Characterization of Different Commercial Dietary Supplements in the Peri-Weaning Period on Consumption and Growth Performance in C57Bl/6J Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Husbandry
2.3. Dietary Supplementation
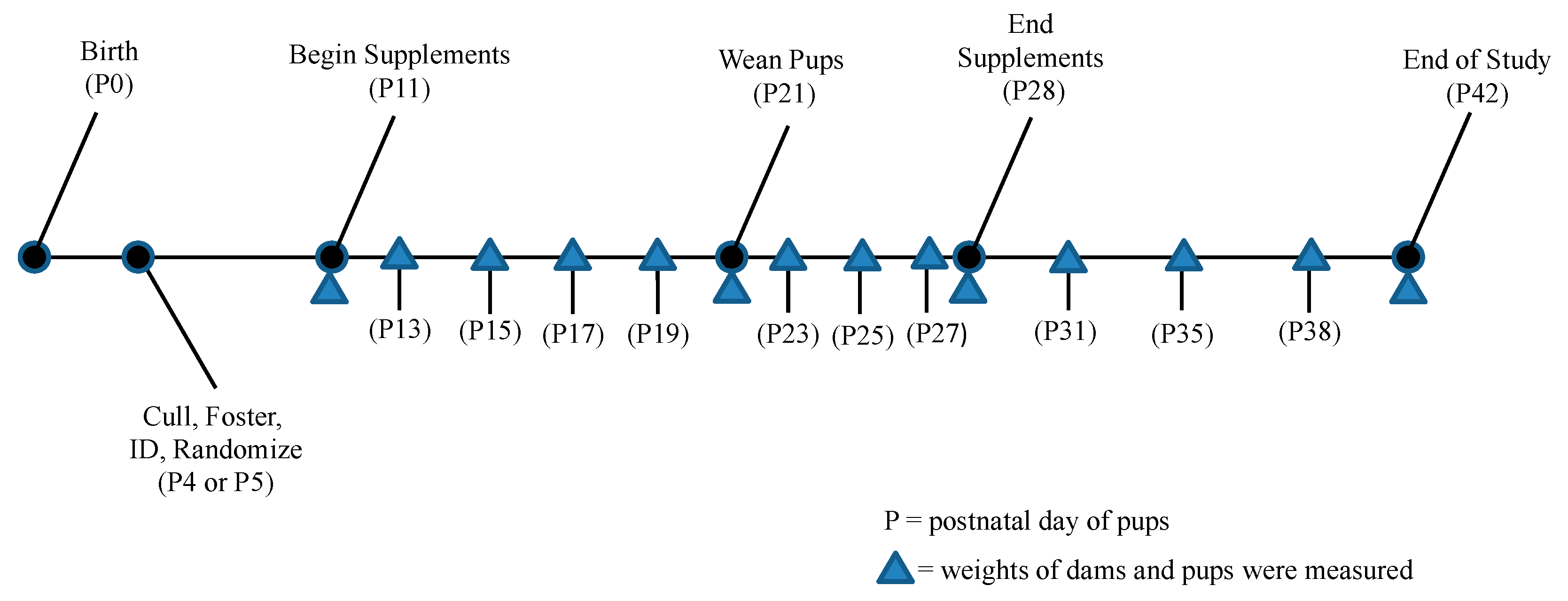
2.4. Data Collection and Animal Handling
2.5. Statistics
3. Results
3.1. Survival and Birth Weights
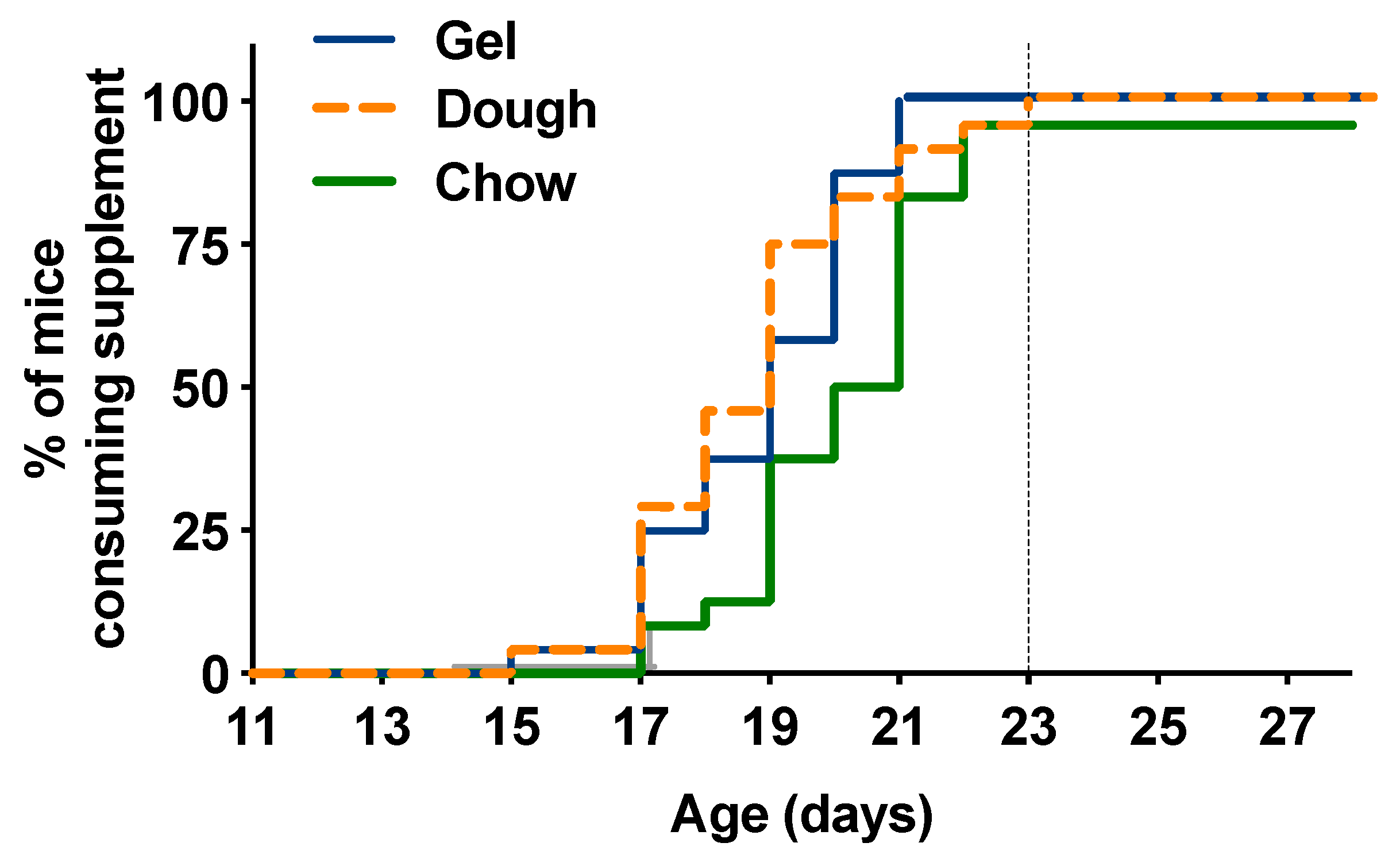
3.2. Time to Consumption for Pups
3.3. Time to Consumption and Weight of Dams
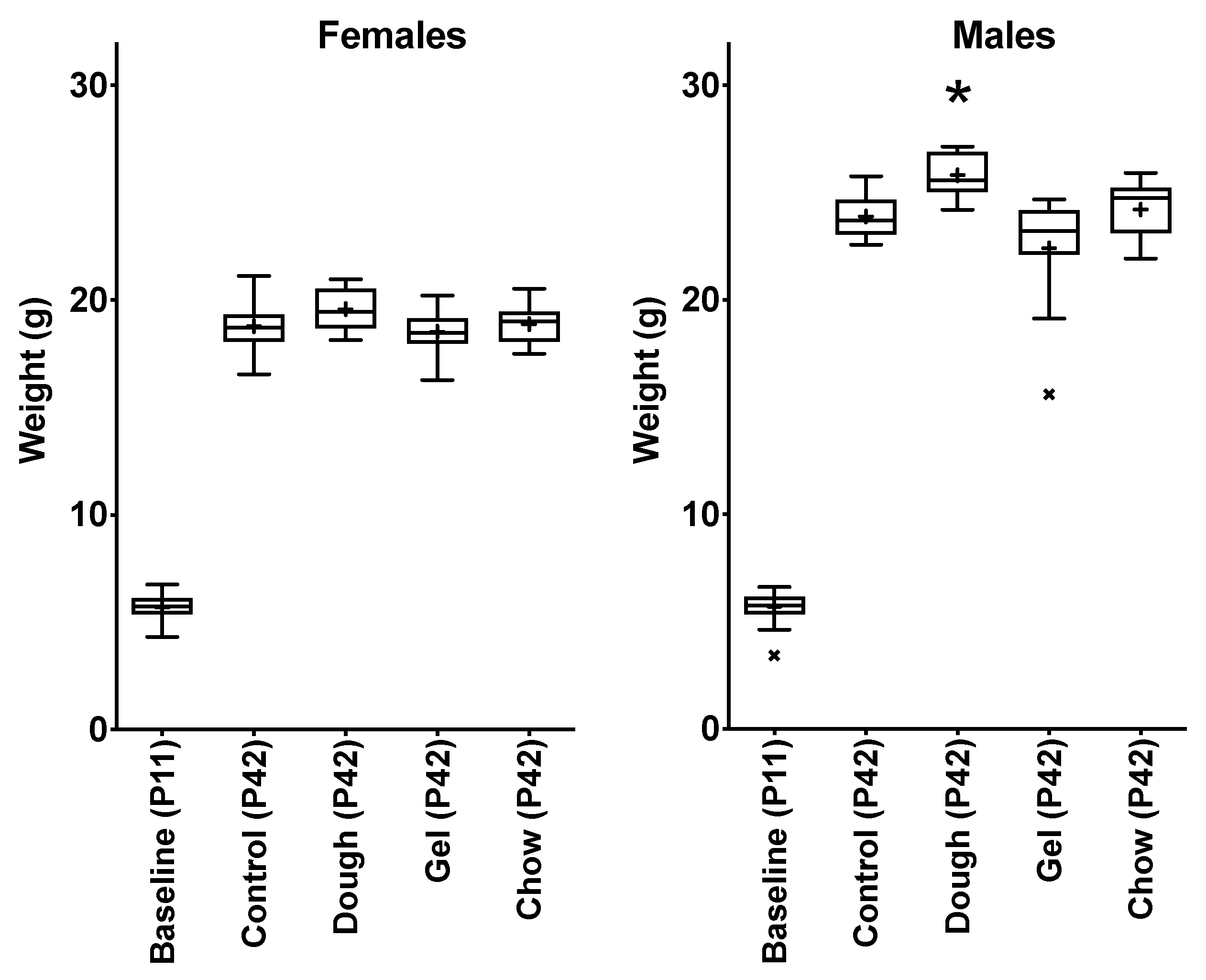
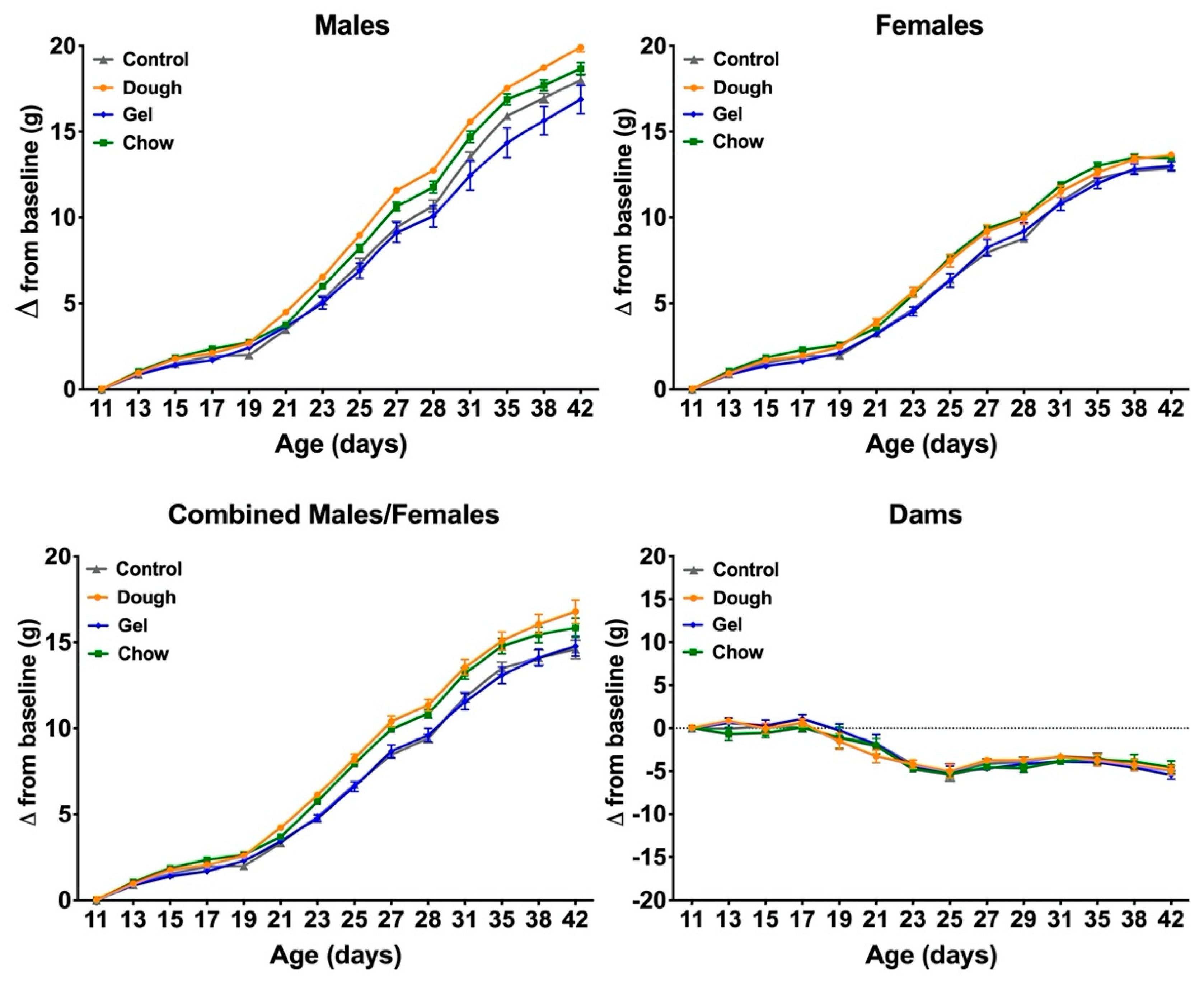
3.4. Comparison of Growth between Pup Groups
4. Discussion
4.1. Supplement Nutritional Profile and Influence on Time to Consumption
4.2. Survival
4.3. Comparison of Weight Gain
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| P | postnatal day |
| EDIM | epizootic diarrhea of infant mice |
| MHV | mouse hepatitis virus |
| MNV | mouse norovirus |
| MPV | mouse parvovirus |
| MVM | minute virus of mice |
| TMEV | Theiler’s encephalomyelitis virus |
References
