Effect of Oral Supplementation of Healthy Pregnant Sows with Sucrosomial Ferric Pyrophosphate on Maternal Iron Status and Hepatic Iron Stores in Newborn Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
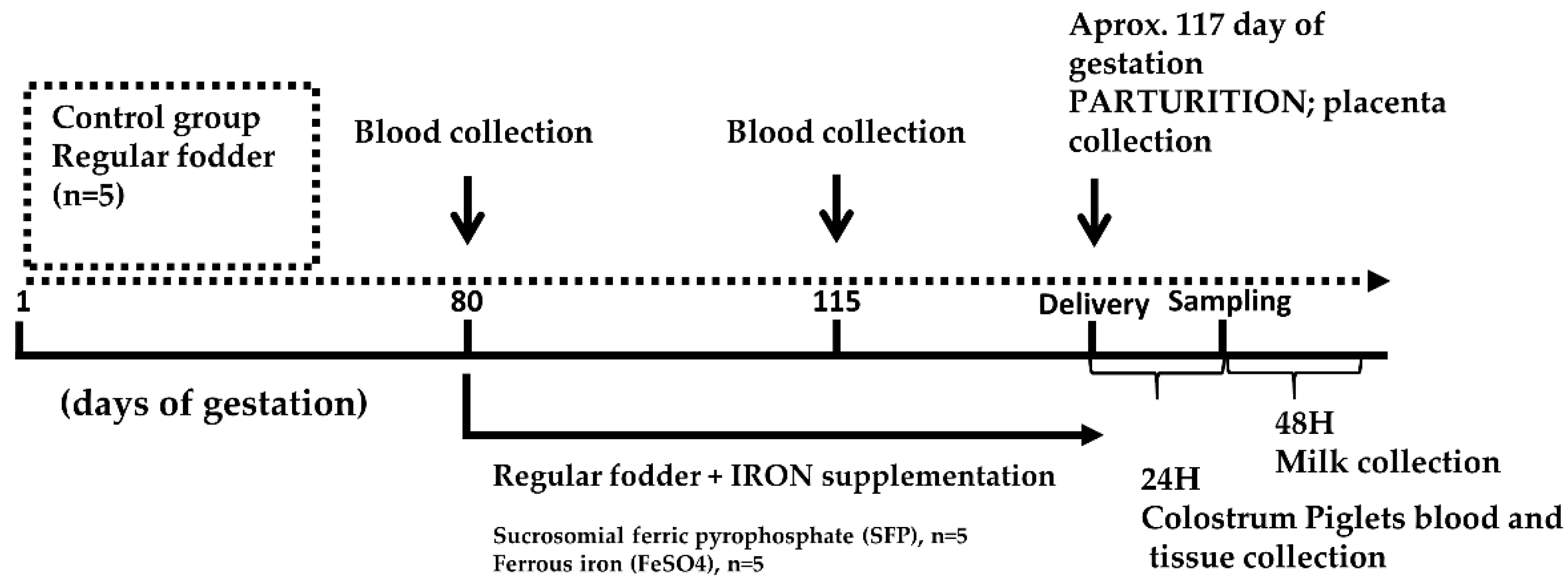
2.1. Sows and Piglets, Experimental Design and Biological Sample Collection
2.2. Measurement of Red Blood Cell Indices and Plasma Iron Level
2.3. Measurement of Non-Heme Iron Content in Tissues
2.4. Plasma Hepcidin-25 Measurement
2.5. Real-Time Quantitative RT-PCR
