Dietary Mannan Oligosaccharides Modulate Gut Inflammatory Response and Improve Duodenal Villi Height in Post-Weaning Piglets Improving Feed Efficiency
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals, and Housing
2.2. Growth Performance and Faecal Microbiological Assay
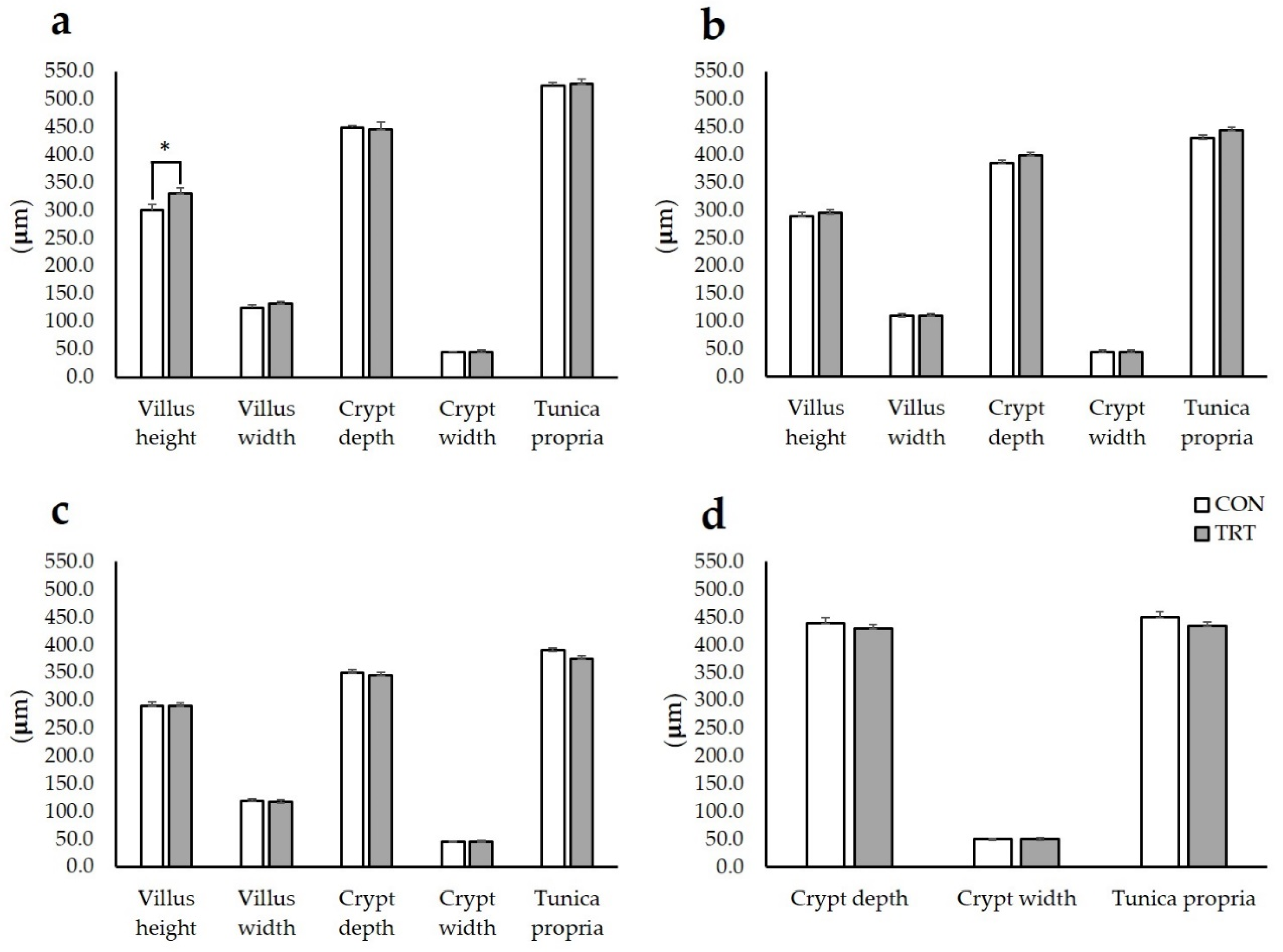
2.3. Intestinal Histometry
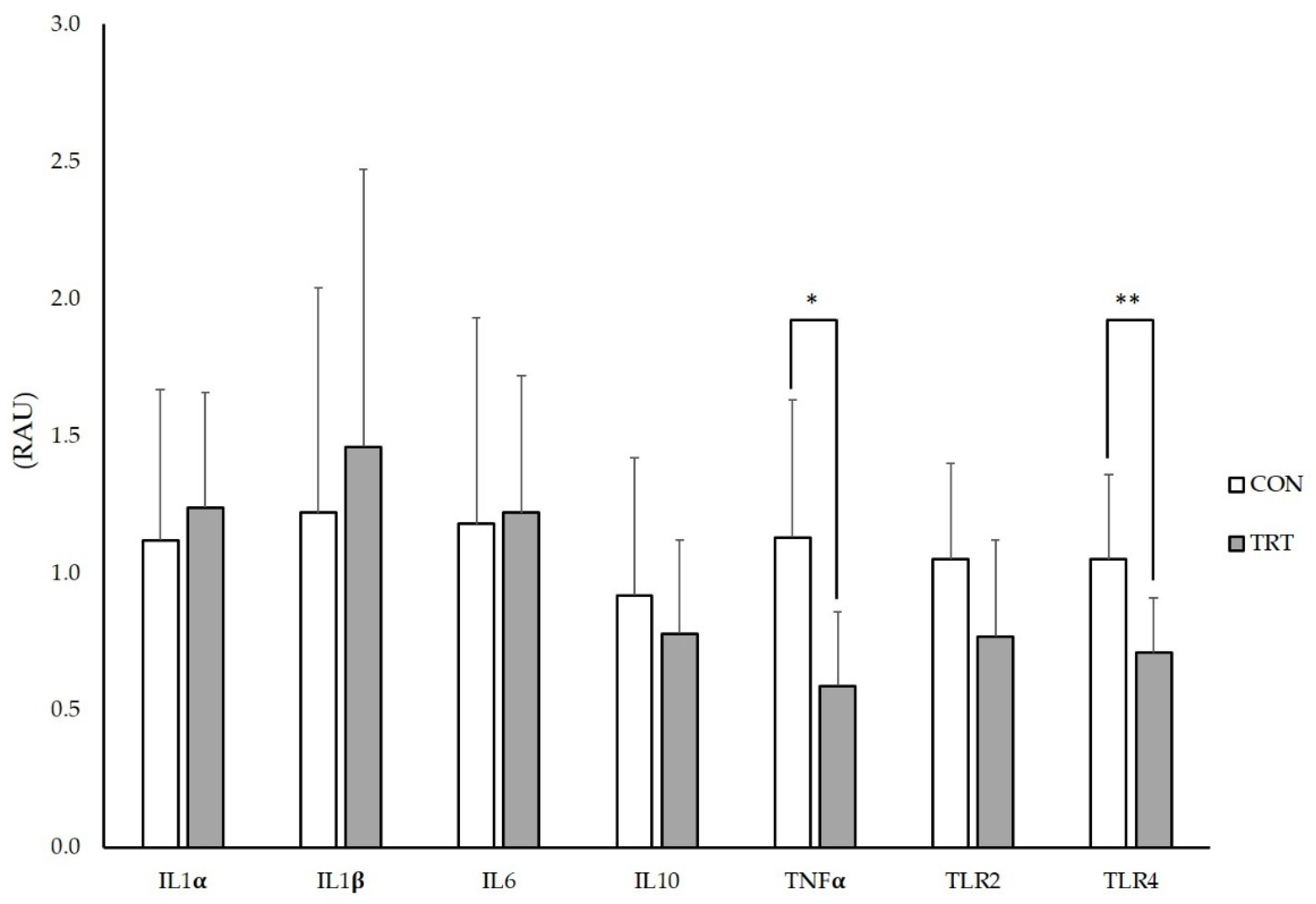
2.4. ILs and TLRs Gene Expression in IPP
2.5. Statistical Analysis
3. Results
3.1. Growth Performance and Faecal Microbiology
3.2. Intestinal Histometry, ILs and TLRs Gene Expression in IPP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A Gene Expression Analyses
References
- Pluske, J.R.; Turpin, D.L.; Kim, J.C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Cowieson, A.; Fru-Nji, F.; Steinert, R.; Kluenter, A.M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, X.; Wang, X.; Tan, B.; Li, T.; Yin, Y. Effects of weaning on intestinal upper villus epithelial cells of piglets. PLoS ONE 2016, 11, e0150216. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Thacker, P.A. Alternatives to antibiotics as growth promoters for use in swine production: A review. J. Anim. Sci. Biotechnol. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Kumar, S.; Verma, A.; Baghel, R. Probiotics as feed additives in weaned pigs: A review. Livest. Res. Int. 2015, 3, 31–39. [Google Scholar]
- Kritas, S.K. Probiotics and prebiotics for the health of pigs and horses. In Probiotics and Prebiotics in Animal Health and Food Safety; Springer: Berlin/Heidelberg, Germany, 2018; pp. 109–126. [Google Scholar]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Perricone, V.; Comi, M.; Bontempo, V.; Lecchi, C.; Ceciliani, F.; Crestani, M.; Ferrari, A.; Savoini, G.; Agazzi, A. Effects of nucleotides administration on growth performance and immune response of post-weaning piglets. Ital. J. Anim. Sci. 2020, 19, 295–301. [Google Scholar] [CrossRef]
- Mohammadi Gheisar, M.; Kim, I.H. Phytobiotics in poultry and swine nutrition—A review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Halas, V.; Nochta, I. Mannan oligosaccharides in nursery pig nutrition and their potential mode of action. Animals 2012, 2, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Spring, P.; Wenk, C.; Connolly, A.; Kiers, A. A review of 733 published trials on Bio-Mos®, a mannan oligosaccharide, and Actigen®, a second generation mannose rich fraction, on farm and companion animals. J. Appl. Anim. Nutr. 2015, 3, e8. [Google Scholar] [CrossRef]