- Moccia, K.D.; Olsen, C.H.; Mitchell, J.M.; Landauer, M.R. Evaluation of Hydration and Nutritional Gels as Supportive Care after Total-Body Irradiation in Mice (Mus musculus). J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 323–328. [Google Scholar] [PubMed]
- Whary, M.T.; Baumgarth, N.; Fox, J.G.; Barthold, S.W. Biology and Diseases of Mice. In Laboratory Animal Medicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 43–149. [Google Scholar]
- Caron, E.; Ciofi, P.; Prevot, V.; Bouret, S.G. Alteration in neonatal nutrition causes perturbations in hypothalamic neural circuits controlling reproductive function. J. Neurosci. 2012, 32, 11486–11494. [Google Scholar] [CrossRef] [PubMed]
- Aubert, R.; Suquet, J.-P.; Lemonnier, D. Long-Term Morphological and Metabolic Effects of Early Under- and Over-Nutrition in Mice. J. Nutr. 1980, 110, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tarry-Adkins, J.L.; Heppolette, C.A.; Palmer, D.B.; Ozanne, S.E. Early-life nutrition influences thymic growth in male mice that may be related to the regulation of longevity. Clin. Sci. 2009, 118, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, A. Fetal programming: Link between early nutrition, DNA methylation, and complex diseases. Nutr. Rev. 2010, 68, 87–98. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Ozanne, S.E.; Hales, C.N. Catch-up growth and obesity in male mice. Nature 2004, 427, 411–412. [Google Scholar] [CrossRef]
- Prescott, S. Early Nutrition as a Major Determinant of ‘Immune Health’: Implications for Allergy, Obesity and Other Noncommunicable Diseases. In Preventive Aspects of Early Nutrition; Karger Publishers: London, UK, 2016; Volume 85, pp. 1–17. [Google Scholar]
- Waterland, R.A.; Jirtle, R.L. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 2004, 20, 63–68. [Google Scholar] [CrossRef]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef]
- Hermann, G.M.; Miller, R.L.; Erkonen, G.E.; Dallas, L.M.; Hsu, E.; Zhu, V.; Roghair, R.D. Neonatal catch up growth increases diabetes susceptibility but improves behavioral and cardiovascular outcomes of low birth weight male mice. Pediatr. Res. 2009, 66, 53–58. [Google Scholar] [CrossRef]
- Ozanne, S.E.; Lewis, R.M.; Jennings, B.J.; Hales, C.N. Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin. Sci. 2004, 106, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Parente, L.B.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Deleterious effects of high-fat diet on perinatal and postweaning periods in adult rat offspring. Clin. Nutr. 2008, 27, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, K.W.; Totoki, K.; Reyes, T.M. Metabolic adaptations to early life protein restriction differ by offspring sex and post-weaning diet in the mouse. Nutr. Metab. Cardiovasc. Dis. 2011, 22, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Barber, H. P85 Influence of nutritional supplements on growth of weanling mice. In Proceedings of the AALAS National Meeting, San Antonio, TX, USA, 19–23 October 2014; p. 571. [Google Scholar]
- Huss, M.C.A.; Nagamine, C. P120 Supplemental Diet Aids Early Weaning of Crl:CD1(ICR) Mouse Pups. In Proceedings of the AALAS National Meeting, San Antonio, TX, USA, 19–23 October 2014; p. 580. [Google Scholar]
- Peveler, J.L.D.H. P121 Evaluation of 6 Industry Diets on the Reproductive Success of DBA-2 Mice. In Proceedings of the AALAS National Meeting, Phoenix, AZ, USA, 1–5 November 2015; p. 598. [Google Scholar]
- Southwell, K.S.P. P91 The Effects of 2 Different Supplemental Gels on C57BL/6J Pup Survival and Weights. In Proceedings of the AALAS National Meeting, Minneapolis, MN, USA, 4–8 November 2012; p. 676. [Google Scholar]
- Overbeek, P.A. Factors affecting transgenic animal production. In Transgenic Animal Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 71–107. [Google Scholar]
- Castelhano-Carlos, M.J.; Sousa, N.; Ohl, F.; Baumans, V. Identification methods in newborn C57BL/6 mice: A developmental and behavioural evaluation. Lab. Anim. 2010, 44, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.C.; Asner, I.N.; Seifert, B.; Bürki, K.; Cinelli, P. Analysis of physiological and behavioural parameters in mice after toe clipping as newborns. Lab. Anim. 2010, 44, 7–13. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals; National Academies Press: Cambridge, MA, USA, 2010. [Google Scholar]
- BioServ. Nutritional Profile Porduct # s3472—Tansgenic Dough Diet, Bacon Flavor, 1 kg/bag Sterile. Available online: https://www.bio-serv.com/pdf/S3472.pdf (accessed on 10 June 2020).
