017. [Google Scholar]
- Prendergast, S.L.; Gibbs, S.J. A comparison of microbial protein synthesis in beef steers fed ad libitum winter ryegrass or fodder beet. Proc. N. Z. Soc. Anim. Prod. 2015, 75, 251–256. [Google Scholar]
- Penner, G.B.; Beauchemin, K.A.; Mutsvangwa, T. Severity of ruminal acidosis in primiparous Holstein cows during the periparturient period. J. Dairy Sci. 2007, 90, 365–375. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Collier, K.; Bryant, M.; Dalley, D.E. Feeding fodder beet (Beta vulgaris L.) with either barley straw or pasture silage to non-lactating dairy cows. N. Z. Vet. J. 2018, 66, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.; Ellis, J.L.; Kebreab, E.; Strathe, A.B.; López, S.; France, J.; Bannink, A. Ruminal pH regulation and nutritional consequences of low pH. Anim. Feed Sci. Technol. 2012, 172, 22–33. [Google Scholar] [CrossRef]
- Rego, O.A.; Cabrita, A.R.; Rosa, H.J.; Alves, S.P.; Duarte, V.; Fonseca, A.J.; Bessa, R.J. Changes in milk production and milk fatty acid composition of cows switched from pasture to a total mixed ration diet and back to pasture. Ital. J. Anim. Sci. 2016, 15, 76–86. [Google Scholar] [CrossRef]
- Senn, S. Cross-Over Trials in Clinical Research; John Wiley & Sons: Hoboken, NJ, USA, 2002; Volume 5. [Google Scholar]
- AOAC. Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (2003.05). J. AOAC Int. 2003, 86, 888–898. [Google Scholar] [CrossRef]
- CSIRO. Nutrient Requirements of Domesticated Ruminants; CSIRO Publishing: Clayton, Australia, 2007. [Google Scholar]
- Heems, D.; Luck, G.; Fraudeau, C.; Vérette, E. Fully automated precolumn derivatization, on-line dialysis and high-performance liquid chromatographic analysis of amino acids in food, beverages and feedstuff. J. Chromatogr. A 1998, 798, 9–17. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Lenth, R.V. Using Lsmeans. CRAN. 2018. Available online: https://cran.r-project.org/web/packages/lsmeans/lsmeans.pdf (accessed on 26 June 2020).
- Krajcarski-Hunt, H.; Plaizier, J.C.; Walton, J.P.; Spratt, R.; McBride, B.W. Effect of subacute ruminal acidosis on in situ fiber digestion in lactating dairy cows. J. Dairy Sci. 2002, 85, 570–573. [Google Scholar] [CrossRef]
- Gozho, G.N.; Krause, D.O.; Plaizier, J.C. Rumen Lipopolysaccharide and Inflammation During Grain Adaptation and Subacute Ruminal Acidosis in Steers. J. Dairy Sci. 2006, 89, 4404–4413. [Google Scholar] [CrossRef]
- Aquilani, R.; Maestri, R.; Boselli, M.; Achilli, M.P.; Arrigoni, N.; Bruni, M.; Barbieri, A. The relationship between plasma amino acids and circulating albumin and haemoglobin in postabsorptive stroke patients. PLoS ONE 2019, 14, e0219756. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, X.; Zou, Y.; Yang, Z.; Li, S.; Cao, Z. Changes in feed intake, nutrient digestion, plasma metabolites, and oxidative stress parameters in dairy cows with subacute ruminal acidosis and its regulation with pelleted beet pulp. J. Anim. Sci. Biotechnol. 2013, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- Cassida, K.A.; Stokes, M.R. Eating and resting salivation in early lactation dairy cows. J. Dairy Sci. 1996, 69, 1282–1292. [Google Scholar] [CrossRef]
