Urinary Proteome of Newborn Calves—New Potential in Non-Invasive Neonatal Diagnostic
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Diets
2.2. Isolation of Urine Proteins
2.3. Sample Preparation and Selection for ProteomicsAnalysis
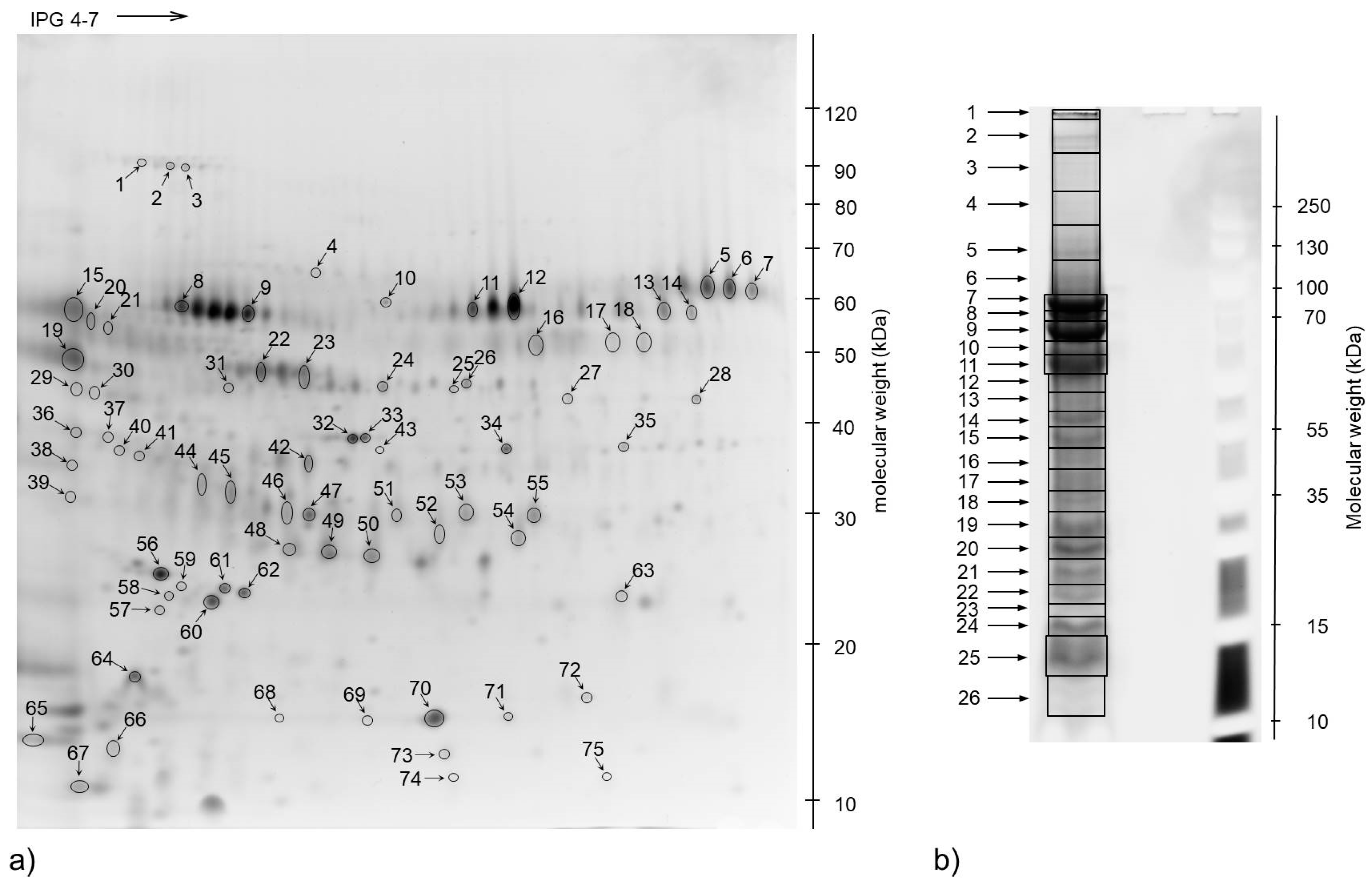
2.4. 2-Dimensional Gel Electrophoresis (2-DE)
2.5. Protein Spot Identification by Mass Spectrometry
2.6. MALDI-TOF-TOF-MS/MS
2.7. GEL LC-MS/MS
2.8. Functional Annotation and Localization of Identified Proteins
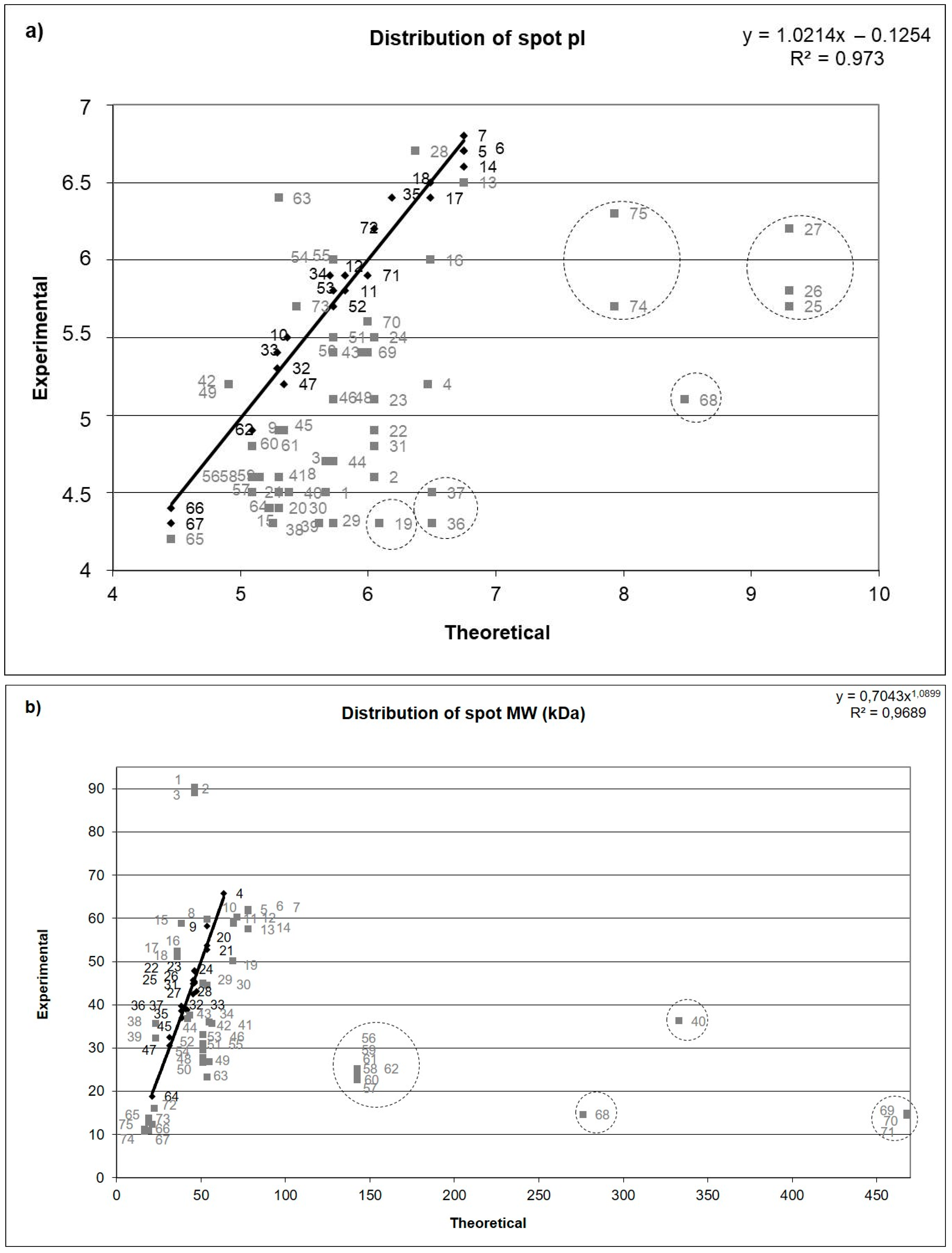
3. Results
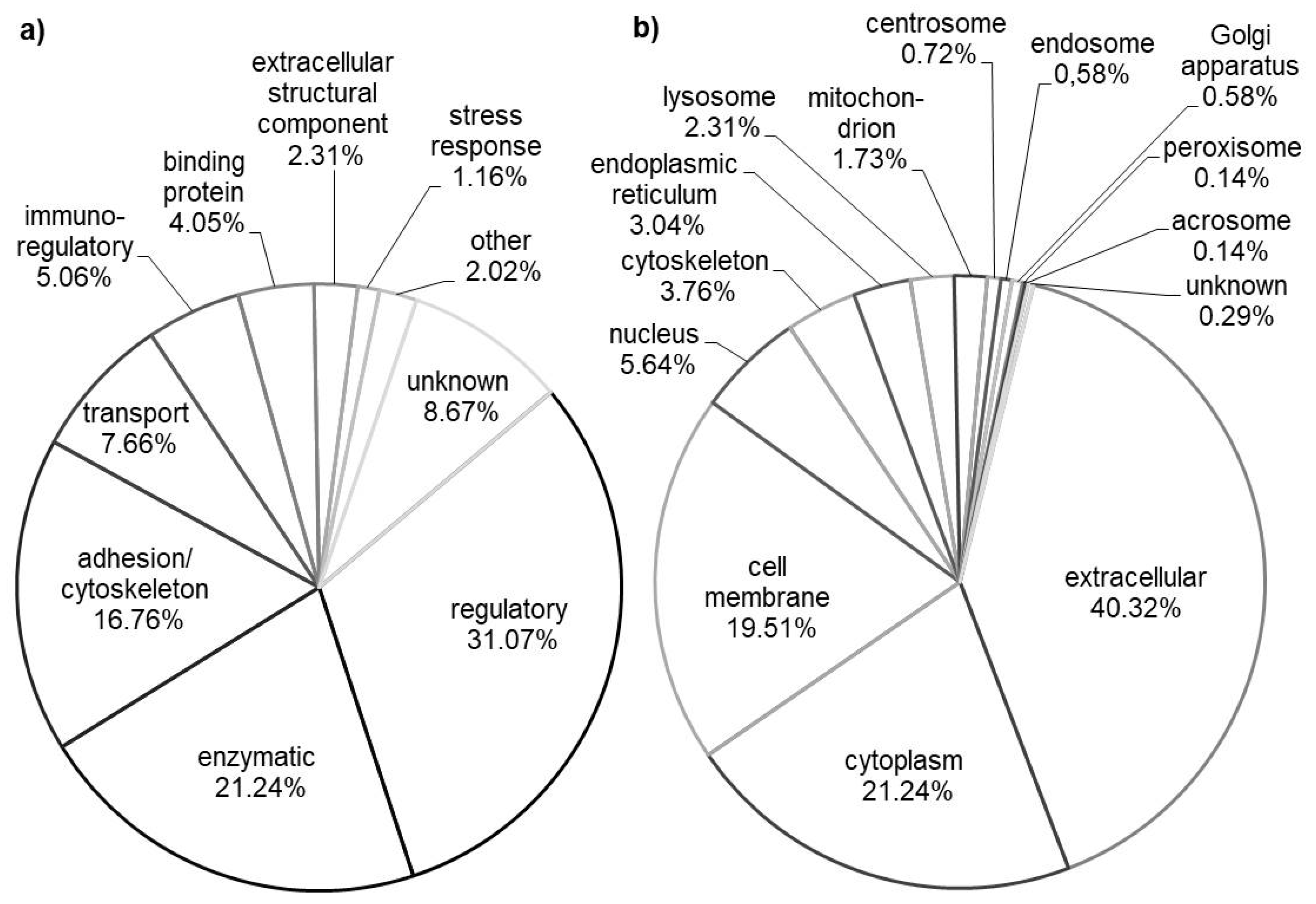
3.1. General Molecular Function and Subcellular Localization
3.2. Tissue Origin and Organs Development
3.3. The Most Abundant Proteins
4. Discussion
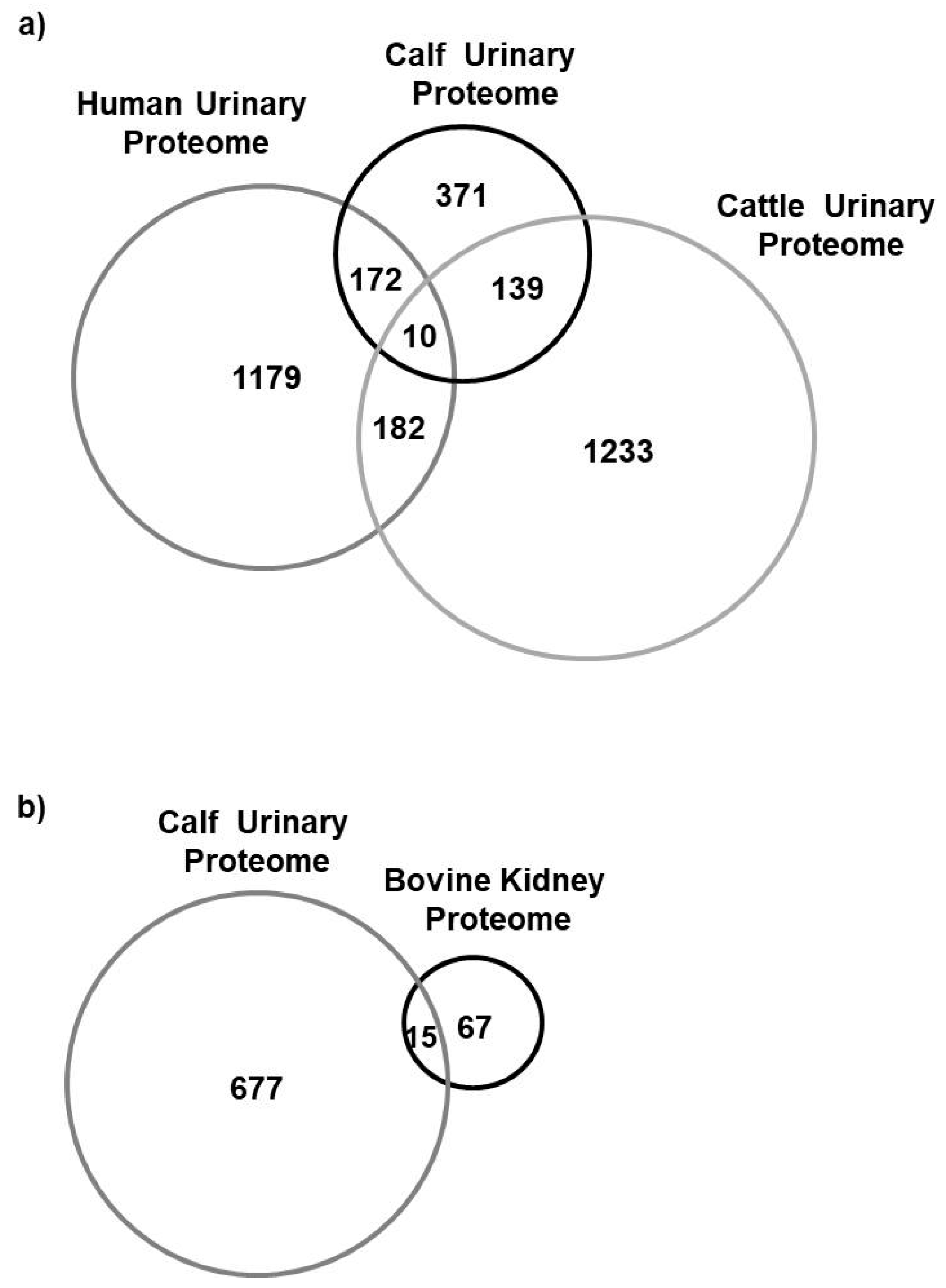
4.1. Proteomics Characterization of Newborn Calf Urine
4.2. Comparison to the Human and Bovine Urinary Proteome and Bovine Kidney Proteome
4.3. The Largest Group of Proteins Involved in Various Regulatory Processes
4.4. Blood Plasma-Derived Proteins in Urine
4.5. Kidney-Specific Urinary Proteins
4.6. Proteins Related to the Mammalian Embryonic Development
4.7. Proteins Involved in Kidney Regulation of Water and Electrolyte Balance
4.8. Proteins Involved in Extrauterine Development and Proteins Characteristic of Various Systems (Organs)
4.9. The Most Abundant Proteins
4.10. Urinary Proteomics in Veterinary Medicine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decramer, S.; Gonzalez de Peredo, A.; Breuil, B.; Mischak, H.; Monsarrat, B.; Bascands, J.L.; Schanstra, J.P. Urine in clinical proteomics. Mol. Cell. Proteomics 2008, 7, 1850–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, L.U.; Schiffer, E.; Zurbig, P.; Good, D.M.; Kellmann, M.; Mouls, L.; Pitt, A.R.; Coon, J.J.; Schmieder, R.E.; Peter, K.H.; et al. Urinary proteomic biomarkers in coronary artery disease. Mol. Cell. Proteomics 2008, 7, 290–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarosiek, I.; Schicho, R.; Blandon, P.; Bashashati, M. Urinary metabolites as noninvasive biomarkers of gastrointestinal diseases: A clinical review. World J. Gastrointest. Oncol. 2016, 8, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Kentsis, A.; Monigatti, F.; Dorff, K.; Campagne, F.; Bachur, R.; Steen, H. Urine proteomics for profiling of human disease using high accuracy mass spectrometry. Proteomics Clin. Appl. 2009, 3, 1052–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bathla, S.; Rawat, P.; Baithalu, R.; Yadav, M.L.; Naru, J.; Tiwari, A.; Kumar, S.; Balhara, A.K.; Singh, S.; Chaudhary, S.; et al. Profiling of urinary proteins in Karan Fries cows reveals more than 1550 proteins. J. Proteomics 2015, 127 Pt A, 193–201. [Google Scholar] [CrossRef]