- Budiño, F.E.L.; Thomaz, M.C.; Kronka, R.N.; Nakaghi, L.S.O.; Tucci, F.M.; Fraga, A.L.; Scandolera, A.J.; Huaynate, R.A.R. Effect of probiotic and prebiotic inclusion in weaned piglet diets on structure and ultra-structure of small intestine. Braz. Arch. Biol. Technol. 2005, 48, 921–929. [Google Scholar] [CrossRef]
- Castillo, M.; Martin-Orue, S.M.; Taylor-Pickard, J.A.; Perez, J.F.; Gasa, J. Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: Effects on microbiota and gut function. J. Anim. Sci. 2008, 86, 94–101. [Google Scholar] [CrossRef]
- Rekiel, A.; Wiecek, J.; Bielecki, W.; Gajewska, J.; Cichowicz, M.; Kulisiewicz, J.; Batorska, M.; Roszkowski, T.; Beyga, K. Effect of addition of feed antibiotic flavomycin or prebiotic BIO-MOS on production results of fatteners, blood biochemical parameters, morphometric indices of intestine and composition of microflora. Arch. Tierz. Dummerstorf 2007, 50, 172–180. [Google Scholar]
- Liu, P.; Piao, X.S.; Kim, S.W.; Wang, L.; Shen, Y.B.; Lee, H.S.; Li, S.Y. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 2008, 86, 2609–2618. [Google Scholar] [CrossRef]
- White, L.A.; Newman, M.C.; Cromwell, G.; Lindemann, M. Brewers dried yeast as a source of mannan oligosaccharides for weanling pigs. J. Anim. Sci. 2002, 80, 2619–2628. [Google Scholar] [CrossRef]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef]
- Valpotić, H.; Samardžija, M.; Terzić, S.; Vince, S.; Šperanda, M.; Lacković, G.; Habrun, B.; Mas, N.; Đuričić, D.; Kočila, P. Effect of mannan oligosaccharide supplementation on blood and intestinal immune cells, bacteria numbers and performance in weaned pigs. Acta Vet. BRNO 2016, 85, 267–276. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Jung, J.H.; Kim, I.H. Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs. J. Anim. Sci. 2012, 90, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.C.; Rodriguez-Zas, S.L.; Pettigrew, J.E. Efficacy of a mannan oligosaccharide (Bio-Mos®) for improving nursery pig performance. J. Swine Health Prod. 2004, 12, 296–307. [Google Scholar]