- Coombe, J.E.; Pyman, M.F.; Mansell, P.D.; Auldist, M.J.; Anderson, G.A.; Wales, W.J.; Conley, M.J.; Manos, S.; Hannah, M.; Fisher, A.D. The effects on ruminal pH and serum haptoglobin after feeding a grain-based supplement to grazing dairy cows as a partial mixed ration or during milking. Vet. J. 2015, 204, 105–109. [Google Scholar] [CrossRef]
- Illius, A.W.; Jessop, N.S. Metabolic constraints on voluntary intake in ruminants. J. Anim. Sci. 1996, 74, 3052–3062. [Google Scholar] [CrossRef]
- Brown, M.S.; Krehbiel, C.R.; Galyean, M.L.; Remmenga, M.D.; Peters, J.P.; Hibbard, B.; Robinson, J.A.; Moseley, W.M. Evaluation of models of acute and subacute acidosis on dry matter intake, ruminal fermentation, blood chemistry, and endocrine profiles of beef steers. J. Anim. Sci. 2000, 78, 3155–3168. [Google Scholar] [CrossRef]
- Arndt, C.; Powell, J.M.; Aguerre, M.J.; Crump, P.M.; Wattiaux, M.A. Feed conversion efficiency in dairy cows: Repeatability, variation in digestion and metabolism of energy and nitrogen, and ruminal methanogens. J. Dairy Sci. 2015, 98, 3938–3950. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited Review: Pathology, Etiology, Prevention and Treatment of Fatty Liver in Dairy Cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Gross, J.; van Dorland, H.A.; Bruckmaier, R.M.; Schwarz, F.J. Milk fatty acid profile related to energy balance in dairy cows. J. Dairy Res. 2011, 78, 479–488. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; & Doreau, M. Ruminant milk fat plasticity: Nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann. Zootech. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Colucci, P.E.; Chase, L.E.; Van Soest, P.J. Feed intake, apparent diet digestibility, and rate of particulate passage in dairy cattle. J. Dairy Sci. 1982, 65, 1445–1456. [Google Scholar] [CrossRef]
- Auldist, M.J.; Grainger, C.; Macmillan, K.L.; Marett, L.C.; Hannah, M.; Leury, B.J.; Wales, W.J. Feed conversion efficiency and marginal milk production responses of pasture-fed dairy cows offered supplementary grain during an extended lactation. Anim. Prod. Sci. 2011, 51, 204–209. [Google Scholar] [CrossRef]
- Laeger, T.; Görs, S.; Metges, C.C.; Kuhla, B. Effect of feed restriction on metabolites in cerebrospinal fluid and plasma of dairy cows. J. Dairy Sci. 2012, 95, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.D.; Heitzman, R.J.; Hibbitt, K.G. Effects of starvation on intermediary metabolism in the lactating cow. A comparison with metabolic changes occurring during bovine ketosis. Biochem. J. 1972, 128, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.; Boer, H.; Van Bruchem, J.; Bruining, M.; Tamminga, S. Absorption of volatile fatty acids from the rumen of lactating dairy cows as influenced by volatile fatty acid concentration, pH and rumen liquid volume. Br. J. Nutr. 1993, 69, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Slyter, L.L.; Rumsey, T.S. Effect of coliform bacteria, feed deprivation, and pH on ruminal D-lactic acid production by steer or continuous-culture microbial populations changed from forage to concentrates. J. Anim. Sci. 1991, 69, 3055–3066. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90 (Suppl. 1), E17–E38. [Google Scholar] [CrossRef]
