Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Treatments
2.2. DAI and Histological Evaluation
2.3. Microbiome Profiling and Analysis (Gut Microbiota Determination)
2.4. Statistical Analysis
3. Results
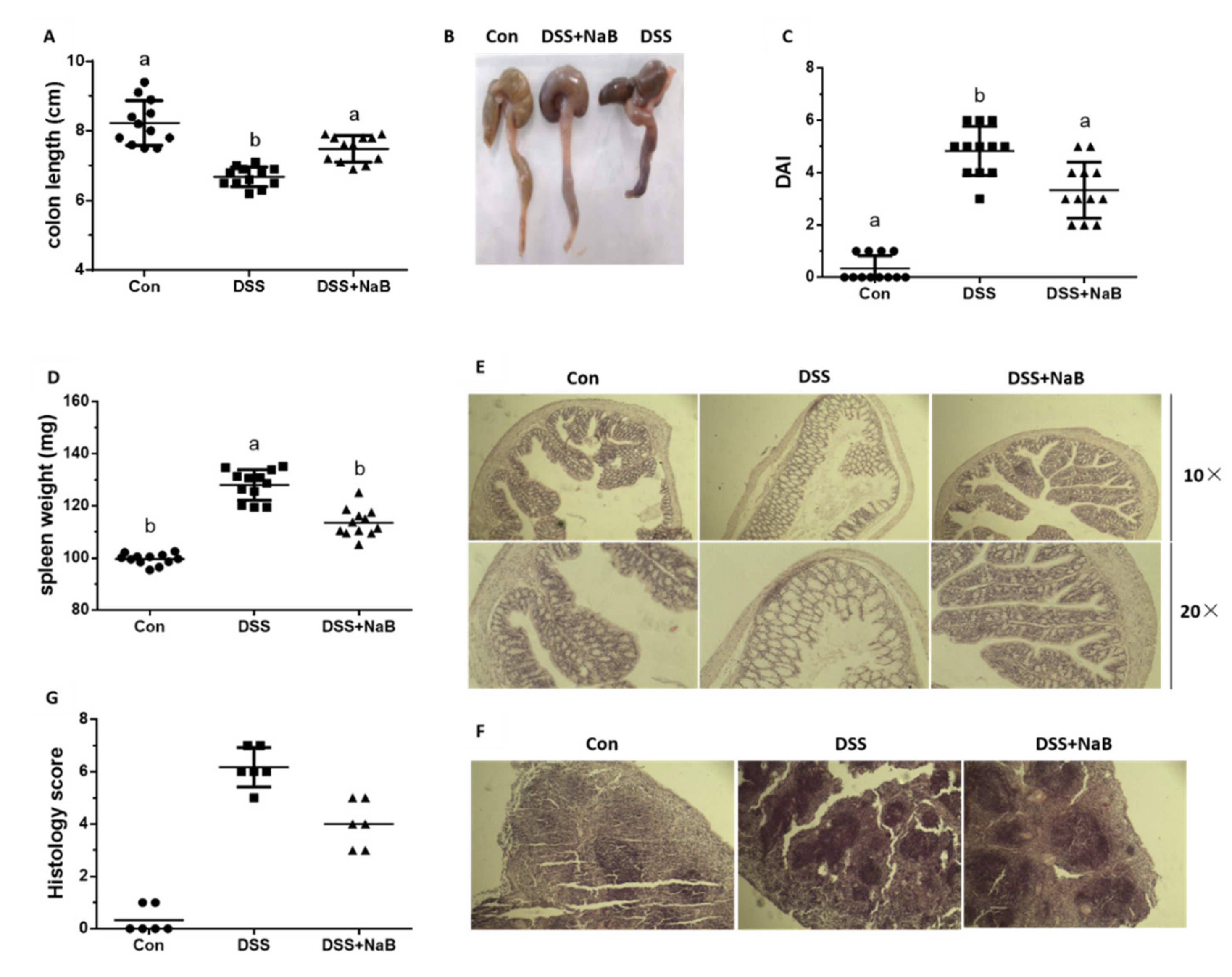
3.1. NaB Ameliorates Colitis Symptoms
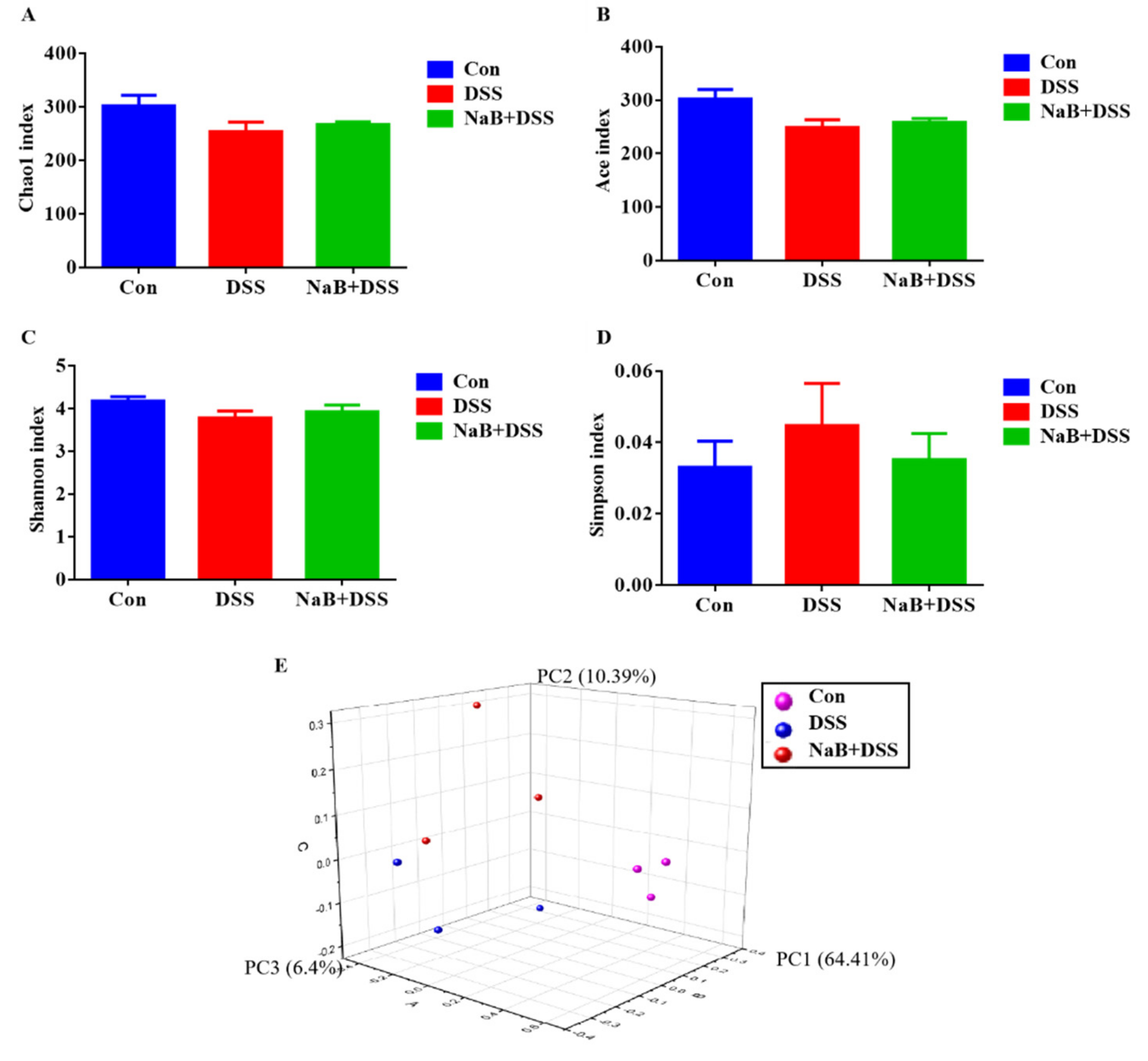
3.2. Dietary NaB Increased Gut Microbiota Diversity to Alleviate Colitis
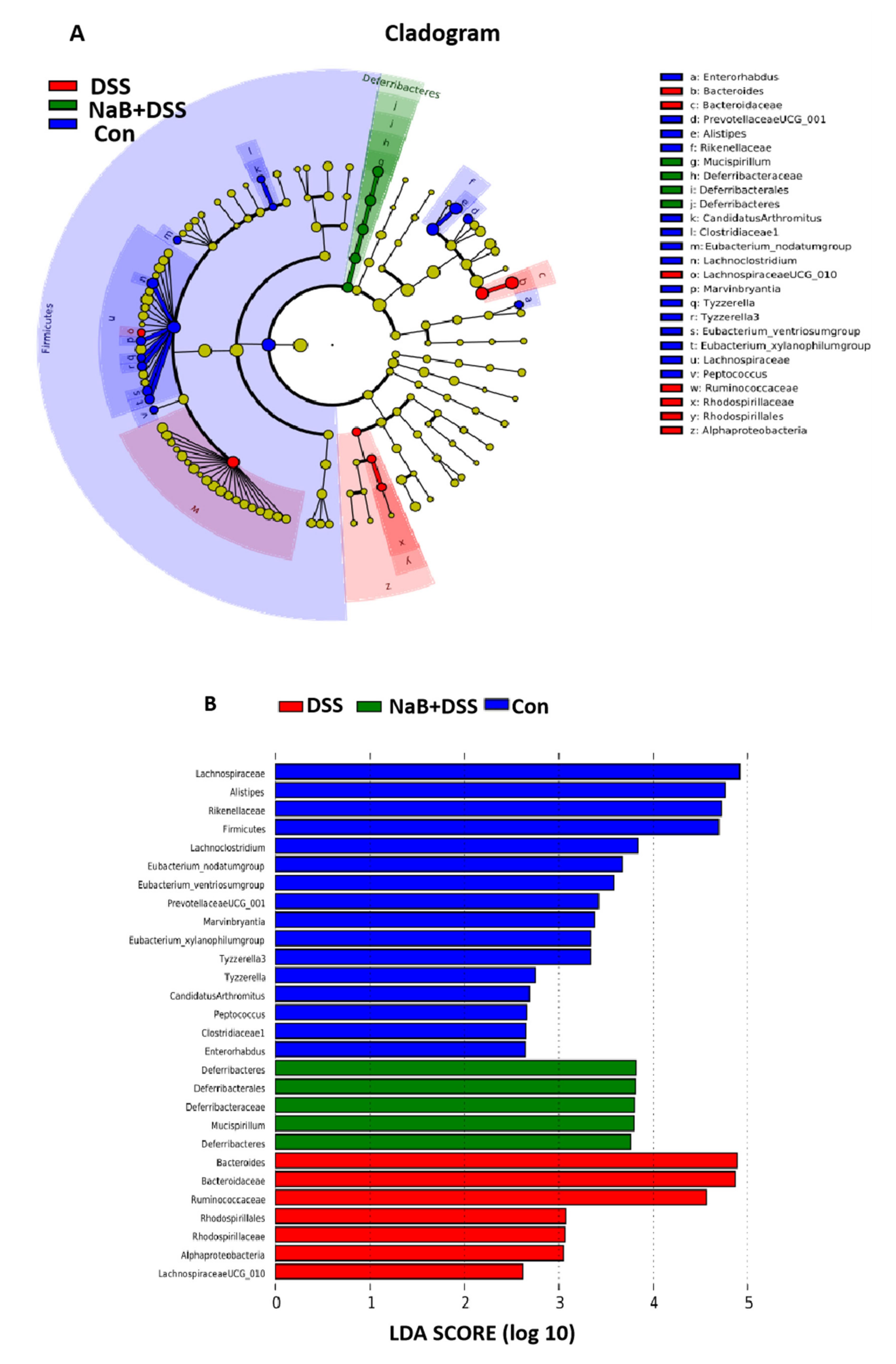
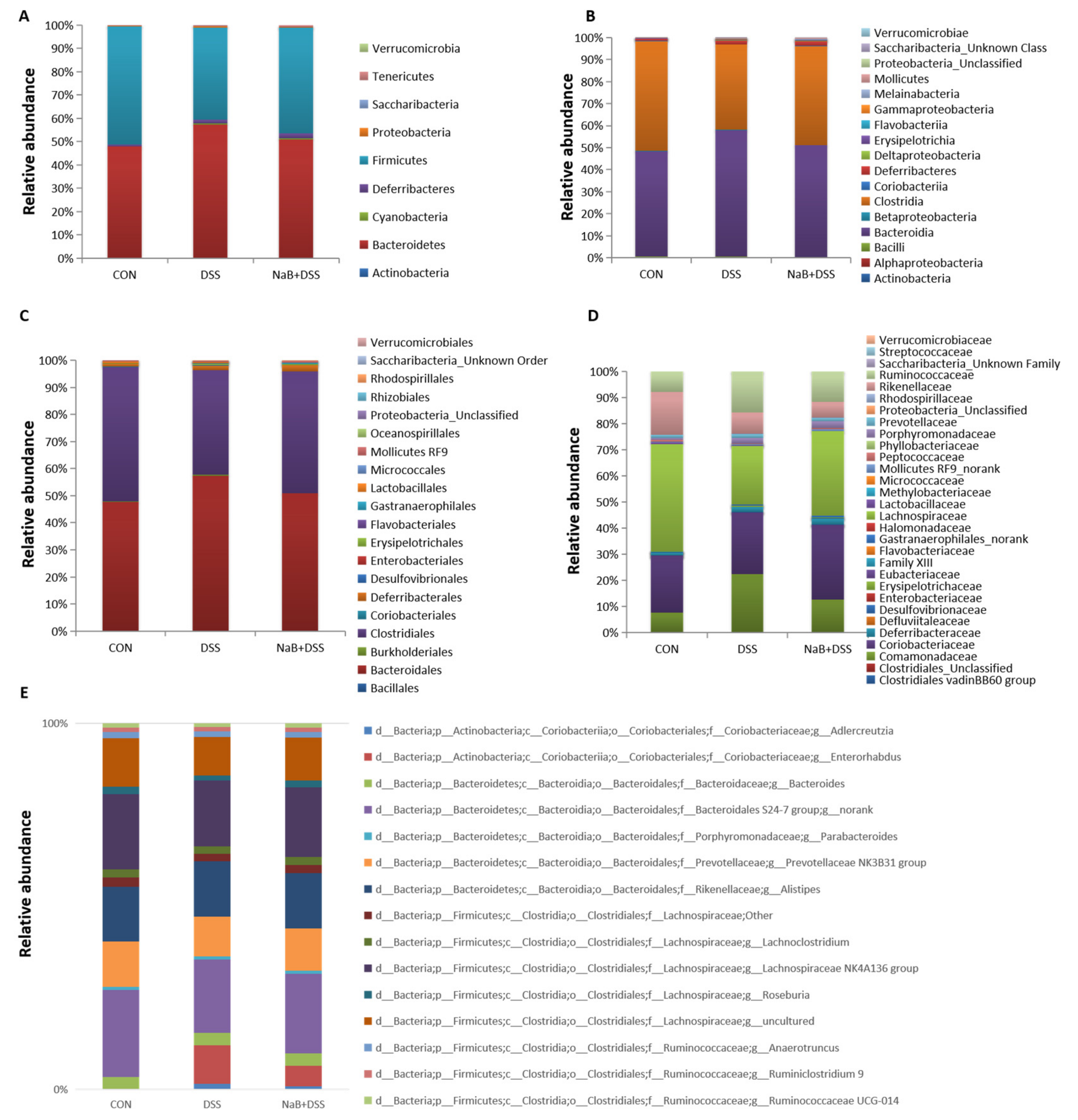
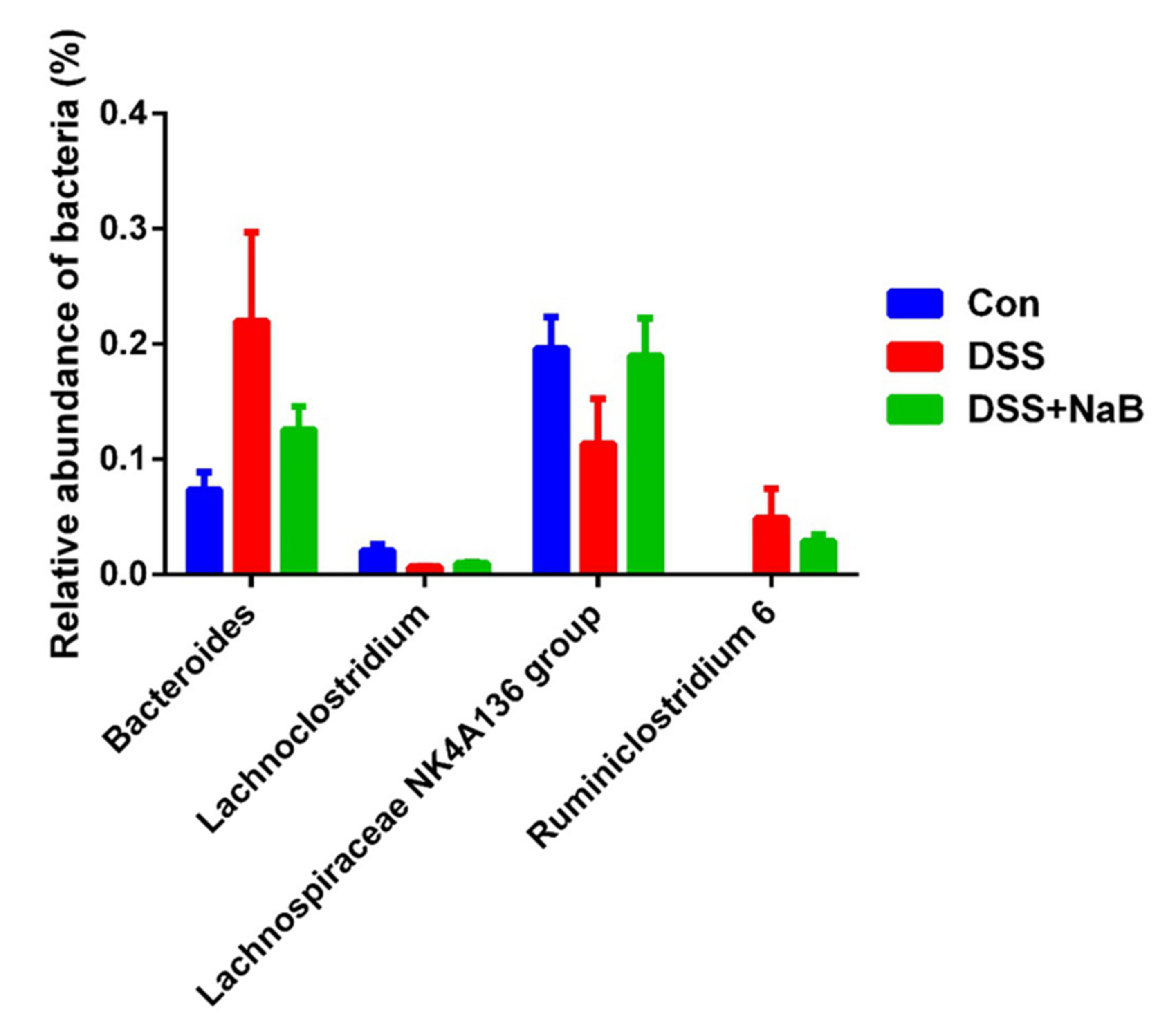
3.3. Dietary NaB Ameliorated Gut Microbial Dysbiosis to Alleviate Colitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matricon, J.; Barnich, N.; Ardid, D. Immunopathogenesis of inflammatory bowel disease. Self Nonself 2010, 1, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Miner-Williams, W.; Moughan, P.J. Intestinal barrier dysfunction: Implications for chronic inflammatory conditions of the bowel. Nutr. Res. Rev. 2016, 29, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Cotter, P. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naïve microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; Leleiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Håkansson, Å.; Tormo-Badia, N.; Baridi, A.; Xu, J.; Molin, G.; Hagslätt, M.-L.; Karlsson, C.; Jeppsson, B.; Cilio, C.M.; Ahrné, S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exp. Med. 2014, 15, 107–120. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, e2960–e2969. [Google Scholar] [CrossRef]
- Borton, M.A.; Sabag-Daigle, A.; Wu, J.; Solden, L.; O’Banion, B.S.; Daly, R.; Wolfe, R.A.; Gonzalez, J.F.; Wysocki, V.H.; Ahmer, B.M.M.; et al. Chemical and pathogen-induced inflammation disrupt the murine intestinal microbiome. Microbiome 2017, 5, 47. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Dou, X.; Gao, N.; Lan, J.; Han, J.; Yang, Y.; Shan, A. TLR2/EGFR are two Sensors for pBD3 and pEP2C induction by Sodium Butyrate independent on HDAC inhibition. J. Agric. Food Chem. 2019, 68, 512–522. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2016, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.; Pedroso, A.A.; Mallo, J.J.; Puyalto, M.; Kim, W.K.; Applegate, T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 2017, 96, 3981–3993. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Jian, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef]
- Vieira, E.L.M.; Leonel, A.J.; Sad, A.P.; Beltrão, N.R.; Costa, T.F.; Ferreira, T.M.; Gomes-Santos, A.C.; Faria, A.C.; Peluzio, M.C.; Cara, D.C.; et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J. Nutr. Biochem. 2012, 23, 430–436. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Genet. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Wang, X.; Wang, S.; Bi, D. The Impact of Lactobacillus plantarumon the Gut Microbiota of Mice with DSS-Induced Colitis. BioMed Res. Int. 2019, 2019, 3921315. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Chen, W.; Fan, H.; Liang, R.; Zhang, R.; Zhang, J.; Zhu, J.-S. Taraxacum officinale extract ameliorates dextran sodium sulphate-induced colitis by regulating fatty acid degradation and microbial dysbiosis. J. Cell. Mol. Med. 2019, 23, 8161–8172. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Dou, X.; Li, J.; Yang, Y.; Xue, C.; Wang, C.; Gao, N.; Shan, A. l-Arginine Ameliorates Lipopolysaccharide-Induced Intestinal Inflammation through Inhibiting the TLR4/NF-κB and MAPK Pathways and Stimulating β-Defensin Expression in Vivo and in Vitro. J. Agric. Food Chem. 2020, 68, 2648–2663. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Ji, G.E.; Sung, M.-K. Dietary Kaempferol Suppresses Inflammation of Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2011, 57, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Navas-Molina, J.A.; Peralta-Sanchez, J.M.; Gonzalez, A.; McMurdie, P.J.; Vazquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Song, S.J.; et al. Advancing Our Understanding of the Human Microbiome Using QIIME. In Microbial Metagenomics, Metatranscriptomics, and Metaproteomics; DeLong, E.F., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 531, pp. 371–444. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.D.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Li, X.; Xue, C.; Zhang, L.; Wang, C.; Xu, X.; Shan, A. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J. Cell. Physiol. 2020, 235. [Google Scholar] [CrossRef]
- Osada, T.; Ohkusa, T.; Okayasu, I.; Yoshida, T.; Shu, H.; Beppu, K.; Shibuya, T.; Sakamoto, N.; Kobayashi, O.; Nagahara, A.; et al. Correlations among total colonoscopic findings, clinical symptoms, and laboratory markers in ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23, CS262–CS267. [Google Scholar] [CrossRef]
- Cai, X.; Han, Y.; Min, G.; Song, M.; Wu, X.; Li, Z.; Fang, L.; Goulette, T.; Hang, X.J.F. Function, Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice. Food Funct. 2019, 10, 6331–6341. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.-E. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef]
- Hu, Q.; Yuan, B.; Wu, X.; Du, H.; Gu, M.; Han, Y.; Yang, W.; Song, M.; Xiao, H. Dietary Intake of Pleurotus eryngii Ameliorated Dextran-Sodium-Sulfate-Induced Colitis in Mice. Mol. Nutr. Food Res. 2019, 63, e1801265. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Hu, Y.; Zhao, L.; Zhang, C. Initial gut microbiota structure affects sensitivity to DSS-induced colitis in a mouse model. Sci. China Life Sci. 2017, 61, 762–769. [Google Scholar] [CrossRef]
