Ejaculate Collection Influences the Salivary Oxytocin Concentrations in Breeding Male Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ejaculate Collection
2.2. Saliva Collection and Oxytocin Measurement
2.3. Measurement of Salivary Oxytocin Concentration
2.4. Experimental Design
2.5. Statistical Analysis
3. Results
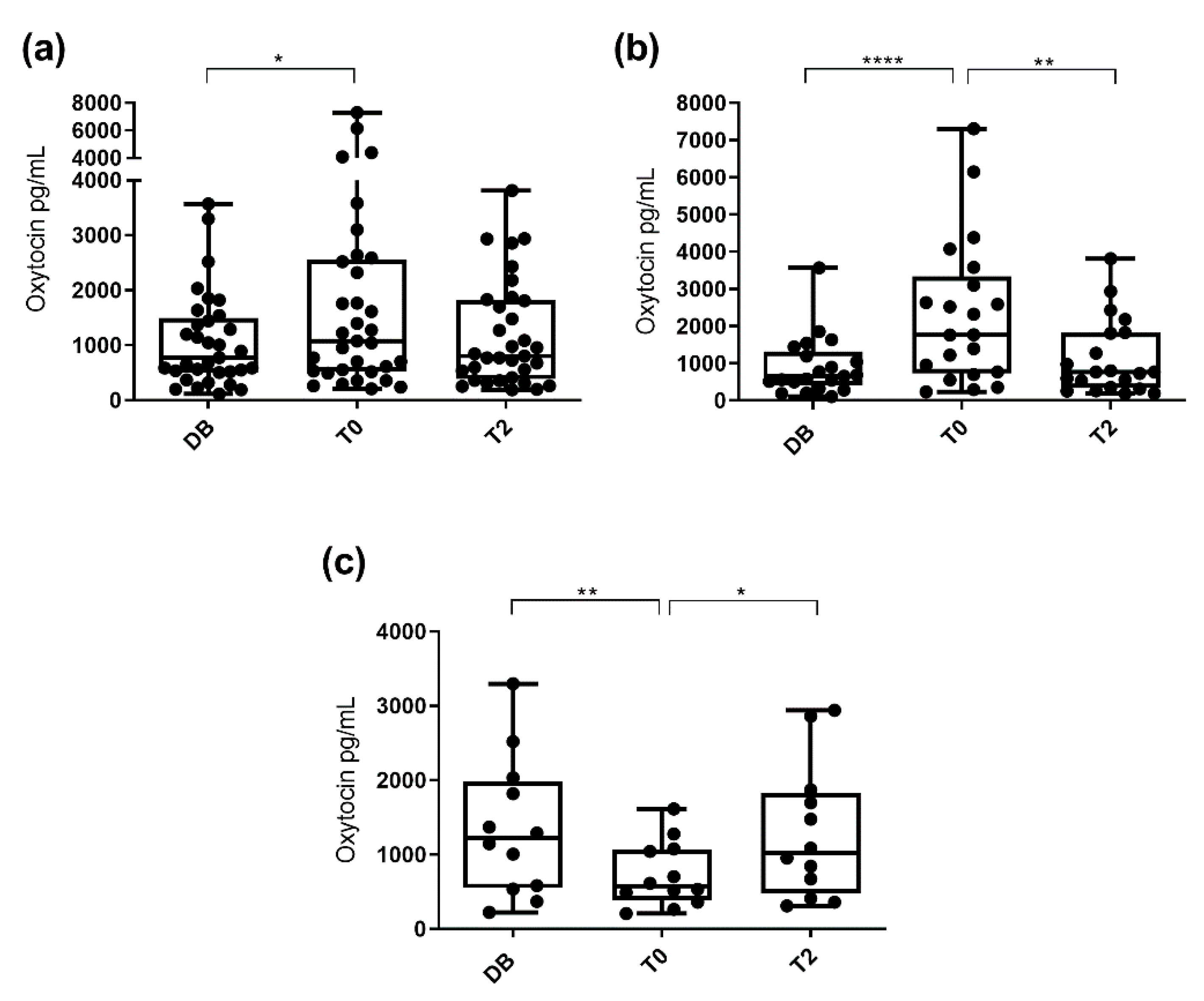
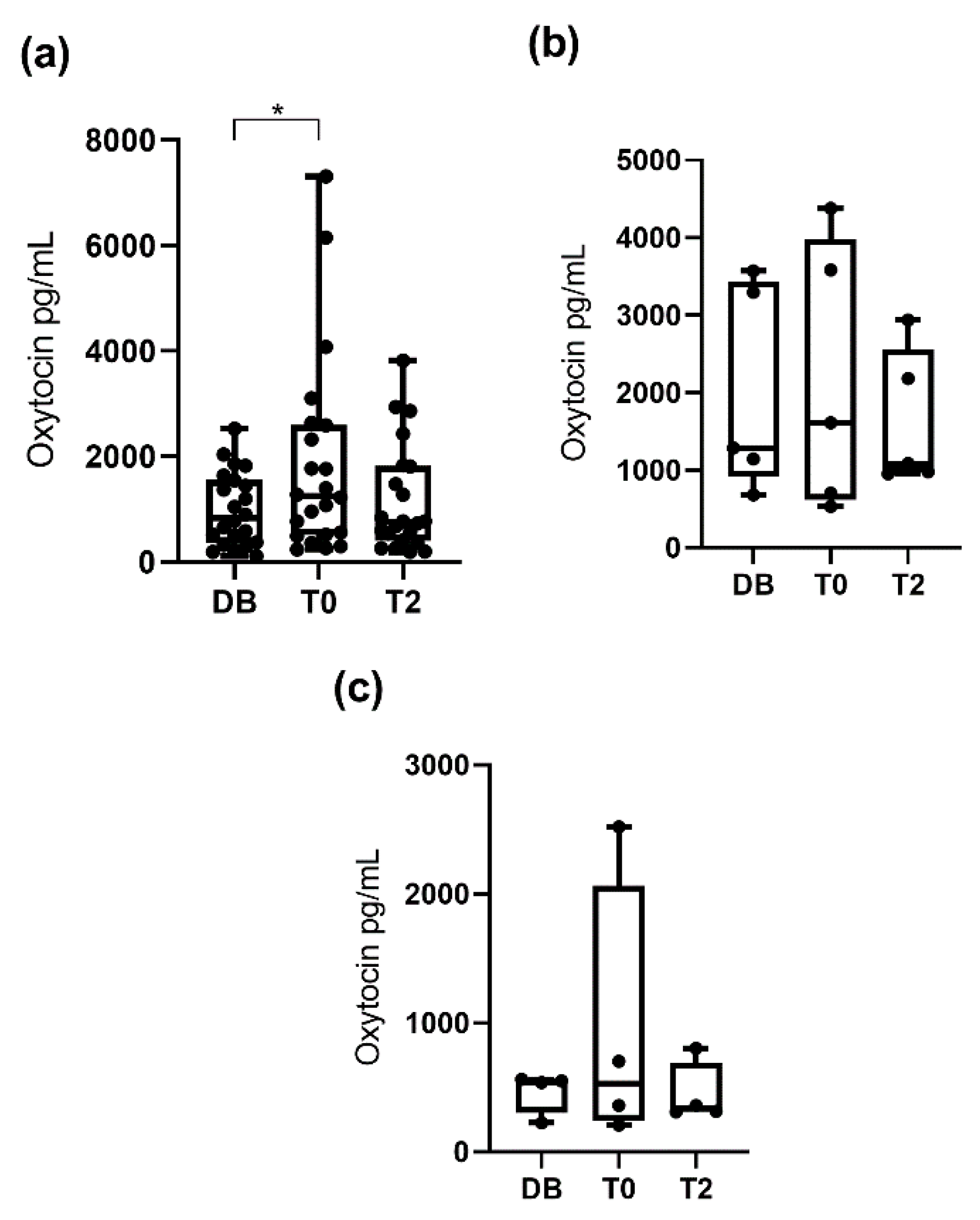
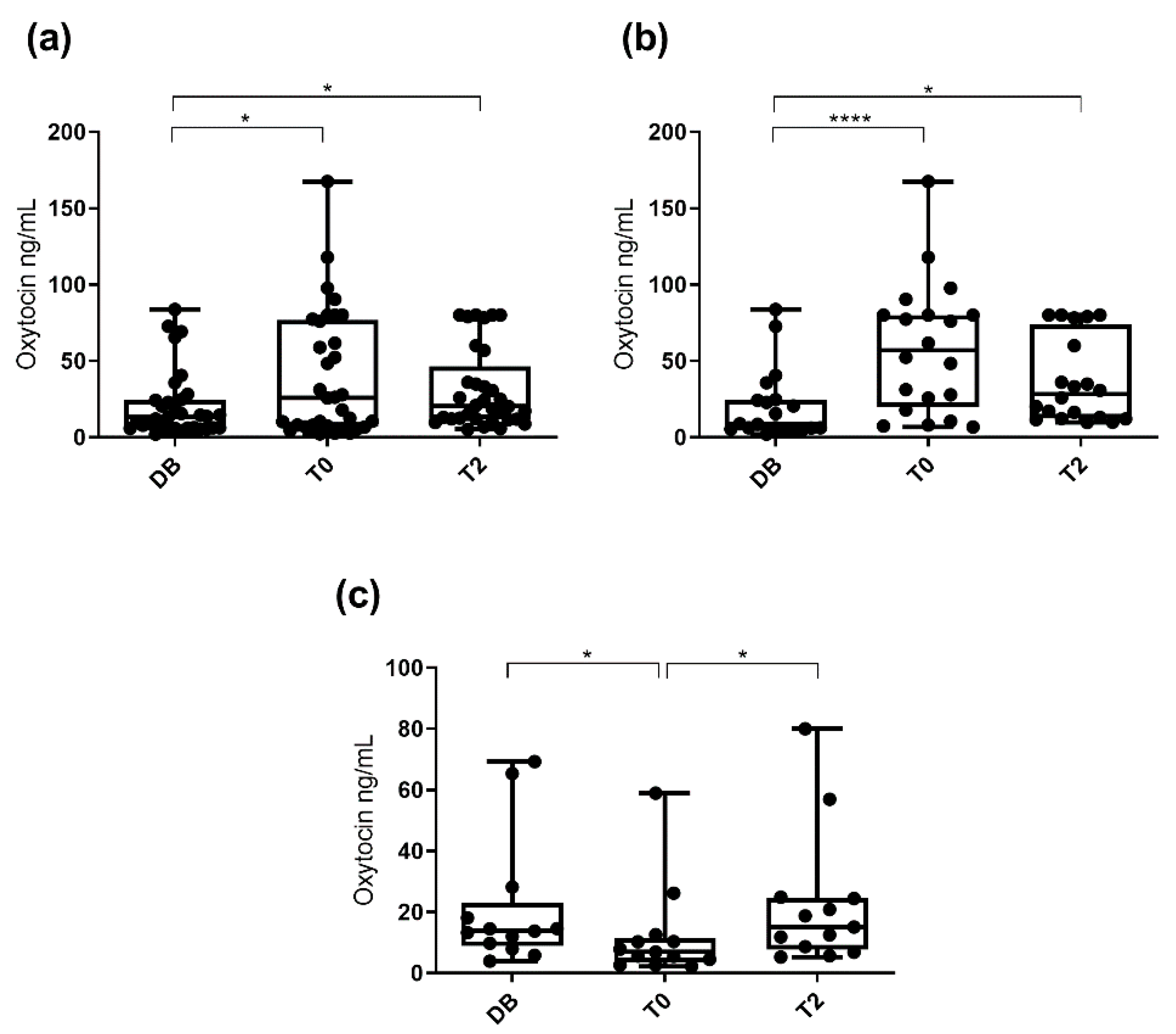
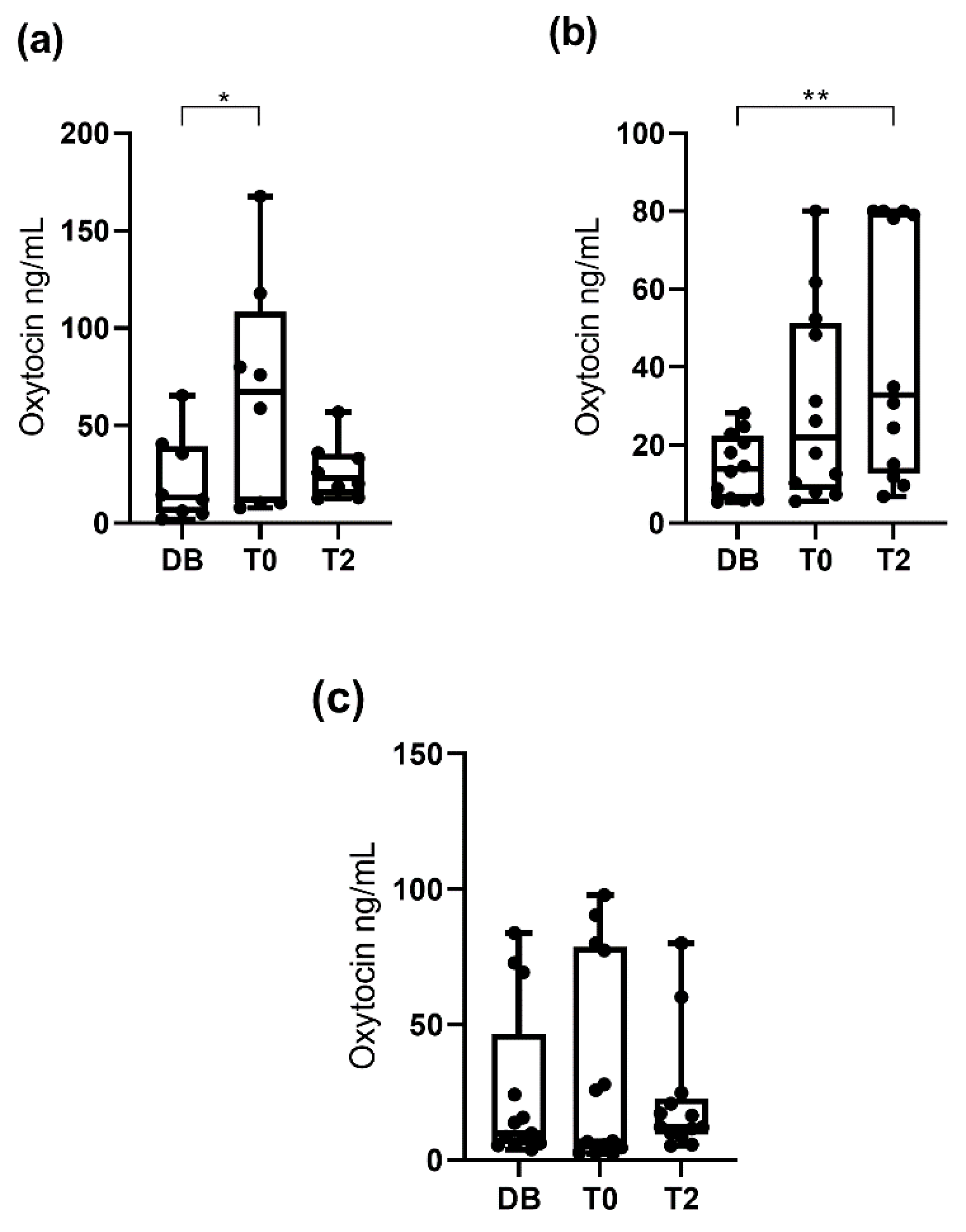
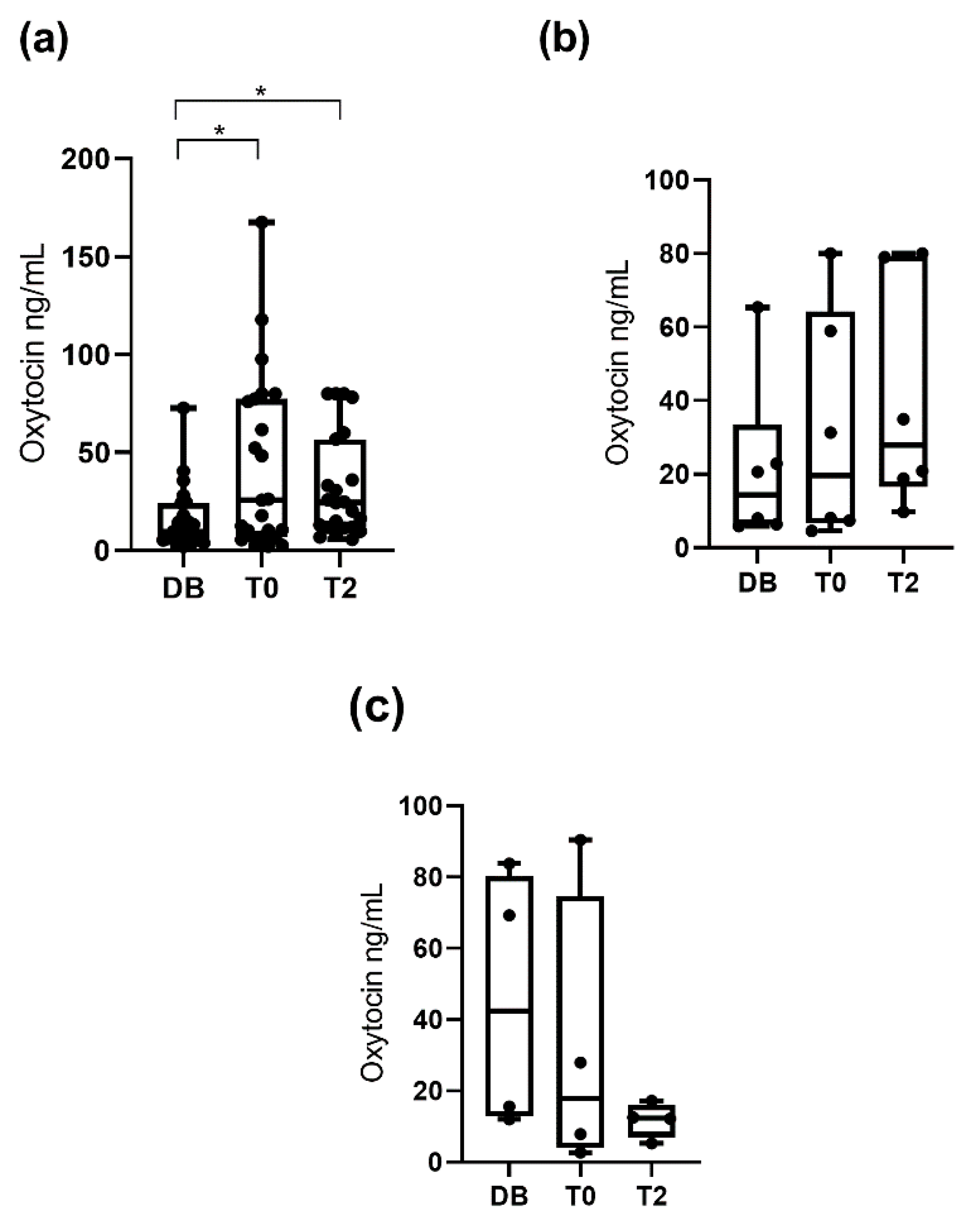
3.1. Oxytocin Concentrations Measured with the Monoclonal Assay
3.2. Oxytocin Concentration Measured with the Polyclonal Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hruby, V.J.; Chow, M.-S. Conformational and structural considerations in oxytocin-receptor binding and biological activity. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 501–534. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A.; Leng, G. Sex, parturition and motherhood without oxytocin? J. Endocrinol. 1998, 157, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Chiodera, P.; Salvarani, C.; Bacchi-Modena, A.; Spallanzani, R.; Cigarini, C.; Alboni, A.; Gardini, E.; Coiro, V. Relationship between Plasma Profiles of Oxytocin and Adrenocorticotropic Hormone during Suckling or Breast Stimulation in Women. Horm. Res. 1991, 35, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.L.; Komleva, Y.K.; Gorina, Y.V.; Olovyannikova, R.Y.; Trufanova, L.V.; Hashimoto, T.; Takahashi, T.; Kikuchi, M.; Minabe, Y.; Higashida, H.; et