- Baldwin, R.; Jesse, B.W. Propionate Modulation of Ruminal Ketogenesis. J. Anim. Sci. 1996, 74, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, R.S.; Gerdes, R. Hydrogen production and transfer in the rumen. Recent Adv. Anim. Nutr. Aust. 1999, 12, 37–44. [Google Scholar]
- Storm, A.C.; Hanigan, M.D.; Kristensen, N.B. Effects of ruminal ammonia and butyrate concentrations on reticuloruminal epithelial blood flow and volatile fatty acid absorption kinetics under washed reticulorumen conditions in lactating dairy cows. J. Dairy Sci. 2011, 94, 3980–3994. [Google Scholar] [CrossRef] [PubMed]
- Schlau, N.; Guan, L.L.; Oba, M. The relationship between rumen acidosis resistance and expression of genes involved in regulation of intracellular pH and butyrate metabolism of ruminal epithelial cells in steers. J. Dairy Sci. 2012, 95, 5866–5875. [Google Scholar] [CrossRef] [PubMed]
- Penner, G.B. Mechanisms of volatile fatty acid absorption and metabolism and maintenance of a stable rumen environment. In Proceedings of the 25th Florida Ruminant Nutrition Symposium, Gainsville, FL, USA, 4 February 2014. [Google Scholar]
- Storm, A.C.; Kristensen, N.B.; Hanigan, M.D. A model of ruminal volatile fatty acid absorption kinetics and rumen epithelial blood flow in lactating Holstein cows. J. Dairy Sci. 2012, 95, 2919–2934. [Google Scholar] [CrossRef]
- Sakata, T.; Tamate, H. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J. Dairy Sci. 1998, 61, 1109–1113. [Google Scholar] [CrossRef]
- Gäbel, G.; Martens, H.; Sündermann, M.; Galfi, P. The effect of diet, intraruminal pH and osmolarity on sodium, chloride and magnesium absorption from the temporarily isolated and washed reticulo-rumen of sheep. J. Exp. Physiol. Transl. Integr. 1987, 72, 501–511. [Google Scholar] [CrossRef]
- Ørskov, E.R. The effect of processing on digestion and utilization of cereals by ruminants. Proc. Nutr. Soc. 1976, 35, 245–252. [Google Scholar] [CrossRef]
- Manns, J.G.; Boda, J.M. Insulin release by acetate, propionate, butyrate, and glucose in lambs and adult sheep. Am. J. Physiol. Leg. Content 1964, 212, 747–755. [Google Scholar] [CrossRef]
- Annison, E.F.; Linzell, J.L. The oxidation and utilization of glucose and acetate by the mammary gland of the goat in relation to their over-all metabolism and to milk formation. J. Physiol. 1964, 175, 372–385. [Google Scholar] [CrossRef]
- Baldwin, R.L. Use of isolated ruminal epithelial cells in the study of rumen metabolism. J. Nutr. 1998, 128, 293–296. [Google Scholar]
- Zhang, F.; Li, D.; Wu, Q.; Sun, J.; Guan, W.; Hou, Y.; Zhu, Y.; Wang, J. Prepartum body conditions affect insulin signaling pathways in postpartum adipose tissues in transition dairy cows. J. Anim. Sci. Biotechnol. 2019, 10, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Penner, G.B.; Stumpff, F.; Gäbel, G. Role of fermentation acid absorption in the regulation of ruminal pH. In Proceedings of the Ruminant Nutrition Symposium, Denver, CO, USA, 10–15 July 2010; pp. 1092–1107. [Google Scholar]
