Hand-Rearing of Three Lesser Flamingo Chicks (Phoeniconaias minor)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
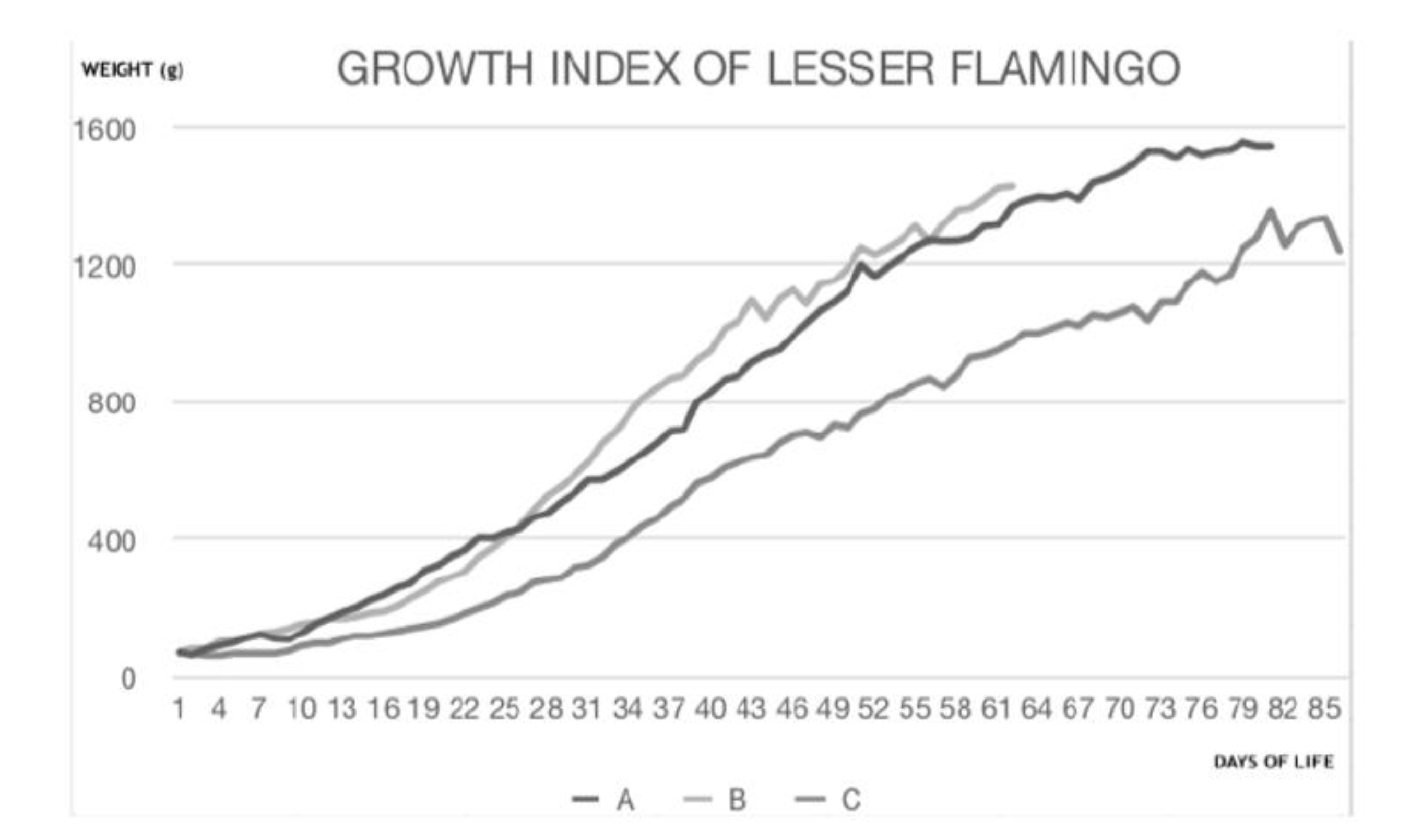
2.1. Animal Collection
2.2. Diet (Formula)
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaggwa, M.; Kruber, M.; Oduor, S.O.; Schagerl, M. A detailed time series assessment of the diet of Lesser Flamingos: Further explanation for their itinerant behaviour. Hydrobiologia 2013, 710, 83–93. [Google Scholar] [CrossRef]
- Krienitz, L.; Dadheech, T.; Hübener, K.; Kotut, W.; Luo, K.; Teubner, W.; Versfeld, D. Food algae for Lesser Flamingos: A stocktaking. Hydrobiologia 2016, 775, 21–50. [Google Scholar] [CrossRef]
- Tuite, C.H. The Lesser Flamingo (Phoeniconaias minor): Aspects of Its Ecology and Behaviour in the East African Rift Valley of Kenya and Northern Tanzania. Ph.D. Thesis, University of Bristol, Bristol, UK, 1978. [Google Scholar]
- Tuite, C.H. The Distribution and Density of Lesser Flamingos in East Africa in Relation to Food Availability and Productivity. Waterbirds 2000, 23, 52–63. [Google Scholar] [CrossRef]
- Simmons, R.E. Declines and Movements of Lesser Flamingos in Africa. Waterbirds 2000, 23, 40–46. [Google Scholar] [CrossRef]
- Kihwele, E.S.; Lugomela, C.; Howell, K.M. Temporal Changes in the Lesser Flamingos Population (Phoenicopterus minor) in Relation to Phytoplankton Abundance in Lake Manyara, Tanzania. Ecology 2014, 4, 145–161. [Google Scholar] [CrossRef]
- Ndetei, R.; Muhandiki, V.S. Mortalities of Lesser Flamingos in Kenyan Rift Valley Saline Lakes and the Implications for Sustainable Management of the Lakes. Lakes Reserv. Res. Manag. 2005, 10, 51–58. [Google Scholar] [CrossRef]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Putz, S.; Metcalf, J.S.; Coddm, G.A.; Pflugmacher, S. Contribution of Hot Spring Cyanobacteria to the Mysterious Deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef]
- Krienitz, L.; Kotut, K. Fluctuating Algal Food Populations and the Occurrence of Lesser Flamingos (Phoeniconaias Minor.) in Three Kenyan Rift Valley Lakes. J. Phycol. 2010, 46, 1088–1096. [Google Scholar] [CrossRef]
- Mlingwa, C.; Baker, N. Lesser Flamingo Phoenicopterus minor Counts in Tanzanian Soda Lakes: Implications for Conservation. In Waterbirds Around the World; Boere, G.C., Galbraith, C.A., Stroud, D.A., Eds.; The Stationery Office: Edinburgh, UK, 2006; pp. 230–233. [Google Scholar]