- Zou, X.; Ji, J.; Qu, H.; Wang, J.; Shu, D.; Wang, Y.; Liu, T.; Li, Y.; Luo, C. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult. Sci. 2019, 98, 4449–4456. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2014, 37, 47–55. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, L.; Zhao, W.; Zhao, W.; Han, X.; Niu, J.; Li, R.; Zhao, C. Flaxseed oil alleviates dextran sulphate sodium-induced ulcerative colitis in rats. J. Funct. Foods. 2020, 64, 103602. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Nunez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, M.; Xiong, W.; Li, J.; An, Y.; Ren, J.; Xie, Y.; Xue, H.; Yan, D.; Li, M.; et al. Saikosaponin-d ameliorates dextran sulfate sodium-induced colitis by suppressing NF-κB activation and modulating the gut microbiota in mice. Int. Immunopharmacol. 2020, 81, 106288. [Google Scholar] [CrossRef] [PubMed]
| Score | Body Weight Loss (%) | Stool Consistency | Fecal Blood |
|---|---|---|---|
| 0 | None | Normal | No blood |
| 1 | 1–5 | Soft but firm | Hemoccult+ |
| 2 | 5–10 | Loose stools | Blood |
| 3 | 10–20 | Diarrhea | Gross blood |
| 4 | >20 |
| Score | Inflammation Severity | Inflammation Extent | Crypt Damage |
|---|---|---|---|
| 0 | None | Normal | None |
| 1 | Mild | Mucosal | Basal 1/3 |
| 2 | Moderate | Mucosal and submucosal | Basal 2/3 |
| 3 | Severe | Transmural | Crypts lost but surface epithelium intact |
| 4 | Crypts and surface epithelium lost |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, X.; Gao, N.; Yan, D.; Shan, A. Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals 2020, 10, 1154. https://doi.org/10.3390/ani10071154
Dou X, Gao N, Yan D, Shan A. Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals. 2020; 10(7):1154. https://doi.org/10.3390/ani10071154
Chicago/Turabian StyleDou, Xiujing, Nan Gao, Di Yan, and Anshan Shan. 2020. "Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis" Animals 10, no. 7: 1154. https://doi.org/10.3390/ani10071154
APA StyleDou, X., Gao, N., Yan, D., & Shan, A. (2020). Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals, 10(7), 1154. https://doi.org/10.3390/ani10071154