- Dieho, K.; Bannink, A.; Geurts, I.A.L.; Schonewille, J.T.; Gort, G.; Dijkstra, J. Morphological adaptation of rumen papillae during the dry period and early lactation as affected by rate of increase of concentrate allowance. J. Dairy Sci. 2016, 99, 2339–2352. [Google Scholar] [CrossRef]
- Dirksen, G.U.; Liebich, H.G.; Mayer, E. Adaptive changes of the ruminal mucosa and their functional and clinical significance. Bov. Pract. 1985, 20, 116–120. [Google Scholar]
- Etschmann, B.; Suplie, A.; Martens, H. Change of ruminal sodium transport in sheep during dietary adaptation. Arch. Anim. Nutr. 2009, 63, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Oba, M. Characteristics of dairy cows with a greater or lower risk of subacute ruminal acidosis: Volatile fatty acid absorption, rumen digestion and expression of genes in rumen epithelial cells. J. Dairy Sci. 2016, 99, 8733–8745. [Google Scholar] [CrossRef]
- Gao, X.; Oba, M. Relationship of severity of subacute ruminal acidosis to rumen fermentation, chewing activities, sorting behavior, and milk production in lactating dairy cows fed a high-grain diet. J. Dairy Sci. 2014, 97, 3006–3016. [Google Scholar] [CrossRef]
- García, S.C.; Pedernera, M.; Fulkerson, W.J.; Horadagoda, A.; Nandra, K. Feeding concentrates based on individual cow requirements improves the yield of milk solids in dairy cows grazing restricted pasture. J. Exp. Agric. 2007, 47, 502–508. [Google Scholar] [CrossRef]
- Proudfoot, K.L.; Veira, D.M.; Weary, D.M.; Von Keyserlingk, M.A.G. Competition at the feed bunk changes the feeding, standing, and social behavior of transition dairy cows. J. Dairy Sci. 2009, 92, 3116–3123. [Google Scholar] [CrossRef]

| Diets | Period 1 | SE 1 | Period 2 | SE | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Herbage | FB Bulb | Herbage | FB Bulb | Diet | Period | |||||
| HO | FBH | FBH | HO | FBH | FBH | |||||
| Pre-grazing (kg DM/ha) | 5497 | 5453 | - | 86 | 3478 | 3596 | - | 61 | 0.14 | *** |
| Post-grazing (kg DM/ha) | 2823 | 3050 | - | 68 | 1953 | 2277 | - | 59 | *** | *** |
| Area (m2/cow/day) | 53.6 | 52.2 | - | 1.06 | 76.3 | 73.4 | - | 1.71 | 0.18 | *** |
| Allocation (kg DM/cow) | 26.4 | 26.0 | - | 0.38 | 29.1 | 28.7 | - | 0.45 | 0.52 | *** |
| Pasture botanical | ||||||||||
| Ryegrass % DM | 91.6 | 95.2 | - | 2.04 | 86.4 | 85.6 | - | 1.96 | 0.31 | 0.26 |
| White clover % DM | 1.36 | 1.14 | - | 1.67 | 5.02 | 6.14 | - | 2.08 | 0.71 | 0.13 |
| Weeds % DM | 3.96 | 3.73 | - | 2.83 | 8.74 | 10.6 | - | 3.39 | 0.62 | 0.06 |
| Dead % DM | 5.27 a | 2.89 | - | 0.707 | 1.52 b | 1.95 | - | 0.914 | 0.38 | 0.14 |
| Chemical composition | ||||||||||
| DM % DM | 14.7 c | 14.2 c | 12.7 a | 0.36 | 17.5 d | 18.1 d | 20.7 b | 0.60 | 0.39 | *** |
| OM % DM | 91.5 b | 91.4 b | 94.2 a | 0.26 | 91.8 b | 91.7 b | 93.7 a | 0.29 | 0.66 | 0.75 |
| ADF % DM | 21.0 c | 21.2 c | 7.81 a | 0.123 | 23.3 d | 23.6 d | 8.15 b | 0.130 | 0.12 | *** |
| NDF % DM | 36.6 d | 37.7 c | 13.0 a | 0.185 | 41.7 e | 41.8 e | 14.0 b | 0.241 | 0.07 | *** |
| WSC % DM | 21.1 b | 20.6 b | 63.9 a | 0.39 | 20.5 b | 20.2 b | 59.4 a | 0.41 | 0.36 | 0.15 |