- Ferlizza, E.; Campos, A.; Neagu, A.; Cuoghi, A.; Bellei, E.; Monari, E.; Dondi, F.; Almeida, A.M.; Isani, G. The effect of chronic kidney disease on the urine proteome in the domestic cat (Felis catus). Vet. J. 2015, 204, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Winiarczyk, D.; Michalak, K.; Adaszek, L.; Winiarczyk, M.; Winiarczyk, S. Urinary proteome of dogs with kidney injury during babesiosis. BMC Vet. Res. 2019, 15, 439. [Google Scholar] [CrossRef] [Green Version]
- Pyo, J.; Hwang, S.I.; Oh, J.; Lee, S.J.; Kang, S.C.; Kim, J.S.; Lim, J. Characterization of a bovine pregnancy-associated protein using two-dimensional gel electrophoresis, N-terminal sequencing and mass spectrometry. Proteomics 2003, 3, 2420–2427. [Google Scholar] [CrossRef]
- Palviainen, M.; Raekallio, M.; Rajamäki, M.M.; Linden, J.; Vainio, O. Kidney-derived proteins in urine as biomarkers of induced acute kidney injury in sheep. Vet. J. 2012, 193, 287–289. [Google Scholar] [CrossRef]
- Alhaider, A.A.; Bayoumy, N.; Argo, E.; Gader, A.G.; Stead, D.A. Survey of the camel urinary proteome by shotgun proteomics using a multiple database search strategy. Proteomics 2012, 12, 3403–3406. [Google Scholar] [CrossRef]
- Dratwa-Chałupnik, A.; Ożgo, M.; Lepczyński, A.; Herosimczyk, A.; Michałek, K. Excessive amount of lactose in the diet of two-week-old calves induces urinary protein changes. Arch. Anim. Breed. 2016, 59, 417–422. [Google Scholar] [CrossRef]
- Lee, R.S.; Monigatti, F.; Lutchman, M.; Patterson, T.; Budnik, B.; Steen, J.A.; Freeman, M.R.; Steen, H. Temporal variations of the postnatal rat urinary proteome as a reflection of systemic maturation. Proteomics 2008, 8, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; Yoon, K.J. An overview of calf diarrhea—infectious etiology, diagnosis, and intervention. J. Vet. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobom, J.; Nordhoff, E.; Mirgorodskaya, E.; Ekman, R.; Roepstorff, P. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 1999, 34, 105–116. [Google Scholar] [CrossRef]