al. Oxytocin and excitation/inhibition balance in social recognition. Neuropepeptides 2018, 72, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Shima, R.; Yoshioka, T. A novel role of oxytocin: Oxytocin-induced well-being in humans. Biophys. Physicobiol. 2019, 16, 132–139. [Google Scholar] [CrossRef]
- Rault, J.L.; van den Munkhof, M.; Buisman-Pijlman, F.T.A. Oxytocin as an indicator of psychological and social well-being in domesticated animals: A critical review. Front. Psychol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Langendijk, P.; Bouwman, E.G.; Schams, D.; Soede, N.M.; Kemp, B. Effects of different sexual stimuli on oxytocin release, uterine activity and receptive behavior in estrous sows. Theriogenology 2003, 59, 849–861. [Google Scholar] [CrossRef]
- Yun, J.; Björkman, S.; Oliviero, C.; Soede, N.; Peltoniemi, O. The effect of farrowing duration and parity on preovulatory follicular size and oxytocin release of sows at subsequent oestrus. Reprod. Domest. Anim. 2018, 53, 776–783. [Google Scholar] [CrossRef]
- Thackare, H.; Nicholson, H.D.; Whittington, K. Oxytocin—Its role in male reproduction and new potential therapeutic uses. Hum. Reprod. Update 2006, 12, 437–448. [Google Scholar] [CrossRef]
- Arletti, R.; Benelli, A.; Bertolini, A. Sexual behavior of aging male rats is stimulated by oxytocin. Eur. J. Pharmacol. 1990, 179, 377–381. [Google Scholar] [CrossRef]