- Tuite, C.H. Standing Crop Densities and Distribution of Spirulina and Benthic Diatoms in East African Alkaline Saline Lakes. Freshw. Biol. 1981, 11, 345–360. [Google Scholar] [CrossRef]
- Berry, H.H. Hand-rearing abandoned lesser flamingos. Int. Zoo Yearb. 1974, 14, 203–210. [Google Scholar] [CrossRef]
- Sabat, P.; Novoa, F. Digestive constraints and nutrient hydrolysis in nestling of two flamingo species. Condor 2001, 103, 396–399. [Google Scholar] [CrossRef]
- Van Bocxstaele, R. Attempts at hand-rearing the Caribbean flamingo. Int. Zoo Yearb. 1974, 14, 199–210. [Google Scholar] [CrossRef]
- Johnson, A.; Cézilly, F. The Greater Flamingo; A&C Black Publishers Ltd.: London, UK, 2007; pp. 263–285. [Google Scholar]
- Burch, L.E.; Gailband, C. Comparison of hand-reared Caribbean flamingos and lesser flamingos at Seaworld California. Waterbirds 2000, 23, 193–197. [Google Scholar] [CrossRef]
- Ward, A.M.; Hunt, A.; Maslanka, M.; Brown, C. Nutrient composition of American flamingo crop milk. In Proceedings of the AZA Nutrition Advisory Group 4th Conference on Zoo and Wildlife Nutrition, Lake Buena Vista, FL, USA, 19–23 September 2001; pp. 187–193. [Google Scholar]
- Brown, C.; King, C.; Mossbarger, S.; Barron, J. Flamingo Husbandry Guidelines; American Zoo and Aquarium Association: Silver Spring, MD, USA, 2005; pp. 68–108. [Google Scholar]
- Kear, J. Notes on keeping flamingos in captivity. Int. Zoo Yearb. 1974, 14, 142–144. [Google Scholar] [CrossRef]
- Hallager, S. Early weaning of hand-reared flamingo chicks at the National Zoological Park. Aviculture 1976, 102, 15–17. [Google Scholar]
- Kunneman, F.; Perry, J. Hand-rearing the Caribbean flamingo, Phoenicopterus r. ruber at the San Antonio Zoo. Int. Zoo Yearb. 1990, 29, 95–99. [Google Scholar] [CrossRef]
- Wackernagel, H. Dietary Requirements; Kear, J., Duplaix-hall, N., Eds.; T&A.D. Poyser: Berckhamsted, UK, 1975; pp. 196–198. [Google Scholar]
- Studer-Thiersch, A. Basle Zoo. In Flamingos; Kear, J., Duplaix-hall, N., Eds.; T&A.D. Poyser: Berckhamsted, UK, 1975; pp. 168–169. [Google Scholar]
- Vareschi, E. The Ecology of Lake Nakuru (Kenya). Oecologia 1978, 32, 11–35. [Google Scholar] [CrossRef]
