Immunohistochemical Assessment of Immune Response in the Dermis of Sarcoptes scabiei—Infested Wild Carnivores (Wolf and Fox) and Ruminants (Chamois and Red Deer)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Immunohistochemistry
2.3. Cell Counting and Statistical Analysis
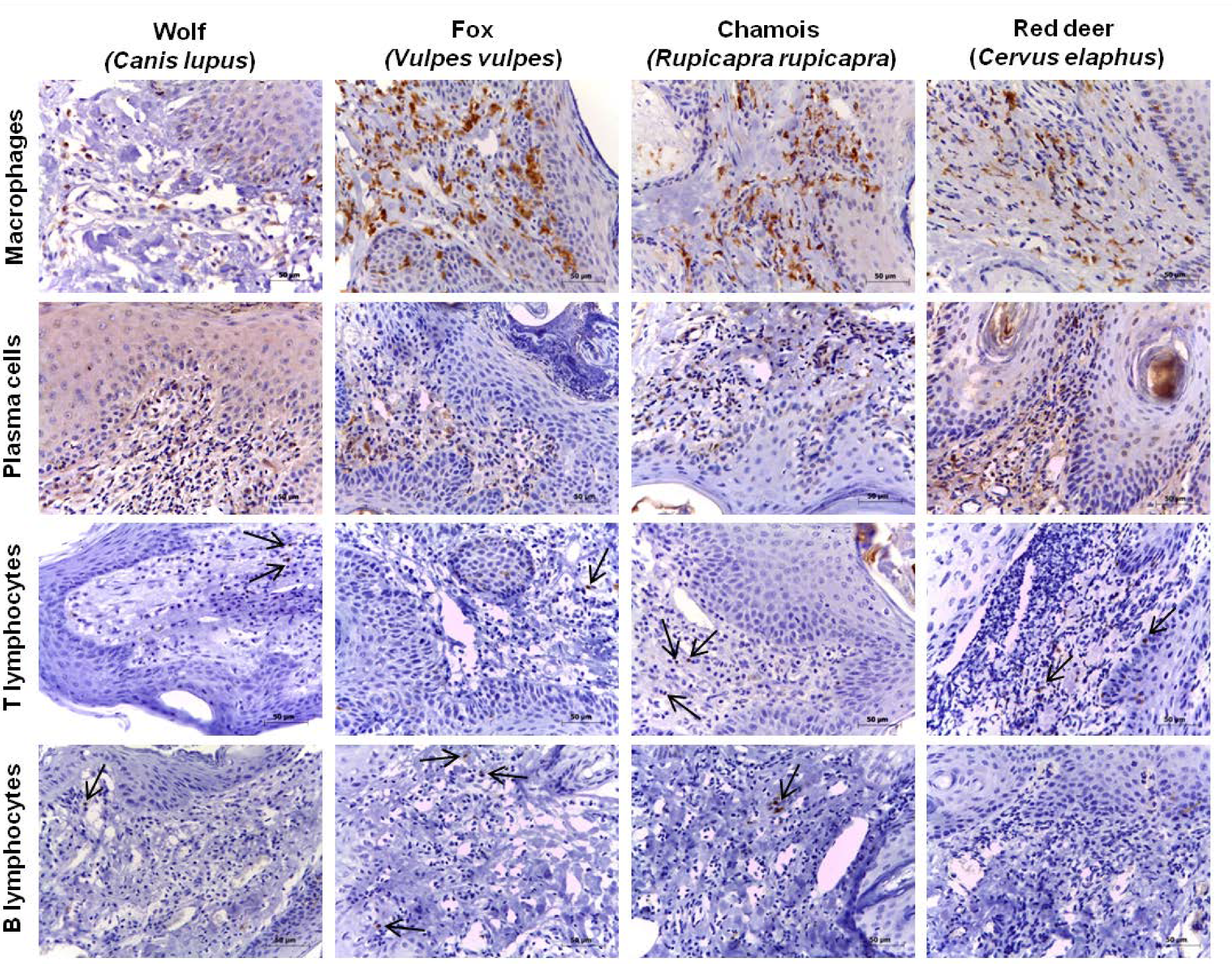
3. Results
3.1. Total Number of Inflammatory Cells
3.2. Relative Proportions of Inflammatory Cell Types within Each Species
3.3. Relative Proportions of Inflammatory Cell Types across Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández-Morán, J.; Gómez, S.; Ballesteros, F.; Quirós, P.; Benito, J.; Feliu, C.; Nieto, J. Epizootiology of sarcoptic mange in a population of cantabrian chamois (Rupicapra pyrenaica parava) in Northwestern Spain. Vet. Parasitol. 1997, 73, 163–171. [Google Scholar] [CrossRef]
- León-Vizcaíno, L.; Cubero, M.; González-Capitel, E.; Simón, M.A.; Pérez, L.; Ruiz de Ybáñez, M.; Ortíz, J.M.; González, M.; Alonso, F. Experimental ivermectin treatment of sarcoptic mange and establishment of a mange-free population of Spanish ibex. J. Wild. Dis. 2001, 37, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Bates, P. Sarcoptic mange (Sarcoptes scabiei var vulpes) in a red fox (Vulpes vulpes) population in north-west Surrey. Vet. Rec. 2003, 152, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, Á.; Casais, R.; Balseiro, A.; Espí, A.; Llaneza, L.; Hartasánchez, A.; Gortázar, C. New techniques for an old disease: Sarcoptic mange in the Iberian wolf. Vet. Parasitol. 2011, 181, 255–266. [Google Scholar] [CrossRef]
- Oleaga, A.; Casais, R.; Prieto, J.M.; Gortázar, C.; Balseiro, A. Comparative pathological and immunohistochemical features of sarcoptic mange in five sympatric wildlife species in Northern Spain. Eur. J. Wild. Res. 2012, 58, 997–1000. [Google Scholar] [CrossRef]
- Nimmervoll, H.; Hoby, S.; Robert, N.; Lommano, E.; Welle, M.; Ryser-Degiorgis, M.P. Pathology of sarcoptic mange in red foxes (Vulpes vulpes): macroscopic and histologic characterization of three disease stages. J. Wild. Dis. 2013, 49, 91–102. [Google Scholar] [CrossRef]
- Oleaga, A.; García, A.; Balseiro, A.; Casais, R.; Mata, E.; Crespo, E. First description of sarcoptic mange in the endangered Iberian lynx (Lynx pardinus): Clinical and epidemiological features. Eur. J. Wild. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, C.; Rocchigiani, G.; Lazzarotti, C.; Formenti, N.; Trogu, T.; Lanfranchi, P.; Zanardello, C.; Citterio, C.; Poli, A. Histological lesions and cellular response in the skin of Alpine chamois (Rupicapra r. rupicapra) spontaneously affected by sarcoptic mange. Biomed. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Vicente, J.; Ferroglio, E.; Pegoraro de Macedo, M.R.; Casais, R.; del Cerro, A.; Espí, A.; García, E.J.; Gortázar, C. Concomitance and interactions of pathogens in the Iberian wolf (Canis lupus). Res. Vet. Sci. 2015, 101, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Arlian, L.G.; Morgan, M.S. A review of Sarcoptes scabiei: Past, present and future. Parasite Vector. 2017, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.; Mörner, T.; Samuel, W.M. Sarcoptes scabiei and sarcoptic mange. In Parasitic Diseases of Wild Mammals; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Iowa State University Press: Ames, IA, USA, 2001; pp. 107–109. [Google Scholar]
- Lalli, P.N.; Morgan, M.S.; Arlian, L.G. Skewed Th1/Th2 immune response to Sarcoptes scabiei. J. Parasitol. 2004, 90, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.F. The immunology of susceptibility and resistance to scabies. Parasite Immunol. 2010, 32, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Rambozzi, L.; Menzano, A.; Lavín, S.; Rossi, L. Biotin-avidin amplified ELISA for detection of antibodies to Sarcoptes scabiei in chamois (Rupicapra spp.). Vet. Res. 2004, 35, 701–708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarasa, M.; Rambozzi, L.; Rossi, L.; Meneguz, P.G.; Serrano, E.; Granados, J.E.; González, F.J.; Fandos, P.; Soriguer, R.C.; González, G.; et al. Sarcoptes scabiei: Specific immune response to sarcoptic mange in the Iberian ibex Capra pyrenaica depends on previous exposure and sex. Exp. Parasitol. 2010, 124, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Skerratt, L.F. Cellular response in the dermis of common wombats (Vombatus ursinus) infected with Sarcoptes scabiei var. wombati. J. Wild. Res. 2003, 39, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Arlian, L.G.; Morgan, M.S.; Rapp, C.M.; Vyszenski-Moher, D.L. The development of protective immunity in canine scabies. Vet. Parasitol. 1996, 62, 133–142. [Google Scholar] [CrossRef]

| Primary Antibody (Dilution) | Target Cell Type | Epitope Demasking | Biotinylated Secondary Antibody (Dilution) |
|---|---|---|---|
| Iba1 (WAKO 019_19741), rabbit polyclonal (1:1000) | Macrophage | Microwave in citrate (pH 6), 20 min | Anti-rabbit (1:200) |
| Lambda (Dako A0193), rabbit polyclonal (1:1000) | Plasma cell | Microwave in citrate (pH 6), 20 min | Anti-rabbit (1:200) |
| CD3 (Novocastra-CL-L-CD3-565), mouse monoclonal (1:500) | pan-T cell | Microwave in citrate (pH 6), 20 min | Anti-mouse (1:200) |