- Melin, P.; Kihlstroem, J.E. Influence of Oxytocin on Sexual Behavior in Male Rabbits. Endocrinology 1963, 73, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.W.; Amundson, S.D.; Brito, L.F.C.; Waldner, C.L.; Barth, A.D. Use of oxytocin and cloprostenol to facilitate semen collection by electroejaculation or transrectal massage in bulls. Anim. Reprod. Sci. 2004, 80, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Gerendai, I.; Csaba, Z.; Csernus, V. Effect of intratesticular administration of somatostatin on testicular function in immature and adult rats. Life Sci. 1996, 59, 859–866. [Google Scholar] [CrossRef]
- Gibson, S.; Tempelman, R.J.; Kirkwood, R.N. Effect of oxytocin-supplemented semen on fertility of sows bred by intrauterine insemination. J. Swine Health Prod. 2004, 12, 182–185. [Google Scholar]
- Okazaki, T.; Ikoma, E.; Tinen, T.; Akiyoshi, T.; Mori, M.; Teshima, H. Addition of oxytocin to semen extender improves both sperm transport to the oviduct and conception rates in pigs following AI. Anim. Sci. J. 2014, 85, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ngula, J.; Manjarín, R.; Martínez-Pastor, F.; Alegre, B.; Tejedor, I.; Brown, T.; Piñán, J.; Kirkwood, R.N.; Domínguez, J.C. A novel semen supplement (SuinFort) improves sow fertility after artificial insemination. Anim. Reprod. Sci. 2019, 210, 106193. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.R.; Seckl, J.R.; Burton, S.; Checkley, S.A.; Lightman, S.L. Changes in oxytocin and vasopressin secretion during sexual activity in men. J. Clin. Endocrinol. Metab. 1987, 65, 738–741. [Google Scholar] [CrossRef]