| CP % DM | 15.6 b | 16.0 b | 8.23 a | 0.27 | 15.7 b | 15.5 b | 9.39 a | 0.37 | 0.69 | 0.29 |
| ME (MJ/kg DM) | 11.2 a | 11.2 a | 13.4 b | 0.03 | 11.1 a | 11.0 a | 13.2 b | 0.03 | *** | *** |
| Fat % DM | 2.30 c | 2.72 b | 0.59 a | 0.084 | 2.12 c | 2.54 b | 0.40 a | 0.088 | *** | 0.13 |
| ∑SFA (mg/g DM) | 4.32 b | 4.49 b | 0.66 a | 0.077 | 4.27 a | 4.60 a | 0.76 b | 0.077 | *** | 0.07 |
| ∑MUFA (mg/g DM) | 1.04 d | 1.17 f | 0.43 b | 0.024 | 0.95b c | 1.09 e | 0.34 a | 0.0243 | *** | *** |
| ∑PUFA (mg/g DM) | 9.97 e | 13.21 f | 1.28 b | 0.100 | 9.07 c | 9.76 d | 0.85 a | 0.100 | *** | *** |
| ∑Total FA (mg/g DM) | 18.9 d | 21.6 f | 3.29 b | 0.47 | 16.8 c | 19.5 e | 1.19 a | 0.47 | *** | *** |
| Adaptation Stage 1 | Stage One | SE 2 | Stage Two | SE | Stage Three | SE | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | HO | FBH | HO | FBH | HO | FBH | Diet | Period | Stage | D × S 5 | |||
| Liveweight (kg) | 478 a | 480 a | 4.2 | 487 a | 488 a | 5.3 | 486 a | 484 a | 6.4 | 0.45 | *** | ** | 0.87 |
| 7d LWT 3 (kg) | 493 a | 494 a | 3.1 | 496 a | 498 a | 3.3 | 497 a | 499 a | 3.5 | 0.40 | *** | ** | 0.78 |
| DMI 4 (kg DM/day) | 13.8 a | 14.5 b | 0.31 | 11.6 c | 15.3 b | 0.42 | 11.6 c | 15.8 d | 0.61 | *** | *** | 0.55 | 0.07 |
| HI (kg DM/day) | 13.9 b | 11.3 a | 0.39 | 11.6 c | 10.5 c | 0.51 | 11.6 c | 10.1 c | 0.747 | *** | ** | * | 0.64 |
| FB (kg DM/day) | 0 b | 3.2 a | 0.12 | 0 b | 4.9 c | 0.21 | 0 b | 5.7 d | 0.31 | ** | *** | ||
| FB utilization % | 94.4 | 2.22 | 80.0 | 2.12 | 82.7 | 1.72 | 0.52 | *** | |||||
| HA (kg DM/d) | 18.5 a | 17.8 a | 0.28 | 16.6 b | 16.4 b | 0.40 | 15.6 b | 16.5 b | 0.61 | 0.27 | 0.38 | *** | 0.28 |
| Fat (%) | 5.09 | 4.57 | 0.155 | 4.47 | 4.55 | 0.220 | 4.46 | 4.59 | 0.311 | 0.13 | 0.19 | 0.57 | 0.20 |
| Fat (kg) | 1.29 a | 1.21 a | 0.034 | 1.06 b | 1.06 b | 0.049 | 1.10 b | 1.08 b | 0.067 | 0.22 | *** | 0.11 | 0.51 |
| Protein (%) | 3.68 | 3.76 | 0.053 | 3.79 | 3.67 | 0.075 | 3.55 | 3.71 | 0.106 | 0.08 | 0.50 | 0.52 | 0.92 |
| Protein (kg) | 0.94 a | 1.00 a | 0.026 | 0.88 b | 0.88 b | 0.036 | 0.86 b | 0.89 b | 0.052 | 0.16 | * | * | 0.68 |
| Lactose (%) | 5.12 a | 5.05 b | 0.023 | 5.14 a | 5.08 b | 0.033 | 5.08 b | 5.04 b | 0.047 | * | 0.48 | ** | 0.88 |
| Lactose (kg) | 1.30 a | 1.34 a | 0.029 | 1.23 b | 1.18 b | 0.041 | 1.23 b | 1.21 b | 0.058 | 0.89 | ** | ** | 0.53 |
| MS (%) | 8.87 | 8.43 | 0.158 | 8.26 | 8.42 | 0.224 | 8.09 | 8.38 | 0.316 | 0.33 | 0.15 | 0.52 | 0.18 |
| MS (kg) | 2.23 a | 2.20 a | 0.046 | 1.94 b | 1.94 b | 0.066 | 1.94 a | 1.99 a | 0.093 | 0.88 | *** | * | 0.87 |
| Milk (kg) | 25.4 a | 26.5 a | 0.56 | 23.9 b | 23.3 b | 0.80 | 24.2 a | 24.0 a | 1.13 | 0.51 | ** | ** | 0.46 |
| MUN 6 | 5.3 a | 7.3 a | 1.09 | 8.2 ab | 4.2a | 1.52 | 4.6 a | 4.0 a | 2.15 | 0.93 | 0.49 | 0.90 | 0.09 |
| FCE (kg milk/kg DMI) | 2.00 b | 1.78 ab | 0.149 | 2.07 b | 1.55 a | 0.154 | 2.10 b | 1.61 a | 0.190 | *** | 0.60 | *** | * |
| Adaptation Stage 1 | Stage 1 | SE 2 | Stage 2 | SE | Stage 3 | SE | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HO | FBH | HO | FBH | HO | FBH | Diet | Stage | Period | D × S 4 | ||||