- Westfall, L.W.; Shearer, M.H.; Jumper, C.A.; White, G.L.; Papin, J.F.; Eberle, R.; Butel, J.S.; Bright, R.K.; Kennedy, R.C. Evidence of simian virus 40 exposure in a colony of captive baboons. Virology 2008, 377, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemperline, D.C.; Scalf, M.; Smith, L.M.; Vierstra, R.D. Morpheus Spectral Counter: A computational tool for label-free quantitative mass spectrometry using the Morpheus search engine. Proteomics 2016, 16, 920–924. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Pinto, S.M.; Getnet, D.; Nirujogi, R.S.; Manda, S.S.; Chaerkady, R.; Madugundu, A.K.; Kelkar, D.S.; Isserlin, R.; Jain, S.; et al. A draft map of the human proteome. Nature 2014, 509, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Al Shweiki, M.R.; Mönchgesang, S.; Majovsky, P.; Thieme, D.; Trutschel, D.; Hoehenwarter, W. Assessment of Label-Free Quantification in Discovery Proteomics and Impact of Technological Factors and Natural Variability of Protein Abundance. J. Proteome Res. 2017, 16, 1410–1424. [Google Scholar] [CrossRef]
- UniProt. Available online: http://www.uniprot.org (accessed on 23 July 2020).
- Euk-mPLoc 2.0. Available online: http://www.csbio.sjtu.edu.cn/bioinf/euk-multi-2/ (accessed on 23 July 2020).
- Rogowska-Wrzesinska, A.; Le Bihan, M.C.; Thaysen-Andersen, M.; Roepstorff, P.J. 2D gels still have a niche in proteomics. Proteomics 2013, 88, 4–13. [Google Scholar] [CrossRef]
- Lee, P.Y.; Saraygord-Afshari, N.; Low, T.Y. The evolution of two-dimensional gel electrophoresis—From proteomics to emerging alternative applications. J. Chromatogr. A. 2020, 1615, 460763. [Google Scholar] [CrossRef]
- Skrzypczak, W.F.; Ozgo, M.; Lepczynski, A.; Herosimczyk, A. Defining the blood plasma protein repertoire of seven day old dairy calves—A preliminary study. J. Physiol. Pharmacol. 2011, 62, 313–319. [Google Scholar]
- Talamo, F.; D’Ambrosio, C.; Arena, S.; Del Vecchio, P.; Ledda, L.; Zehender, G.; Ferrara, L.; Scaloni, A. Proteins from bovine tissues and biological fluids: Defining a reference electrophoresis map for liver, kidney, muscle, plasma and red blood cells. Proteomics 2003, 3, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Wait, R.; Miller, I.; Eberini, I.; Cairoli, F.; Veronesi, C.; Battocchio, M.; Gemeiner, M.; Gianazza, E. Strategies for proteomics with incompletely characterized genomes: The proteome of Bos taurus serum. Electrophoresis 2002, 23, 3418–3427. [Google Scholar] [CrossRef]
- Dratwa, A. Atrial natriuretic peptide and renal haemodynamics in newborn calves. Acta Vet. Brno 2006, 75, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Dratwa, A.; Skrzypczak, W.F.; Ożgo, M. Atrial natriuretic peptide and volemia regulation in newborn calves. EJPAU 2004, 7, 2. [Google Scholar]