- Ogawa, S.; Kudo, S.; Kitsunai, Y.; Fukuchi, S. Increase in Oxytocin Secretion at Ejaculation in Male. Clin. Endocrinol. 1980, 13, 95–97. [Google Scholar] [CrossRef]
- López-Arjona, M.; Mateo, S.V.; Manteca, X.; Escribano, D.; Cerón, J.J.; Martínez-Subiela, S. Oxytocin in saliva of pigs: An assay for its measurement and changes after farrowing. Domest. Anim. Endocrinol. 2020, 70, 106384. [Google Scholar] [CrossRef]
- Lürzel, S.; Bückendorf, L.; Waiblinger, S.; Rault, J.L. Salivary oxytocin in pigs, cattle, and goats during positive human-animal interactions. Psychoneuroendocrinology 2020, 115, 104636. [Google Scholar] [CrossRef]
- de Jong, T.R.; Menon, R.; Bludau, A.; Grund, T.; Biermeier, V.; Klampfl, S.M.; Jurek, B.; Bosch, O.J.; Hellhammer, J.; Neumann, I.D. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology 2015, 62, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, M.S.; Humbert, R.; Dixen, J.; Palmisano, G.; Greenleaf, W.; Davidson, J.M. Plasma oxytocin increases in the human sexual response. J. Clin. Endocrinol. Metab. 1987, 64, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.D.; Malven, P.V.; Singleton, W.L.; Weesner, G.D. Hormonal and behavioral correlates of emotional states in sexually trained boars. J. Anim. Sci. 1999, 77, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- López-Arjona, M.; Mateo, S.V.; Escribano, D.; Tecles, F.; Cerón, J.J.; Martínez-Subiela, S. Effect of reduction and alkylation treatment in three different assays used for the measurement of oxytocin in saliva of pigs. Domest. Anim. Endocrinol. 2020, 74, 106498. [Google Scholar] [CrossRef] [PubMed]