- Envigo. 2019 Teklad Global 19% Protein Extruded Rodent Diet. Available online: https://insights.envigo.com/hubfs/resources/data-sheets/2019-datasheet-0915.pdf (accessed on 10 June 2020).
- H20 C. DietGel Boost Purified High Energy Dietary Supplement. Available online: https://www.clearh2o.com/wp-content/uploads/2020/03/DietGelBoost-Sheet.pdf?x28536 (accessed on 10 June 2020).
- Assaad, H.I.; Hou, Y.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables for two-way ANOVA. SpringerPlus 2015, 4, 33. [Google Scholar] [CrossRef]
- Assaad, H.I.; Zhou, L.; Carroll, R.J.; Wu, G.J.S. Rapid publication-ready MS-Word tables for one-way ANOVA. SpringerPlus 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef]
- Myagmarjalbuu, B.; Moon, M.J.; Heo, S.H.; Jeong, S.I.; Park, J.-S.; Jun, J.Y.; Jeong, Y.Y.; Kang, H.K. Establishment of a Protocol for Determining Gastrointestinal Transit Time in Mice Using Barium and Radiopaque Markers. Korean J. Radiol. 2013, 14, 45–50. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Grosse, J.; Asad, A.B.M.A.; Radda, G.K.; Golay, X. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 2013, 3, 60. [Google Scholar] [CrossRef]
- Schwarz, R.; Kaspar, A.; Seelig, J.; Künnecke, B. Gastrointestinal transit times in mice and humans measured with27Al and19F nuclear magnetic resonance. Magn. Reson. Med. 2002, 48, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Nutrient Requirements of Laboratory Animals: 1995; National Academies Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Grippo, R.M.; Tang, Q.; Zhang, Q.; Chadwick, S.R.; Gao, Y.; Altherr, E.B.; Sipe, L.; Purohit, A.M.; Purohit, N.M.; Sunkara, M.D.; et al. Dopamine Signaling in the Suprachiasmatic Nucleus Enables Weight Gain Associated with Hedonic Feeding. Curr. Biol. 2020, 30, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Londei, T.; Calci, M.; Leone, V.G. First experiences with solid food by mouse pups: Effects of age and the presence of the mother. Bolletino di Zool. 1988, 55, 155–159. [Google Scholar] [CrossRef]
- Galef, B.G.; Sherry, D.F. Mother’s milk: A medium for transmission of cues reflecting the flavor of mother’s diet. J. Comp. Physiol. Psychol. 1973, 83, 374. [Google Scholar] [CrossRef] [PubMed]
- Reproductive Performance Survey, Females of 33 Inbred Strains of Mice. MPD:14943. 1991. Available online: https://phenome.jax.org/projects/Jax3 (accessed on 20 October 2017).
- Koopman, J.P.; Scholten, P.M.; Brink, M.E.V.D.; Beynen, A.C. Availability of solid feed and growth rates of pre-weaned mice of different litter size. Z. fur Vers. 1990, 33, 217–219. [Google Scholar]
- Mousel, E.; Wright, C.; Walker, J.; Gessner, H. Creep Feeding Beef Calves. SDSU Extension Extra Archives. 2010; 94. Available online: https://openprairie.sdstate.edu/extension_extra/94 (accessed on 20 October 2017).