| g/100g of FA | |||||||||||||
| Σ Small chain | 7.26 a | 7.41 a | 0.108 | 7.42 a | 6.81 b | 0.153 | 7.26 a | 7.23 a | 0.216 | 0.37 | 0.22 | 0.11 | * |
| Σ Long chain | 32.8 b | 28.2 a | 1.47 | 32.4 b | 28.0 a | 2.07 | 26.4 a | 23.5 a | 2.93 | * | 0.08 | 0.86 | 0.94 |
| Σ Med chain | 52.3 a | 55.8 ab | 1.24 | 52.6 a | 57.4 ab | 1.75 | 58.7 b | 60.6 b | 2.47 | * | * | 0.82 | 0.80 |
| Σ Branched | 2.02 a | 1.88 a | 0.060 | 2.94 a | 1.97 a | 0.084 | 1.75 ab | 1.71 ab | 0.119 | 0.27 | 0.08 | 0.79 | 0.54 |
| Σ Trans | 3.58 | 3.49 | 0.234 | 3.77 | 3.17 | 0.331 | 2.57 | 2.67 | 0.468 | 0.42 | 0.06 | 0.13 | 0.59 |
| Σ Saturated | 69.8 b | 72.5 a | 1.13 | 69.8 b | 73.2 a | 1.60 | 75.3 a | 75.8 a | 2.26 | * | 0.06 | 0.84 | 0.75 |
| Σ MUFA | 22.0 b | 19.1 a | 1.11 | 21.7 b | 18.8 a | 1.56 | 17.1 ab | 16.0 a | 2.21 | * | 0.09 | 0.75 | 0.87 |
| Σ PUFA | 2.35 | 2.35 | 0.093 | 2.56 | 2.19 | 0.132 | 2.10 | 2.07 | 0.186 | 0.29 | 0.19 | 0.38 | 0.30 |
| C4 | 3.63 a | 3.37 b | 0.104 | 3.66 a | 3.10 b | 0.147 | 3.50 a | 3.42 ab | 0.208 | ** | 0.61 | * | 0.33 |
| C6 | 2.26 b | 2.43 a | 0.038 | 2.33 a | 2.28 a | 0.054 | 2.33 a | 2.35 a | 0.076 | * | 0.73 | 0.38 | 0.06 |
| C8 | 1.38 b | 1.61 a | 0.044 | 1.44 a | 1.42 a | 0.062 | 1.43 a | 1.46 a | 0.087 | ** | 0.50 | 0.53 | 0.07 |
| C10 | 3.47 b | 4.38 a | 0.177 | 3.63 a | 3.89 a | 0.251 | 3.93 a | 4.33a | 0.355 | ** | 0.50 | 0.56 | 0.32 |
| C10:1 | 0.26 b | 0.32 a | 0.013 | 0.28 a | 0.28 a | 0.019 | 0.28 a | 0.28 a | 0.027 | * | 0.79 | 0.53 | 0.15 |
| C12 | 4.32 b | 5.49 a | 0.285 | 4.25 a | 4.92 a | 0.404 | 5.01 a | 5.67 a | 0.571 | ** | 0.32 | 0.32 | 0.72 |
| C14 | 12.3 a | 13.5 b | 0.36 | 12.4 a | 13.7 b | 0.51 | 14.0 b | 14.4 bc | 0.73 | ** | 0.09 | 0.36 | 0.78 |
| C14:1 cis 9 | 0.65 a | 0.90 b | 0.063 | 0.76 a | 0.84 b | 0.089 | 0.59 a | 0.86 b | 0.126 | * | 0.73 | 0.18 | 0.37 |
| C16:0 | 29.7 a | 30.7 a | 0.71 | 30.0 a | 32.7 a | 0.98 | 33.9 b | 34.4 b | 1.38 | 0.08 | ** | 0.84 | 0.57 |
| C16:1 | 1.11 | 1.12 | 0.066 | 1.10 | 1.11 | 0.093 | 0.99 | 1.13 | 0.132 | 0.69 | 0.81 | 0.06 | 0.76 |
| C18:0 | 9.90 a | 8.34 b | 0.424 | 9.62 a | 8.56 a | 0.599 | 8.57 a | 7.00 a | 0.847 | ** | 0.14 | 0.77 | 0.89 |
| C18:1 trans 9 | 0.14 | 0.13 | 0.004 | 0.13 | 0.12 | 0.006 | 0.12 | 0.11 | 0.008 | 0.06 | 0.07 | 0.06 | 0.90 |
| C18:1 trans 11 | 3.44 | 3.36 | 0.232 | 3.64 | 3.05 | 0.328 | 2.45 | 2.55 | 0.464 | 0.43 | 0.07 | 0.14 | 0.60 |
| C18:1 cis 9 | 16.3 b | 13.5 a | 1.03 | 15.8 a | 13.5 a | 1.46 | 12.5 a | 11.2 a | 2.07 | * | 0.20 | 0.56 | 0.90 |
| C18:1 cis 11 | 0.60 b | 0.52 a | 0.025 | 0.57 a | 0.52 a | 0.025 | 0.55 a | 0.51 a | 0.050 | * | 0.80 | 0.34 | 0.79 |
| C18:2 cis 9, 12 | 0.49 | 0.46 | 0.018 | 0.51 | 0.46 | 0.026 | 0.53 | 0.54 | 0.036 | 0.09 | 0.17 | 0.66 | 0.63 |
| C18:3 cis 9, 12, 15 | 0.69 | 0.66 | 0.033 | 0.76 | 0.67 | 0.047 | 0.79 | 0.69 | 0.067 | 0.17 | 0.39 | 0.98 | 0.66 |
| CLA 3 | 1.18 a | 1.23 a | 0.080 | 1.29 a | 1.06 a | 0.113 | 0.80 b | 0.85 b | 0.160 | 0.79 | * | 0.26 | 0.35 |
| Adaptation Stage 1 | Stage One | SE 2 | Stage Two | SE | Stage Three | SE | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HO | FBH | HO | FBH | HO | FBH | Diet | Stage | Period | Cow | D × S3 | ||||