- Rosen, G.D. Holo-analysis of the efficacy of Bio-Mos in broiler nutrition. Br. Poult. Sci. 2007, 48, 21–26. [Google Scholar] [CrossRef] [PubMed]
- LeMieux, F.M.; Southern, L.L.; Bidner, T.D. Effect of mannan oligosaccharides on growth performance of weanling pigs. J. Anim. Sci. 2003, 81, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hyun, Y.; Sohn, K.; Kim, T.; Woo, H.; Han, I. Effects of mannanoligosaccharide and protein levels on growth performance and immune status in pigs weaned at 21 days of age. Korean J. Anim. Sci. 2000, 42, 489–498. [Google Scholar]
- Davis, M.E.; Maxwell, C.V.; Brown, D.C.; de Rodas, B.Z.; Johnson, Z.B.; Kegley, E.B.; Hellwig, D.H.; Dvorak, R.A. Effect of dietary mannan oligosaccharides and(or) pharmacological additions of copper sulfate on growth performance and immunocompetence of weanling and growing/finishing pigs. J. Anim. Sci. 2002, 80, 2887–2894. [Google Scholar] [CrossRef]
- Duan, X.; Chen, D.; Zheng, P.; Tian, G.; Wang, J.; Mao, X.; Yu, J.; He, J.; Li, B.; Huang, Z. Effects of dietary mannan oligosaccharide supplementation on performance and immune response of sows and their offspring. Anim. Feed Sci. Technol. 2016, 218, 17–25. [Google Scholar] [CrossRef]
- Duan, X.; Tian, G.; Chen, D.; Huang, L.; Zhang, D.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z. Mannan oligosaccharide supplementation in diets of sow and (or) their offspring improved immunity and regulated intestinal bacteria in piglet. J. Anim. Sci. 2019, 97, 4548–4556. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Bontempo, V.; Jiang, X.R.; Cheli, F.; Lo Verso, L.; Mantovani, G.; Vitari, F.; Domeneghini, C.; Agazzi, A. Administration of a novel plant extract product via drinking water to post-weaning piglets: Effects on performance and gut health. Animal 2014, 8, 721–730. [Google Scholar] [CrossRef]
- Abu-Tarboush, H.M.; Al-Saiady, M.Y.; El-Din, A.H.K. Evaluation of diet containing lactobacilli on performance, fecal coliform, and lactobacilli of young dairy calves. Anim. Feed Sci. Technol. 1996, 57, 39–49. [Google Scholar] [CrossRef]