| CD20 (ThermoFisher-PA516701), rabbit polyclonal (1:200) | pan-B cell | Steamer in citrate (pH 6), 20 min | Anti-rabbit (1:200) |
| Species | n | Mean ± SD * | Median | IQR |
|---|---|---|---|---|
| Wolf | 5 | 1175.2 ± 135.9 a | 1179 | 1044.0–1304.5 |
| Fox | 5 | 1636.4 ± 195.8 ab | 1725 | 1431.5–1797.0 |
| Chamois | 5 | 1242.4 ± 232.4 b | 1192 | 1088.5–1421.5 |
| Red deer | 5 | 1293.6 ± 137.3 | 1228 | 1206.5–1413.5 |
| Biomarker (Target Cell Type) | Animal Species | % Positive Cells | ||
|---|---|---|---|---|
| n | Mean * | SD | ||
| Iba1 (macrophages) | Wolf | 5 | 35.59 a | 4.11 |
| Fox | 5 | 29.11 | 7.94 | |
| Chamois | 5 | 25.70 | 9.60 | |
| Red deer | 5 | 22.57 a | 3.96 | |
| Lambda chain (plasma cells) | Wolf | 5 | 6.61 b | 0.97 |
| Fox | 5 | 6.56 c | 1.29 | |
| Chamois | 5 | 2.99 bc | 0.28 | |
| Red deer | 5 | 6.31 | 3.19 | |
| CD3 (T lymphocytes) | Wolf | 5 | 4.88 | 1.88 |
| Fox | 5 | 3.39 | 1.53 | |
| Chamois | 5 | 7.15 | 3.59 | |
| Red deer | 5 | 5.59 | 1.49 | |
| CD20 (B lymphocytes) | Wolf | 5 | 5.60 | 2.08 |
| Fox | 5 | 3.33 | 0.90 | |
| Chamois | 5 | 3.31 | 0.93 | |
| Red deer | 5 | 4.42 | 1.87 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, I.Z.; Oleaga, Á.; Sojo, I.; García-Iglesias, M.J.; Pérez-Martínez, C.; García Marín, J.F.; Balseiro, A. Immunohistochemical Assessment of Immune Response in the Dermis of Sarcoptes scabiei—Infested Wild Carnivores (Wolf and Fox) and Ruminants (Chamois and Red Deer). Animals 2020, 10, 1146. https://doi.org/10.3390/ani10071146
Martínez IZ, Oleaga Á, Sojo I, García-Iglesias MJ, Pérez-Martínez C, García Marín JF, Balseiro A. Immunohistochemical Assessment of Immune Response in the Dermis of Sarcoptes scabiei—Infested Wild Carnivores (Wolf and Fox) and Ruminants (Chamois and Red Deer). Animals. 2020; 10(7):1146. https://doi.org/10.3390/ani10071146
Chicago/Turabian StyleMartínez, Ileana Z., Álvaro Oleaga, Irene Sojo, María José García-Iglesias, Claudia Pérez-Martínez, Juan F. García Marín, and Ana Balseiro. 2020. "Immunohistochemical Assessment of Immune Response in the Dermis of Sarcoptes scabiei—Infested Wild Carnivores (Wolf and Fox) and Ruminants (Chamois and Red Deer)" Animals 10, no. 7: 1146. https://doi.org/10.3390/ani10071146
APA StyleMartínez, I. Z., Oleaga, Á., Sojo, I., García-Iglesias, M. J., Pérez-Martínez, C., García Marín, J. F., & Balseiro, A. (2020). Immunohistochemical Assessment of Immune Response in the Dermis of Sarcoptes scabiei—Infested Wild Carnivores (Wolf and Fox) and Ruminants (Chamois and Red Deer). Animals, 10(7), 1146. https://doi.org/10.3390/ani10071146