| Nadir pH | 5.62 a | 5.57 a | 0.04 | 5.64 a | 5.47 b | 0.04 | 5.71 a | 5.48 b | 0.05 | *** | 0.41 | *** | *** | * |
| Max pH | 6.35 b | 6.43 a | 0.02 | 6.46 c | 6.48 c | 0.03 | 6.46 c | 6.55 d | 0.04 | 0.15 | *** | *** | *** | ** |
| Mean pH | 6.01 b | 6.03 b | 0.002 | 6.09 d | 6.04 c | 0.003 | 6.16 f | 6.08 e | 0.004 | *** | *** | *** | *** | *** |
| pH < 6.0 | 516 b | 475 a | 2.8 | 392 d | 441 c | 3.2 | 349 f | 369 e | 3.7 | *** | *** | *** | *** | *** |
| pH < 5.8 | 142 b | 126 a | 1.5 | 118 d | 134 c | 1.7 | 99 f | 108 e | 1.8 | *** | *** | *** | *** | *** |
| pH < 5.6 | 12.2 b | 20.9 a | 0.7 | 10.9 d | 30.0 c | 0.9 | 10.9 d | 20.8 a | 0.8 | *** | *** | *** | *** | *** |
| Adaptation Stage 1 | 1 | SE 2 | 2 | SE | 3 | SE | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | HO | FBH | HO | FBH | HO | FBH | Diet | Day | Time | Period | D × D × T 5 | |||
| Lactate (mol/L) | 0.94 a | 2.60 a | 1.851 | 3.20 a | 84.7c | 27.0 | 0.70 a | 4.35 b | 2.212 | ** | *** | *** | ** | *** |
| Ammonia (mmol/L) | 4.58 a | 4.04 a | 0.368 | 3.42 c | 2.28 b | 0.270 | 3.59 c | 1.53 b | 0.231 | ** | *** | *** | 0.07 | * |
| tVFA 3 (mmol/L) | 117 a | 118 a | 2.5 | 119 a | 103 b | 2.6 | 118 a | 107 b | 2.6 | *** | * | *** | * | 0.81 |
| Molar Proportions | ||||||||||||||
| Acetate | 60.8 a | 59 b | 0.31 | 60.5 a | 55.0 b | 0.28 | 63.3 a | 56.5 b | 0.29 | *** | *** | *** | ** | *** |
| Propionate | 21.1 a | 21.5 a | 0.22 | 21.2 a | 20.6 b | 0.22 | 20.0 b | 19.8 c | 0.21 | *** | * | *** | 0.64 | * |
| Iso -butyrate | 0.73 a | 0.78 b | 0.013 | 0.72 a | 0.50 c | 0.011 | 0.71 a | 0.55 c | 0.011 | *** | *** | *** | *** | *** |
| Butyrate | 13.3 a | 14.2 b | 0.20 | 14.0 b | 17.6 d | 0.21 | 12.7 e | 15.4 c | 0.19 | *** | *** | *** | 0.42 | ** |
| Iso-valerate | 1.06 a | 1.13 ab | 0.027 | 1.05 a | 0.68 b | 0.020 | 1.05 a | 0.59 b | 0.019 | *** | *** | *** | 0.48 | *** |
| Valerate | 1.46 a | 1.32 ab | 0.050 | 1.35 a | 2.38 b | 0.070 | 1.43 a | 1.25 c | 0.073 | *** | *** | *** | 0.71 | 1.00 |
| Hexanoic | 0.55 a | 0.60 a | 0.019 | 0.53 a | 0.82 b | 0.023 | 0.50 a | 0.88 c | 0.023 | *** | *** | *** | * | 0.16 |
| A + B/P 4 | 3.54 a | 3.47 a | 0.058 | 3.49 a | 3.62 ab | 0.062 | 3.8 1b | 3.81 b | 0.067 | 0.66 | *** | *** | ** | 0.06 |
| Adaptation Stage 1 | 1 | SE 2 | 2 | SE | 3 | SE | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | HO | FBH | HO | FBH | HO | FBH | Diet | Stage | Period | D × S 3 | |||
| Glutamic acid (μmol/L) | 73.7 a | 74.8 a | 2.57 | 80.0 ab | 87.0 b | 2.57 | 78.0 b | 85.9 b | 2.66 | * | *** | 1.00 | 0.35 |
| Aspartate | 8.11 a | 9.72 a | 0.765 | 11.26 b | 11.33 b | 0.765 | 10.69 b | 12.28 b | 0.793 | 0.10 | ** | ** | 0.52 |
| Cysteine | 99.3 | 99.5 | 3.99 | 106 | 103 | 3.99 | 105 | 101 | 4.13 | 0.47 | 0.42 | * | 0.85 |
| Asparagine | 8.11a | 9.72 a | 0.765 | 11.26 b | 11.33 b | 0.765 | 10.69 b | 12.28 b | 0.793 | 0.10 | ** | ** | 0.52 |
| Serine | 43.0 a | 47.9 a | 3.29 | 44.1 a | 43.5 a | 3.29 | 42.0 a | 35.6 b | 3.34 | 0.63 | ** | 0.18 | * |
| Glutamine | 103 | 94.8 | 9.04 | 99.6 | 103 | 9.04 | 93.7 | 86.0 | 9.33 | 0.50 | 0.36 | 0.36 | 0.73 |