- Kozink, D.M.; Estienne, M.J.; Harper, A.F.; Knight, J.W. The effect of lutalyse on the training of sexually inexperienced boars for semen collection. Theriogenology 2002, 58, 1039–1045. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin, EEUU: Boston, MA, USA, 2003. [Google Scholar]
- Hughes, A.M.; Everitt, B.J.; Lightman, S.L.; Todd, K. Oxytocin in the central nervous system and sexual behaviour in male rats. Brain Res. 1987, 414, 133–137. [Google Scholar] [CrossRef]
- Stoneham, M.D.; Everitt, B.J.; Hansen, S.; Lightman, S.L.; Todd, K. Oxytocin and sexual behaviour in the male rat and rabbit. J. Endocrinol. 1985, 107, 97–106. [Google Scholar] [CrossRef]
- Carter, C.S.; Pournajafi-Nazarloo, H.; Kramer, K.M.; Ziegler, T.E.; White-Traut, R.; Bello, D.; Schwertz, D. Oxytocin: Behavioral associations and potential as a salivary biomarker. Ann. N. Y. Acad. Sci. 2007, 1098, 312–322. [Google Scholar] [CrossRef]
- Landgraf, R.; Neumann, I.D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004, 25, 150–176. [Google Scholar] [CrossRef]
- Gröschl, M. Current Status of Salivary Hormone Analysis. Clin. Chem. 2008, 54, 1759–1769. [Google Scholar] [CrossRef]