- Dratwa-Chałupnik, A.; Herosimczyk, A. The regulation of chloride balance in calves during the first week of life. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2014, 312, 63–68. [Google Scholar]
- Dratwa-Chałupnik, A.; Ożgo, M.; Herosimczyk, A.; Lepczyński, A.; Michałek, K.; Skrzypczak, W.F. Renal regulation of potassium homeostasis in calves in the first week of life including the role of atrial natriuretic peptide. Folia Biol. 2011, 59, 157–161. [Google Scholar]
- Lepczyński, A.; Herosimczyk, A.; Ożgo, M.; Skrzypczak, W.F. Feeding milk replacer instead of whole milk affects blood plasma proteome and lipid profile in preruminant calves. Pol. J. Vet. Sci. 2015, 18, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Thongboonkerd, V.; Malasit, P. Renal and urinary proteomics: Current applications and challenges. Proteomics 2005, 5, 1033–1042. [Google Scholar] [CrossRef]
- Bouley, J.; Chambon, C.; Picard, B. Mapping of bovine skeletal muscle proteins using two-dimensional gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 1811–1824. [Google Scholar] [CrossRef]
- Pieper, R.; Gatlin, C.L.; McGrath, A.M.; Makusky, A.J.; Mondal, M.; Seonarain, M.; Field, E.; Schatz, C.R.; Estock, M.A.; Ahmed, N.; et al. Characterization of the human urinary proteome: A method for high-resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1400 distinct protein spots. Proteomics 2004, 4, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Kumar, C.; Zhang, Y.; Olsen, J.V.; Mann, M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pasha, S.; Inui, T.; Chapple, I.; Harris, S.; Holcombe, L.; Grant, M.M. The Saliva Proteome of Dogs Variations Within and Between Breeds and Between Species. Proteomics 2018, 18, 1700293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozgo, M.; Skrzypczak, W.; Drzezdzon, D.; Lepczynski, A.; Dratwa-Chalupnik, A.; Michalek, K.; Herosimczyk, A. Urinary excretion of low molecular weight proteins in goats during the neonatal period. J. Physiol. Pharmacol. 2009, 60, 119–125. [Google Scholar] [PubMed]
- Jeong, H.W.; Jeon, U.S.; Koo, B.K.; Kim, W.Y.; Im, S.K.; Shin, J.; Cho, Y.; Kim, J.; Kong, Y.Y. Inactivation of Notch signaling in the renal collecting duct causes nephrogenic diabetes insipidus in mice. J. Clin. Investig. 2009, 119, 3290–3300. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, S.; Kudo, Y.; Yoshida, M.; Tsunematsu, T.; Yoshiko, Y.; Uchida, T.; Ogawa, I.; Miyauchi, M.; Takata, T. Ameloblastin regulates osteogenic differentiation by inhibiting Src kinase via cross talk between integrin beta1 and CD63. Mol. Cell. Biol. 2011, 31, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Goossens, K.; Van Soom, A.; Van Zeveren, A.; Favoreel, H.; Peelman, L.J. Quantification of fibronectin 1 (FN1) splice variants, including two novel ones, and analysis of integrins as candidate FN1 receptors in bovine preimplantation embryos. BMC Dev. Biol. 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Rauhauser, A.A.; Dai, J.; Sakthivel, R.; Igarashi, P.; Jetten, A.M.; Attanasio, M. Increased hedgehog signaling in postnatal kidney results in aberrant activation of nephron developmental programs. Hum. Mol. Genet. 2011, 20, 4155–4166. [Google Scholar] [CrossRef] [Green Version]
- Forterre, S.; Raila, J.; Schweigert, F.J. Protein profiling of urine from dogs with renal disease using ProteinChip analysis. J. Vet. Diagn. Investig. 2004, 16, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobori, H.; Ozawa, Y.; Suzaki, Y.; Prieto-Carrasquero, M.C.; Nishiyama, A.; Shoji, T.; Cohen, E.P.; Navar, L.G. Young Scholars Award Lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am. J. Hypertens. 2006, 19, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Kobori, H.; Nishiyama, A.; Harrison-Bernard, L.M.; Navar, L.G. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension 2003, 41, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison-Bernard, L.M. The renal renin-angiotensin system. Adv. Physiol. Educ. 2009, 33, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Peti-Peterdi, J.; Warnock, D.G.; Bell, P.D. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J. Am. Soc. Nephrol. 2002, 13, 1131–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, K.D.; Hiremath, S. Urinary angiotensinogen as a biomarker of chronic kidney disease: Ready for prime time? Nephrol. Dial. Transplant. 2012, 27, 3010–3013. [Google Scholar] [CrossRef] [PubMed]
- Rampoldi, L.; Scolari, F.; Amoroso, A.; Ghiggeri, G.; Devuyst, O. The rediscovery of uromodulin (Tamm–Horsfall protein): From tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011, 80, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Michałek, K.; Grabowska, M. Investigating cellular location of aquaporins in the bovine kidney. A new view on renal physiology in cattle. Res. Vet. Sci. 2019, 125, 162–169. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-1 facilitates cell migration in kidney proximal tubule. J. Am. Soc. Nephrol. 2006, 17, 39–45. [Google Scholar] [CrossRef]