- Sulabo, R.C.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; DeRouchey, J.M.; Nelssen, J.L. Effects of varying creep feeding duration on the proportion of pigs consuming creep feed and neonatal pig performance1. J. Anim. Sci. 2010, 88, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Danson, A.; Marzi, S.J.; Lowe, R.; Holland, M.L.; Rakyan, V.K. Early life diet conditions the molecular response to post-weaning protein restriction in the mouse. BMC Boil. 2018, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.P.; Scholten, P.M.; Beynen, A.C. Hardness of diet pellets and growth of pre-weaned mice: Separation of direct effects on the young and indirect effects mediated by the lactating females. Z. fur Vers. 1989, 32, 257. [Google Scholar]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain, Behav. Immun. 2014, 37, 134–141. [Google Scholar] [CrossRef]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Layé, S.; Ferreira, G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef]
- Hoevenaars, F.P.M.; Keijer, J.; Swarts, H.J.; Snaas-Alders, S.; Bekkenkamp-Grovenstein, M.; Van Schothorst, E.M. Effects of dietary history on energy metabolism and physiological parameters in C57BL/6J mice. Exp. Physiol. 2013, 98, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.E.; Czarnecka, M.; Kitlinska, J.B.; Tilan, J.U.; Kvetnansky, R.; Zukowska, Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann. New York Acad. Sci. 2008, 1148, 232. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, S.L.; Scott, A.N.; Bale, T.L. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 2009, 162, 924–932. [Google Scholar] [CrossRef] [PubMed]
| Manufacturer (Product ID) | Protein % | Fat % | Carbohydrate % | Moisture % | Energy Density a Kcal/gram | |
|---|---|---|---|---|---|---|
| Dough | Bio-Serv (Transgenic Dough Diet) | 21.2 | 12.4 | 46.5 | <12 | 3.83 |
| Gel | ClearH2O (DietGel Boost) | 9.9 | 21.6 | 37.8 | 25–30 | 3.69 |
| Chowb | Teklad (2919) | 19.0 | 9.0 | 44.9 | 47–59 c | 3.3 |
| Phase | Control | Dough (DNS) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| F (n = 16) | M (n = 8) | F (n = 12) | M (n = 12) | Diet | Sex | D◊S 1 | |
| P11 | 5.9 ± 0.1 | 5.9 ± 0.2 | 5.9 ± 0.2 | 5.9 ± 0.1 | 0.95 | 0.86 | 0.92 |
| P21 | 9.2 ± 0.2 b | 9.3 ± 0.3 a,b | 9.8 ± 0.4 a,b | 10.4 ± 0.2 a | 0.001 | 0.14 | 0.44 |
| P28 | 14.7 ± 0.3 c | 16.5 ± 0.5 b | 15.9 ± 0.5 b,c | 18.6 ± 0.2 a | <0.001 | <0.001 | 0.20 |
| P42 | 18.8 ± 0.3 c | 23.9 ± 0.4 b | 19.6 ± 0.3 c | 25.8 ± 0.3 a | <0.001 | <0.001 | 0.07 |
| Phase | Control | Moist (MNS) | p-Value | ||||
| F (n = 16) | M (n = 8) | F (n = 13) | M (n = 11) | Diet | Sex | D◊S 1 | |