| Histidine | 27.0 a | 28.0 a | 1.30 | 26.6 b | 24.1 b | 1.30 | 21.3 c | 19.3 c | 1.34 | 0.24 | *** | *** | 0.28 |
| Glycine | 97.4 a | 112 a | 13.9 | 79.4 a | 182 c | 13.9 | 86.6 a | 110 b | 14.4 | *** | * | 0.22 | ** |
| Threonine | 98.3 b | 103 b | 8.70 | 103 b | 120 bc | 8.70 | 91.0 a | 89.5 a | 8.94 | 0.29 | * | 0.27 | 0.42 |
| Arginine | 49.9 | 53.2 | 4.51 | 54.7 | 54.2 | 4.51 | 54.8 | 48.5 | 4.68 | 0.73 | 0.77 | ** | 0.57 |
| Alanine | 122 a | 138 a | 12.9 | 162 b | 153 b | 12.9 | 131 a | 114 a | 13.4 | 0.60 | ** | ** | 0.23 |
| Taurine | 23.3 a | 23.8 a | 1.24 | 24.5 a | 30.4 b | 1.20 | 23.8 a | 26.7 a | 1.28 | ** | ** | *** | 0.07 |
| Tyrosine | 42.2 a | 44.2 a | 4.44 | 47.4 a | 43.2 a | 4.44 | 37.0 b | 33.6 b | 4.60 | 0.52 | 0.06 | 0.81 | 0.73 |
| Valine | 148 | 167 | 13.8 | 158 | 168 | 13.8 | 147 | 133 | 14.3 | 0.66 | 0.23 | 0.37 | 0.46 |
| Methionine | 18.7 a | 19.1 a | 1.70 | 20.0 a | 21.4 a | 1.70 | 15.3 b | 14.9 b | 1.76 | 0.79 | ** | 0.92 | 0.88 |
| Tryptophan | 42.2 a | 44.4 a | 4.44 | 47.4 a | 43.2 a | 4.44 | 37.0 b | 33.6 b | 5.60 | 0.52 | 0.06 | 0.81 | 0.73 |
| Phenylalanine | 41.3 a | 44.3 a | 3.83 | 46.5 a | 43.8 a | 3.83 | 38.2 ab | 32.6 ab | 3.97 | 0.53 | 0.04 | 0.52 | 0.54 |
| Isoleucine | 93.6 | 103 | 9.76 | 96.1 | 128 | 9.76 | 91.8 | 93.8 | 8.9 | 0.09 | 0.15 | 0.86 | 0.31 |
| Lysine | 31.9 | 41.9 | 6.36 | 35.8 | 39.6 | 6.36 | 24.4 | 20.5 | 6.60 | 0.58 | * | 0.28 | 0.57 |
| Leucine | 93.5 | 104 | 10.2 | 95.4 | 93.2 | 10.2 | 86.8 | 71.4 | 10.6 | 0.74 | 0.15 | 0.81 | 0.47 |
| Proline | 71.0 | 71.8 | 5.11 | 73.4 | 77.2 | 5.11 | 75.3 | 73.9 | 5.25 | 0.75 | 0.61 | 0.49 | 0.82 |
| NEFA (mmol/L) | 0.08 a | 0.08 a | 0.0064 | 0.08 a | 0.05 b | 0.0064 | 0.07 a | 0.06 ab | 0.0066 | * | * | 0.60 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, A.; Garrett, K.; Froehlich, K.; Beck, M.; Bryant, R.H.; Edwards, G.; Gregorini, P. Supplementation of Spring Pasture with Harvested Fodder Beet Bulb Alters Rumen Fermentation and Increases Risk of Subacute Ruminal Acidosis during Early Lactation. Animals 2020, 10, 1307. https://doi.org/10.3390/ani10081307
Fleming A, Garrett K, Froehlich K, Beck M, Bryant RH, Edwards G, Gregorini P. Supplementation of Spring Pasture with Harvested Fodder Beet Bulb Alters Rumen Fermentation and Increases Risk of Subacute Ruminal Acidosis during Early Lactation. Animals. 2020; 10(8):1307. https://doi.org/10.3390/ani10081307
Chicago/Turabian StyleFleming, Anita, Konagh Garrett, Kelly Froehlich, Matthew Beck, Racheal H. Bryant, Grant Edwards, and Pablo Gregorini. 2020. "Supplementation of Spring Pasture with Harvested Fodder Beet Bulb Alters Rumen Fermentation and Increases Risk of Subacute Ruminal Acidosis during Early Lactation" Animals 10, no. 8: 1307. https://doi.org/10.3390/ani10081307
APA StyleFleming, A., Garrett, K., Froehlich, K., Beck, M., Bryant, R. H., Edwards, G., & Gregorini, P. (2020). Supplementation of Spring Pasture with Harvested Fodder Beet Bulb Alters Rumen Fermentation and Increases Risk of Subacute Ruminal Acidosis during Early Lactation. Animals, 10(8), 1307. https://doi.org/10.3390/ani10081307