- Shenolikar, S.; Weinman, E.J. NHERF: Targeting and trafficking membrane proteins. Am. J. Physiol. Renal Physiol. 2001, 280, F389–F395. [Google Scholar] [CrossRef]
- Chromek, M.; Slamova, Z.; Bergman, P.; Kovacs, L.; Podracka, L.; Ehren, I.; Hokfelt, T.; Gudmundsson, G.H.; Gallo, R.L.; Agerberth, B.; et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006, 12, 636–641. [Google Scholar] [CrossRef]
- Simon, S.L.; Lamoureux, L.; Plews, M.; Stobart, M.; LeMaistre, J.; Ziegler, U.; Graham, C.; Czub, S.; Groschup, M.; Knox, J.D. The identification of disease-induced biomarkers in the urine of BSE infected cattle. Proteome Sci. 2008, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Péré-Brissaud, A.; Blanchet, X.; Delourme, D.; Pélissier, P.; Forestier, L.; Delavaud, A.; Duprat, N.; Picard, B.; Maftah, A.; Brémaud, L. Expression of SERPINA3s in cattle: Focus on bovSERPINA3-7 reveals specific involvement in skeletal muscle. Open Biol. 2015, 5, 150071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froehlich, J.W.; Vaezzadeh, A.R.; Kirchner, M.; Briscoe, A.C.; Hofmann, O.; Hide, W.; Steen, H.; Lee, R.S. An in-depth comparison of the male pediatric and adult urinary proteomes. Biochim. Biophys. Acta 2014, 1844, 1044–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidaka, S.; Kranzlin, B.; Gretz, N.; Witzgall, R. Urinary clusterin levels in the rat correlate with the severity of tubular damage and may help to diff erentiate between glomerular and tubular injuries. Cell Tissue Res. 2002, 310, 289–296. [Google Scholar] [CrossRef]
- Saeidi, B.; Koralkar, R.; Griffin, R.L.; Halloran, B.; Ambalavanan, N.; Askenazi, D.J. Impact of gestational age, sex, and postnatal age on urine biomarkers in premature neonates. Pediatr. Nephrol. 2015, 30, 2037–2044. [Google Scholar] [CrossRef]
- Dratwa-Chałupnik, A.; Herosimczyk, A.; Lepczyński, A.; Skrzypczak, W. Calves with diarrhea and a water-electrolyte balance. Med. Weter. 2012, 68, 5–8. [Google Scholar]
| Blood Plasma Proteins Detected in Normal Urine | |
| alpha-1-acid glycoprotein | hemogloglobin alpha and beta chain |
| alpha-1-antiproteinase | immunoglobulins |
| alpha-1B-glycoprotein | kininogen-2 |
| aminopeptidase N | leucine-rich alpha-2-glycoprotein precursor |
| angiotensinogen | retinol-binding protein 4 |
| apolipoproteins | serotransferrin |
| clusterin | serum albumin |
| Proteins Detected in Bovine Blood Plasma/Serum | |
| 78 kDa glucose-regulated protein | haptoglobin |