- Quintana, D.S.; Westlye, L.T.; Smerud, K.T.; Mahmoud, R.A.; Andreassen, O.A.; Djupesland, P.G. Saliva oxytocin measures do not reflect peripheral plasma concentrations after intranasal oxytocin administration in men. Horm. Behav. 2018, 102, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Kagerbauer, S.M.; Gempt, J.; Podtschaske, A.; Hapfelmeier, A.; Schneider, G. Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. J. Neuroendocrinol. 2018, 30, e12596. [Google Scholar] [CrossRef] [PubMed]
- Frayne, J.; Nicholson, H.D. Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: Evidence for a physiological role of oxytocin in the male. Mol. Hum. Reprod. 1998, 4, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Ivell, R.; Balvers, M.; Rust, W.; Bathgate, R.; Einspanier, A. Oxytocin and male reproductive function. Adv. Exp. Med. Biol. 1997, 424, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.L.; Kumar, V. Basic Pathology, 4th ed.; W.B Saunders Company: Philadelphia, PA, USA, 1987. [Google Scholar]
- Hiller, J. Speculations on the links between feelings, emotions and sexual behaviour: Are vasopressin and oxytocin involved? Sex. Relatsh. Ther. 2004, 19, 393–412. [Google Scholar] [CrossRef]
- Mitsui, S.; Yamamoto, M.; Nagasawa, M.; Mogi, K.; Kikusui, T.; Ohtani, N.; Ohta, M. Urinary oxytocin as a noninvasive biomarker of positive emotion in dogs. Horm. Behav. 2011, 60, 239–243. [Google Scholar] [CrossRef]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.R.; Levy, K.; Martin, W.L.; Carter, C.S. Effects of affiliative human-animal interaction on dog salivary and plasma oxytocin and vasopressin. Front. Psychol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Grewen, K.M.; Davenport, R.E.; Light, K.C. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology 2010, 47, 625–632. [Google Scholar] [CrossRef]