| P11 | 5.9 ± 0.1 a | 5.9 ± 0.2 a,b | 5.4 ± 0.1 b | 5.5 ± 0.1 a,b | 0.002 | 0.73 | 0.53 |
| P21 | 9.2 ± 0.2 | 9.3 ± 0.3 | 9.0 ± 0.2 | 9.3 ± 0.2 | 0.63 | 0.23 | 0.71 |
| P28 | 14.7 ± 0.3 c | 16.5 ± 0.5 a,b | 15.5 ± 0.2 b,c | 17.3 ± 0.4 a | 0.003 | <0.001 | 0.98 |
| P42 | 18.8 ± 0.3 b | 23.9 ± 0.4 a | 18.9 ± 0.3 b | 24.2 ± 0.4 a | 0.012 | <0.001 | 0.72 |
| Phase | Control | Gel (GNS) | p-Value | ||||
| F (n = 16) | M (n = 8) | F (n = 13) | M (n = 11) | Diet | Sex | D◊S 1 | |
| P11 | 5.9 ± 0.1 | 5.9 ± 0.2 | 5.5 ± 0.2 | 5.5 ± 0.3 | 0.07 | 0.926 | 0.92 |
| P21 | 9.2 ± 0.2 | 9.3 ± 0.3 | 8.7 ± 0.4 | 9.2 ± 0.4 | 0.34 | 0.332 | 0.68 |
| P28 | 14.7 ± 0.3 | 16.5 ± 0.5 | 14.7 ± 0.6 | 15.6 ± 0.8 | 0.73 | 0.018 | 0.37 |
| P42 | 18.8 ± 0.3 b | 23.9 ± 0.4 a | 18.5 ± 0.3 b | 22.4 ± 0.8 a | 0.68 | <0.001 | 0.21 |
| Females | Control (n = 16) | Gel (n = 13) | Dough (n = 12) | Chow (n = 13) |
|---|---|---|---|---|
| ADG 11–21 | 0.32 ± 0.01 b | 0.32 ± 0.01 b | 0.39 ± 0.02 a | 0.36 ± 0.01 a,b |
| ADG 22–28 | 0.79 ± 0.02 a,b | 0.86 ± 0.06 a,b | 0.87 ± 0.03 a,b | 0.93 ± 0.02 a |
| ADG-29–42 | 0.29 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.03 | 0.24 ± 0.01 |
| Cumulative ADG 11–42 | 0.42 ± 0.01 | 0.42 ± 0.01 | 0.47 ± 0.06 | 0.43 ± 0.01 |
| Males | Control (n = 8) | Gel (n = 11) | Dough (n = 12) | Chow (n = 11) |
| ADG 11–21 | 0.35 ± 0.02 b | 0.36 ± 0.02 b | 0.45 ± 0.01 a | 0.38 ± 0.01 b |
| ADG 22–28 | 1.03 ± 0.03 a,b | 0.92 ± 0.08 b | 1.18 ± 0.01 a | 1.15 ± 0.04 a |
| ADG-29–42 | 0.52 ± 0.02 | 0.49 ± 0.03 | 0.51 ± 0.01 | 0.49 ± 0.01 |
| Cumulative ADG 11–42 | 0.58 ± 0.01 a,b | 0.54 ± 0.03 b | 0.64 ± 0.01 a | 0.6 ± 0.01 a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craig, A.M.; Graham, M.L. Characterization of Different Commercial Dietary Supplements in the Peri-Weaning Period on Consumption and Growth Performance in C57Bl/6J Mice. Animals 2020, 10, 1284. https://doi.org/10.3390/ani10081284
Craig AM, Graham ML. Characterization of Different Commercial Dietary Supplements in the Peri-Weaning Period on Consumption and Growth Performance in C57Bl/6J Mice. Animals. 2020; 10(8):1284. https://doi.org/10.3390/ani10081284
Chicago/Turabian StyleCraig, Angela M., and Melanie L. Graham. 2020. "Characterization of Different Commercial Dietary Supplements in the Peri-Weaning Period on Consumption and Growth Performance in C57Bl/6J Mice" Animals 10, no. 8: 1284. https://doi.org/10.3390/ani10081284
APA StyleCraig, A. M., & Graham, M. L. (2020). Characterization of Different Commercial Dietary Supplements in the Peri-Weaning Period on Consumption and Growth Performance in C57Bl/6J Mice. Animals, 10(8), 1284. https://doi.org/10.3390/ani10081284