- Alvarenga, R.R.; Rodrigues, P.B.; Cantarelli, V.S.; Zangeronimo, M.G.; Silva, J.W.; Silva, L.R.; Santos, L.S.; Pereira, L.J. Energy values and chemical composition of spirulina (Spirulina platensis) evaluated with broilers. Rev. Bras. Zootec. 2011, 40, 992–996. [Google Scholar] [CrossRef]
- Mirzaie, S.; Zirak-Khattab, F.; Hosseini, S.A.; Donyaei-Darian, H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas. J. Anim. Sci. 2018, 31, 556–563. [Google Scholar] [CrossRef]
- Ross, E.; Dominy, W. The Nutritional Value of Dehydrated, Blue-Green Algae (Spirulina plantensis) for Poultry. Poult. Sci. 1990, 69, 794–800. [Google Scholar] [CrossRef]
- Mühling, M.; Belay, A.; Whitton, B.A. Variation in Fatty Acid Composition of Arthrospira (Spirulina) Strains. J. Appl. Phycol. 2005, 17, 137–146. [Google Scholar] [CrossRef]
- Tokusoglu, O.; üUnal, M.K. Biomass Nutrient Profiles of Microalgae: Spirulina platensis, Chlorella Vulgaris and Isochrisis galbana. Food Chem. Toxicol. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Karásková, K.; Suchý, P.; Straková, E. Current use of phytogenic feed additives in animal nutrition: A review. Czech J. Anim. Sci. 2015, 60, 521–530. [Google Scholar] [CrossRef]
- Childress, B.; Nagy, S.; Hughes, B.; Abebe, Y.B. International Single Species Action Plan for the Conservation of Lesser Flamingo; UNEP/CMS Secretariat: Bonn, Germany, 2008; Techniacl Series No. 18 (CMS) and 34 (AEWA). [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorucci, L.; Grande, F.; Macrelli, R.; Schnitzer, P.; Crosta, L. Hand-Rearing of Three Lesser Flamingo Chicks (Phoeniconaias minor). Animals 2020, 10, 1251. https://doi.org/10.3390/ani10081251
Fiorucci L, Grande F, Macrelli R, Schnitzer P, Crosta L. Hand-Rearing of Three Lesser Flamingo Chicks (Phoeniconaias minor). Animals. 2020; 10(8):1251. https://doi.org/10.3390/ani10081251
Chicago/Turabian StyleFiorucci, Letizia, Francesco Grande, Roberto Macrelli, Petra Schnitzer, and Lorenzo Crosta. 2020. "Hand-Rearing of Three Lesser Flamingo Chicks (Phoeniconaias minor)" Animals 10, no. 8: 1251. https://doi.org/10.3390/ani10081251
APA StyleFiorucci, L., Grande, F., Macrelli, R., Schnitzer, P., & Crosta, L. (2020). Hand-Rearing of Three Lesser Flamingo Chicks (Phoeniconaias minor). Animals, 10(8), 1251. https://doi.org/10.3390/ani10081251