- Seltzer, L.J.; Ziegler, T.E.; Pollak, S.D. Social vocalizations can release oxytocin in humans. Proc. R. Soc. B Biol. Sci. 2010, 277, 2661–2666. [Google Scholar] [CrossRef]
- Rault, J.L. Effects of positive and negative human contacts and intranasal oxytocin on cerebrospinal fluid oxytocin. Psychoneuroendocrinology 2016, 69, 60–66. [Google Scholar] [CrossRef]
- Oh, S.H.; See, M.T.; Long, T.E.; Galvin, J.M. Estimates of genetic correlations between production and semen traits in boar. Asian Australas. J. Anim. Sci. 2006, 19, 160–164. [Google Scholar] [CrossRef]
- Savić, R.; Petrović, M. Variability in ejaculation rate and libido of boars during reproductive exploitation. S. Afr. J. Anim. Sci. 2015, 45, 355–361. [Google Scholar] [CrossRef]
- Savic, R.; Marcos, R.; Petrovic, M.; Radojkovic, D.; Radovic, C.; Gogic, M. Fertility of boars—What is important to know. Biotechnol. Anim. Husb. 2017, 33, 135–149. [Google Scholar] [CrossRef]
- Kondracki, S. Breed differences in semen characteristics of boars used in artificial insemination in Poland. Pig News Inform. 2003, 24, 119N–122N. [Google Scholar]
- Jankeviciute, N.; Zilinskas, H. Influence of some factors on semen quality of different breeds of boars. Vet. Ir. Zootech. 2002, 19, 2130. [Google Scholar]