| actin, cytoplasmic 1 | immunoglobulin gamma 1 heavy chain constant region |
| alpha-1-antiproteinase | kininogen-1 |
| alpha-2-HS-glycoprotein | kininogen-2 |
| apolipoprotein A-I | plasminogen |
| apolipoprotein A-IV | prothrombin |
| apolipoprotein C-III | retinol-binding protein 4 |
| apolipoprotein E | serotransferrin |
| clusterin | serpin A3-1 |
| fibrinogen alpha chain | serum albumin |
| fibrinogen beta chain | transthyretin |
| fibrinogen-like protein 1 | vitamin D-binding protein |
| gelsolin | |
| Proteins Obtained in Blood Plasma of Seven-Day-Old Calves | |
| 78 kDa glucose-regulated protein | fibrinogen-like protein 1 |
| actin, cytoplasmic 1 | gelsolin |
| alpha-1-antiproteinase | prothrombin |
| apolipoprotein A-I | serotransferrin |
| apolipoprotein A-IV | serum albumin |
| apolipoprotein E | vitamin D-binding protein |
| clusterin | von Willebrand factor |
| fibrinogen alpha chain | |
| Proteins Detected in Bovine Kidney | |
| acyl-protein thioesterase 1 | heat shock cognate 71 kDa protein |
| alcohol dehydrogenase | peroxiredoxin-2 |
| aldose reductase | phosphoglycerate kinase 1 |
| apolipoprotein A-I | serum albumin |
| calbindin | superoxide dismutase (Cu-Zn) |
| carbonic anhydrase 2 | triosephosphate isomerase |
| glutathione S-transferase P | vimentin |
| glyceraldehyde-3-phosphate dehydrogenase | |
| Proteins Detected Only During Neonatal Period | |
| ameloblastin | gelsolin |
| Delta-like protein | lysyl oxidase |
| embryo-specific fibronectin 1 transcript variant | |
| Mammalian Embryonic Development | |
| ameloblastin | embryo-specific fibronectin 1 transcript variant |
| alpha-fetoprotein | Indian hedgehog homolog |
| Delta-like protein | |
| Assessment of the Renal Tubules Function | |
| retinol-binding protein | uromodulin |
| Regulation of Water and Electrolyte Balance in Kidneys | |
| angiotensinogen | ezrin |
| angiotensin-converting enzyme | uromodulin |
| aquaporin-1 | |
| Cardiovascular System Development | |
| actin, alpha skeletal muscle | erythropoietin receptor (Fragment) |
| actin, cytoplasmic 1 | glucose-6-phosphate isomerase |
| alpha-2-antiplasmin | glypican 3 |
| angiogenin-2 | hypothetical LOC506714 |
| angiopoietin-4 | junction plakoglobin |
| angiopoietin-like 3 | lactadherin |
| angiotensinogen | lysosomal alpha-glucosidase |
| annexin A2 | myoglobin |
| apolipoprotein E | retinol-binding protein 4 |
| aquaporin-1 | roundabout homolog 4 |
| ATP synthase subunit beta, mitochondrial | serine peptidase inhibitor |