- Giannenas, I.; Doukas, D.; Karamoutsios, A.; Tzora, A.; Bonos, E.; Skoufos, I.; Tsinas, A.; Christaki, E.; Tontis, D.; Florou-Paneri, P. Effects of Enterococcus faecium, mannan oligosaccharide, benzoic acid and their mixture on growth performance, intestinal microbiota, intestinal morphology and blood lymphocyte subpopulations of fattening pigs. Anim. Feed Sci. Technol. 2016, 220, 159–167. [Google Scholar] [CrossRef]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Berri, M.; Estelle, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.J.; Chevaleyre, C.; Billon, Y.; Dore, J.; et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ. Microbiol. Rep. 2015, 7, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Chen, Y.P.; Chen, R.; Su, Y.; Zhang, R.Q.; He, Q.F.; Wang, K.; Wen, C.; Zhou, Y.M. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019, 98, 4767–4776. [Google Scholar] [CrossRef]
- Teng, P.Y.; Kim, W.K. Review: Roles of Prebiotics in Intestinal Ecosystem of Broilers. Front. Vet. Sci. 2018, 5, 245. [Google Scholar] [CrossRef]
- Poeikhampha, T.; Bunchasak, C. Comparative effects of sodium gluconate, mannan oligosaccharide and potassium diformate on growth performances and small intestinal morphology of nursery pigs. Asian Australas. J. Anim. Sci. 2011, 24, 844–850. [Google Scholar] [CrossRef]
- Dos Anjos, C.M.; Gois, F.D.; dos Anjos, C.M.; de Souza Rocha, V.; e Castro, D.E.d.S.; Allaman, I.B.; Silva, F.L.; de Oliveira Carvalho, P.L.; Meneghetti, C.; Costa, L.B. Effects of dietary beta-glucans, glucomannans and mannan oligosaccharides or chlorohydroxyquinoline on the performance, diarrhea, hematological parameters, organ weight and intestinal health of weanling pigs. Livest. Sci. 2019, 223, 39–46. [Google Scholar] [CrossRef]
- Subramaniam, M.D.; Kim, I.H. Clays as dietary supplements for swine: A review. J. Anim. Sci. Biotechnol. 2015, 6, 38. [Google Scholar] [CrossRef]
- Pie, S.; Lalles, J.P.; Blazy, F.; Laffitte, J.; Seve, B.; Oswald, I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef]
- Pluske, J.; Kim, J.; Black, J. Manipulating the immune system for pigs to optimise performance. Anim. Prod. Sci. 2018, 58, 666–680. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C. The development and role of microbial-host interactions in gut mucosal immune development. J. Anim. Sci. Biotechnol. 