- Prevost, M.; Zelkowitz, P.; Tulandi, T.; Hayton, B.; Feeley, N.; Carter, C.S.; Joseph, L.; Pournajafi-Nazarloo, H.; Ping, E.Y.; Abenhaim, H.; et al. Oxytocin in pregnancy and the postpartum: Relations to labor and its management. Fron. Public Health 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Behnia, B.; Heinrichs, M.; Bergmann, W.; Jung, S.; Germann, J.; Schedlowski, M.; Hartmann, U.; Kruger, T.H.C. Differential effects of intranasal oxytocin on sexual experiences and partner interactions in couples. Horm. Behav. 2014, 65, 308–318. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Arjona, M.; Padilla, L.; Roca, J.; Cerón, J.J.; Martínez-Subiela, S. Ejaculate Collection Influences the Salivary Oxytocin Concentrations in Breeding Male Pigs. Animals 2020, 10, 1268. https://doi.org/10.3390/ani10081268
López-Arjona M, Padilla L, Roca J, Cerón JJ, Martínez-Subiela S. Ejaculate Collection Influences the Salivary Oxytocin Concentrations in Breeding Male Pigs. Animals. 2020; 10(8):1268. https://doi.org/10.3390/ani10081268
Chicago/Turabian StyleLópez-Arjona, Marina, Lorena Padilla, Jordi Roca, José Joaquín Cerón, and Silvia Martínez-Subiela. 2020. "Ejaculate Collection Influences the Salivary Oxytocin Concentrations in Breeding Male Pigs" Animals 10, no. 8: 1268. https://doi.org/10.3390/ani10081268
APA StyleLópez-Arjona, M., Padilla, L., Roca, J., Cerón, J. J., & Martínez-Subiela, S. (2020). Ejaculate Collection Influences the Salivary Oxytocin Concentrations in Breeding Male Pigs. Animals, 10(8), 1268. https://doi.org/10.3390/ani10081268