| beta-1,4-galactosyltransferase 1 | thy-1 cell surface antigen |
| cadherin-2 | tyrosine-protein kinase receptor Tie-1 |
| collagen alpha-1(I) chain | ephrin-A1 |
| connective tissue growth factor | erythropoietin receptor (Fragment) |
| ephrin-A1 | |
| Bone Development | |
| cartilage oligomeric matrix protein | lactoferrin |
| cell adhesion molecule 1 | latent-transforming growth factor beta-binding protein 2 |
| collagen alpha-1(I) chain | secreted protein acidic and rich in cysteine (SPARC) |
| discoidin domain receptor family, member 2 (DDR2 protein) | |
| Kidney Development | |
| angiotensin-converting enzyme | glypican 3 |
| angiotensinogen | nidogen 1 |
| aquaporin-1 | pro-cathepsin H |
| calbindin | |
| Digestive System Development | |
| adipocyte-specific adhesion molecule (ASAM protein) | hypothetical LOC506714 |
| gastrin-releasing peptide | insulin-like growth factor 2 preproprotein |
| Nervous System | |
| 14-3-3 protein epsilon | NAGLU protein |
| midkine | |
| Cardiovascular System | |
| angiogenin-2 | glutamyl aminopeptidase |
| angiopoietin | lactadherin (BP47) |
| ephrin-a1 | lysosomal alpha-glucosidase |
| Skeletal System | |
| collagen alpha-1(I) chain | secreted phosphoprotein 24 |
| collagen triple helix repeat containing 1 | tripeptidyl-peptidase 1 |
| glypican 3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dratwa-Chałupnik, A.; Wojdyła, K.; Ożgo, M.; Lepczyński, A.; Michałek, K.; Herosimczyk, A.; Rogowska-Wrzesińska, A. Urinary Proteome of Newborn Calves—New Potential in Non-Invasive Neonatal Diagnostic. Animals 2020, 10, 1257. https://doi.org/10.3390/ani10081257
Dratwa-Chałupnik A, Wojdyła K, Ożgo M, Lepczyński A, Michałek K, Herosimczyk A, Rogowska-Wrzesińska A. Urinary Proteome of Newborn Calves—New Potential in Non-Invasive Neonatal Diagnostic. Animals. 2020; 10(8):1257. https://doi.org/10.3390/ani10081257
Chicago/Turabian StyleDratwa-Chałupnik, Alicja, Katarzyna Wojdyła, Małgorzata Ożgo, Adam Lepczyński, Katarzyna Michałek, Agnieszka Herosimczyk, and Adelina Rogowska-Wrzesińska. 2020. "Urinary Proteome of Newborn Calves—New Potential in Non-Invasive Neonatal Diagnostic" Animals 10, no. 8: 1257. https://doi.org/10.3390/ani10081257
APA StyleDratwa-Chałupnik, A., Wojdyła, K., Ożgo, M., Lepczyński, A., Michałek, K., Herosimczyk, A., & Rogowska-Wrzesińska, A. (2020). Urinary Proteome of Newborn Calves—New Potential in Non-Invasive Neonatal Diagnostic. Animals, 10(8), 1257. https://doi.org/10.3390/ani10081257