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Levast, B.; De Monte, M.; Melo, S.; Chevaleyre, C.; Berri, M.; Salmon, H.; Meurens, F. Differences in transcriptomic profile and IgA repertoire between jejunal and ileal Peyer’s patches. Dev. Comp. Immunol. 2010, 34, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Karmakar, S.; Babu, S.P. TLR2 and TLR4 mediated host immune responses in major infectious diseases: A review. Braz. J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef]
- Domeneghini, C.; Di Giancamillo, A.; Savoini, G.; Paratte, R.; Bontempo, V.; Dell’Orto, V. Structural patterns of swine ileal mucosa following L-glutamine and nucleotide administration during the weaning period. An histochemical and histometrical study. Histol. Histopathol. 2004, 19, 49–58. [Google Scholar] [CrossRef]
- Kreuzer, S.; Rieger, J.; Strucken, E.M.; Thaben, N.; Hunigen, H.; Nockler, K.; Janczyk, P.; Plendl, J.; Brockmann, G.A. Characterization of CD4+ subpopulations and CD25+ cells in ileal lymphatic tissue of weaned piglets infected with Salmonella Typhimurium with or without Enterococus faecium feeding. Vet. Immunol. Immunopathol. 2014, 158, 143–155. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.; Smidt, H.; Rebel, J.M.; Smits, M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef]
- Connell, H.; Agace, W.; Hedlund, M.; Klemm, P.; Shembri, M.; Svanborg, C. Fimbriae-mediated adherence induces mucosal inflammation and bacterial clearance. Consequences for anti-adhesion therapy. Adv. Exp. Med. Biol. 1996, 408, 73–80. [Google Scholar]
- Tamandl, D.; Bahrami, M.; Wessner, B.; Weigel, G.; Ploder, M.; Furst, W.; Roth, E.; Boltz-Nitulescu, G.; Spittler, A. Modulation of toll-like receptor 4 expression on human monocytes by tumor necrosis factor and interleukin-6: Tumor necrosis factor evokes lipopolysaccharide hyporesponsiveness, whereas interleukin-6 enhances lipopolysaccharide activity. Shock 2003, 20, 224–229. [Google Scholar] [CrossRef]
- Che, T.M.; Song, M.; Liu, Y.; Johnson, R.W.; Kelley, K.W.; Van Alstine, W.G.; Dawson, K.A.; Pettigrew, J.E. Mannan oligosaccharide increases serum concentrations of antibodies and inflammatory mediators in weanling pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2012, 90, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Rocadembosch, J.; Amador, J.; Bernaus, J.; Font, J.; Fraile, L.J. Production parameters and pig production cost: Temporal evolution 2010–2014. Porc. Health Manag. 2016, 2, 11. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (% as Fed) | Dietary Treatment | |
|---|---|---|
| CON | TRT | |
| Wheat meal | 33.80 | 33.60 |
| Corn meal | 24.00 | 24.00 |
| Soybean meal, 44% CP | 22.10 | 22.10 |
| Wheat bran | 10.00 | 10.00 |
| Soy protein concentrate 1 | 3.00 | 3.00 |
| Soy oil | 3.00 | 3.00 |
| Dicalcium phosphate, 18% | 1.00 | 1.00 |
| Animal fat | 1.00 | 1.00 |
| Calcium carbonate, 37% | 0.80 | 0.80 |
| L-Lysine HCl, 78% | 0.30 | 0.30 |
| Sodium chloride | 0.30 | 0.30 |
| Vitamin premix 2 | 0.30 | 0.30 |
| Mannan oligosaccharides 3 | - | 0.20 |
| L-Threonine, 98% | 0.15 | 0.15 |
| DL-Methionine, 98% | 0.10 | 0.10 |
| Sweetener 4 | 0.05 | 0.05 |
| Triptophane, 98% | 0.05 | 0.05 |
| Flavour 5 | 0.05 | 0.05 |
| Chemical composition 6,7 (% DM) | ||
| Moisture | 11.50 | 11.42 |
| Crude protein | 22.33 | 22.11 |
| Ether Extract | 4.82 | 4.90 |
| Crude Fiber | 3.15 | 2.97 |
| Ash | 6.10 | 7.01 |
| Ca | 0.76 | 0.75 |
| Total P | 0.71 | 0.70 |
| Lysine | 1.42 | 1.44 |
| Methionine | 0.51 | 0.50 |
| ME (Mcal/kg) | 3.82 | 3.78 |
| Item | Days of Treatment | Dietary Treatment | p-Value | ||
|---|---|---|---|---|---|
| CON | TRT | SEM | |||
| BW (kg) | 0 | 13.58 | 13.54 | 0.65 | 0.96 |
| 7 | 13.84 | 14.05 | 0.66 | 0.83 | |
| 14 | 16.68 | 16.72 | 0.87 | 0.98 | |
| 21 | 22.06 | 21.66 | 1.12 | 0.80 | |
| 28 | 27.07 | 27.4 | 1.18 | 0.85 | |
| 35 | 33.73 | 34.43 | 1.51 | 0.75 | |
| BWG (kg) | 0–7 | 0.26 | 0.51 | 0.21 | 0.41 |
| 7–14 | 2.84 | 2.67 | 0.29 | 0.68 | |
| 14–21 | 5.38 | 4.94 | 0.36 | 0.40 | |
| 21–28 | 5.01 | 5.74 | 0.29 | 0.09 | |
| 28–35 | 6.66 | 7.03 | 0.48 | 0.60 | |
| 0–35 | 20.15 | 20.89 | 1.12 | 0.65 | |
| FI (kg) | 0–7 | 2.84 | 2.86 | 0.21 | 0.94 |
| 7–14 | 4.99 | 5.21 | 0.08 | 0.07 | |
| 14–21 | 7.61 | 7.34 | 0.27 | 0.48 | |
| 21–28 | 9.35 | 8.93 | 0.39 | 0.46 | |
| 28–35 | 13.51 | 12.28 | 0.47 | 0.08 | |
| 0–35 | 38.30 | 36.63 | 1.17 | 0.32 | |
| G:F | 0–7 | 0.06 | 0.14 | 0.08 | 0.47 |
| 7–14 | 0.57 | 0.51 | 0.05 | 0.43 | |
| 14–21 | 0.70 | 0.67 | 0.03 | 0.55 | |
| 21–28 | 0.54 | 0.65 | 0.03 | 0.03 | |
| 28–35 | 0.49 | 0.57 | 0.025 | 0.04 | |
| 0–35 | 0.52 | 0.57 | 0.02 | 0.08 | |
| Bacterial Classes Isolated (log CFU/g) | Days of Treatment | Dietary Treatment | |||
|---|---|---|---|---|---|
| CON | TRT | SEM | p-Value | ||
| Clostridia | 0 | 5.61 | 5.82 | 0.30 | 0.63 |
| 14 | 3.79 | 3.00 | 0.26 | 0.05 | |
| 35 | 3.24 | 3.00 | 0.17 | 0.33 | |
| Lactobacilli | 0 | 9.47 | 8.52 | 0.23 | <0.01 |
| 14 | 9.51 | 10.02 | 0.18 | 0.06 | |
| 35 | 9.00 | 9.41 | 0.16 | 0.08 | |
| Coliforms | 0 | 8.05 | 8.48 | 0.37 | 0.42 |
| 14 | 7.16 | 7.54 | 0.39 | 0.50 | |
| 35 | 6.45 | 6.85 | 0.29 | 0.34 | |
| Lactobacilli/Coliforms | 0 | 1.43 | 0.04 | 0.53 | 0.02 |
| 14 | 2.35 | 2.48 | 0.63 | 0.85 | |
| 35 | 2.55 | 2.57 | 0.44 | 0.96 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agazzi, A.; Perricone, V.; Omodei Zorini, F.; Sandrini, S.; Mariani, E.; Jiang, X.-R.; Ferrari, A.; Crestani, M.; Nguyen, T.X.; Bontempo, V.; et al. Dietary Mannan Oligosaccharides Modulate Gut Inflammatory Response and Improve Duodenal Villi Height in Post-Weaning Piglets Improving Feed Efficiency. Animals 2020, 10, 1283. https://doi.org/10.3390/ani10081283
Agazzi A, Perricone V, Omodei Zorini F, Sandrini S, Mariani E, Jiang X-R, Ferrari A, Crestani M, Nguyen TX, Bontempo V, et al. Dietary Mannan Oligosaccharides Modulate Gut Inflammatory Response and Improve Duodenal Villi Height in Post-Weaning Piglets Improving Feed Efficiency. Animals. 2020; 10(8):1283. https://doi.org/10.3390/ani10081283
Chicago/Turabian StyleAgazzi, Alessandro, Vera Perricone, Fabio Omodei Zorini, Silvia Sandrini, Elena Mariani, Xian-Ren Jiang, Alessandra Ferrari, Maurizio Crestani, Thi Xuan Nguyen, Valentino Bontempo, and et al. 2020. "Dietary Mannan Oligosaccharides Modulate Gut Inflammatory Response and Improve Duodenal Villi Height in Post-Weaning Piglets Improving Feed Efficiency" Animals 10, no. 8: 1283. https://doi.org/10.3390/ani10081283
APA StyleAgazzi, A., Perricone, V., Omodei Zorini, F., Sandrini, S., Mariani, E., Jiang, X.-R., Ferrari, A., Crestani, M., Nguyen, T. X., Bontempo, V., Domeneghini, C., & Savoini, G. (2020). Dietary Mannan Oligosaccharides Modulate Gut Inflammatory Response and Improve Duodenal Villi Height in Post-Weaning Piglets Improving Feed Efficiency. Animals, 10(8), 1283. https://doi.org/10.3390/ani10081283