2.6. Immunofluorescence (IF) Analysis and Confocal Microscopy of Placental Sections
2.7. Placental Samples and Fixation for Transmission Electron Microscopy (TEM)
2.8. Ethic Statement
2.9. Statistical Analysis
3. Results
3.1. Preganant Sows Supplemented with SFP and FeSO4 Show No Changes in Hematological and Iron Status Compared with Non-Suplemented Controls
3.2. Supplementation of Pregnant Sows with Iron Did Not Influence Iron Content in the Colostrum and Milk
3.3. Red Blood Cell Indices and Plasma Iron Status in Piglets from Control and Iron-Supplemented Sows
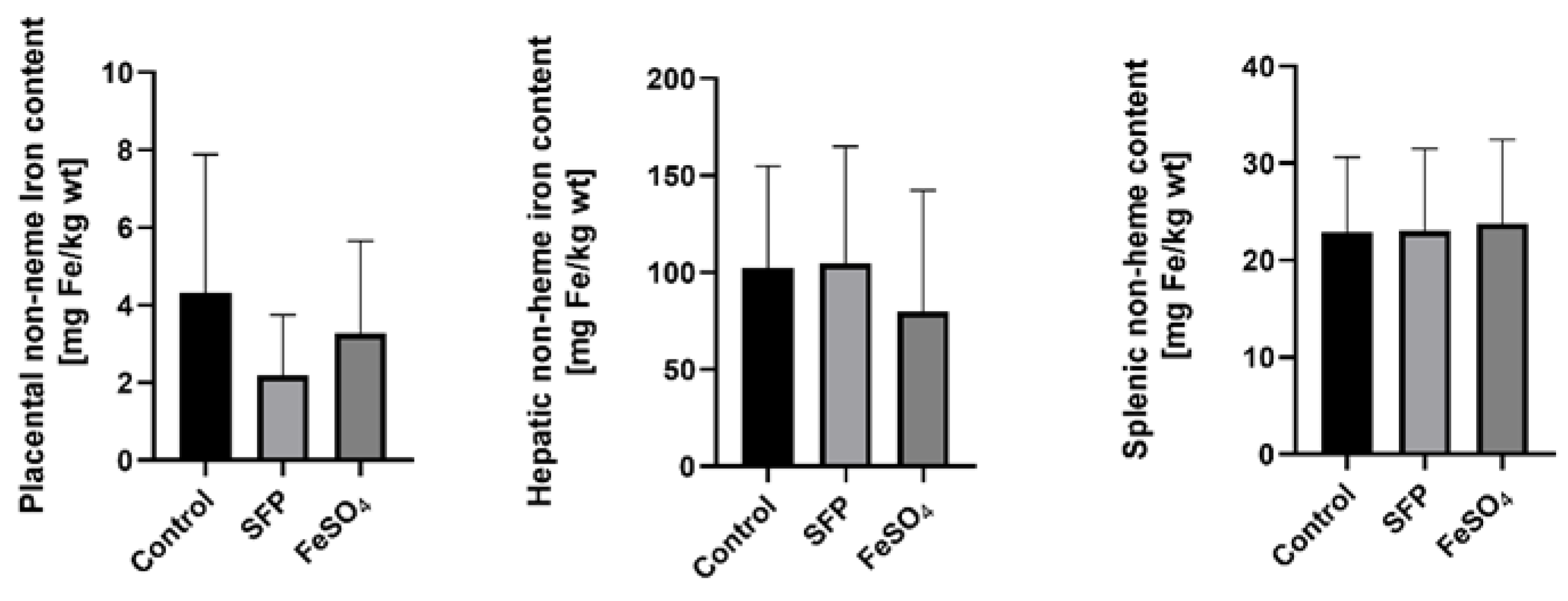
3.4. Supplementation of Pregnant Sows with SFP or FeSO4 Does Not Alter neither Iron Content in the Placenta nor Hepatic and Splenic Iron Status of their Progeny
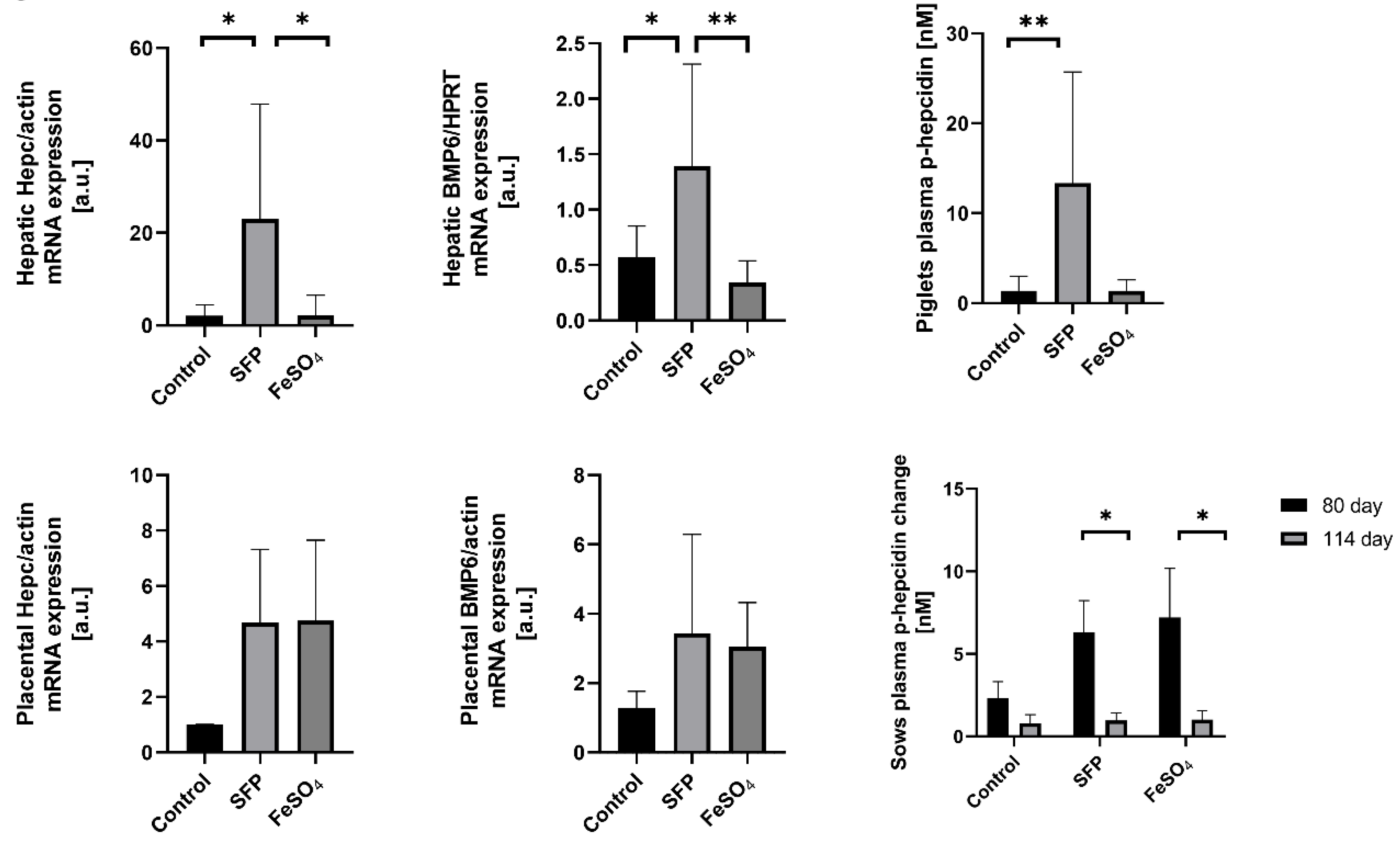
3.5. Increased Hepcidin and Bone Morphogenetic Protein 6 (BMP6) Expression in the Placenta and in the Liver of Piglets from Sows Supplemented with SFP
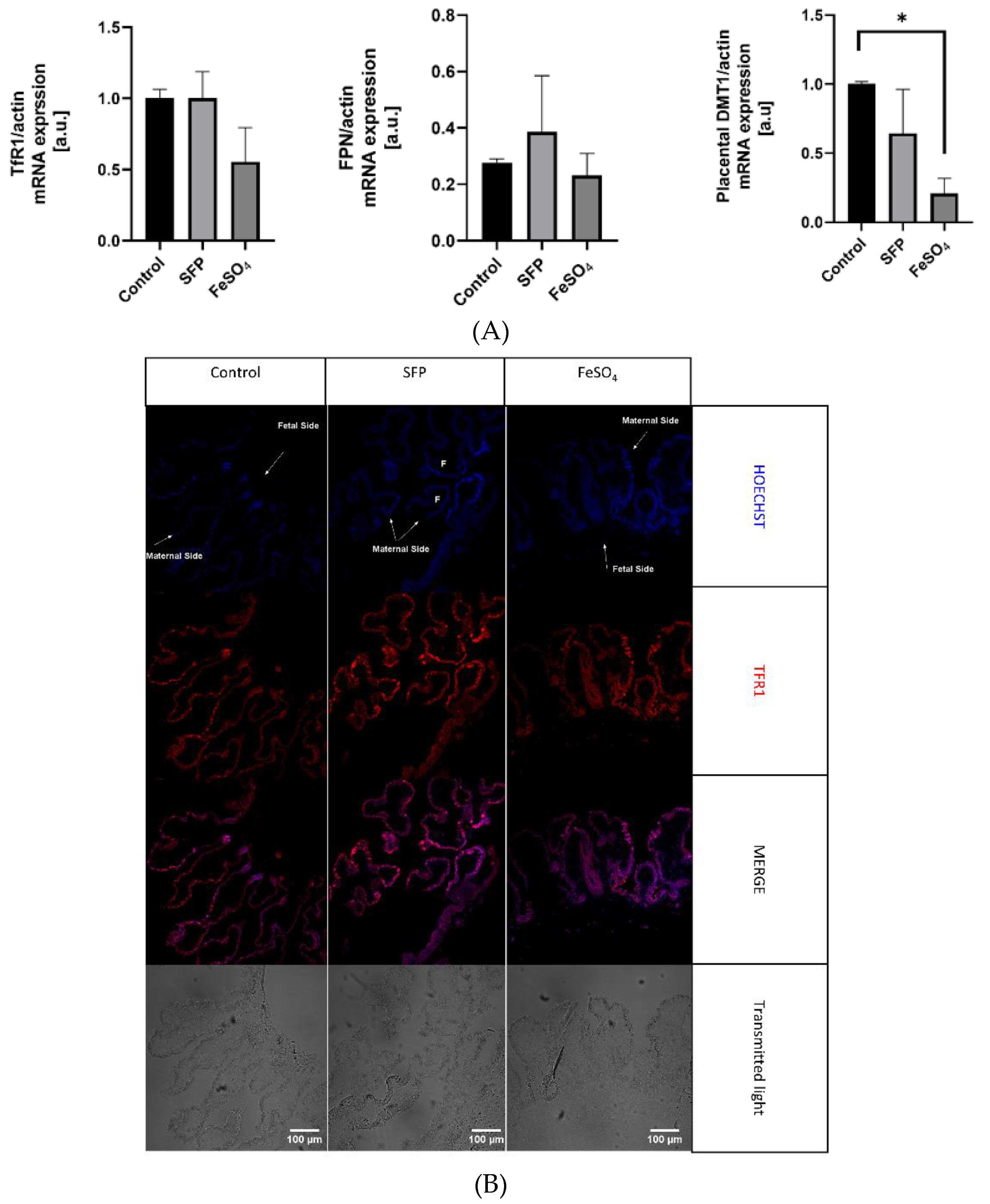
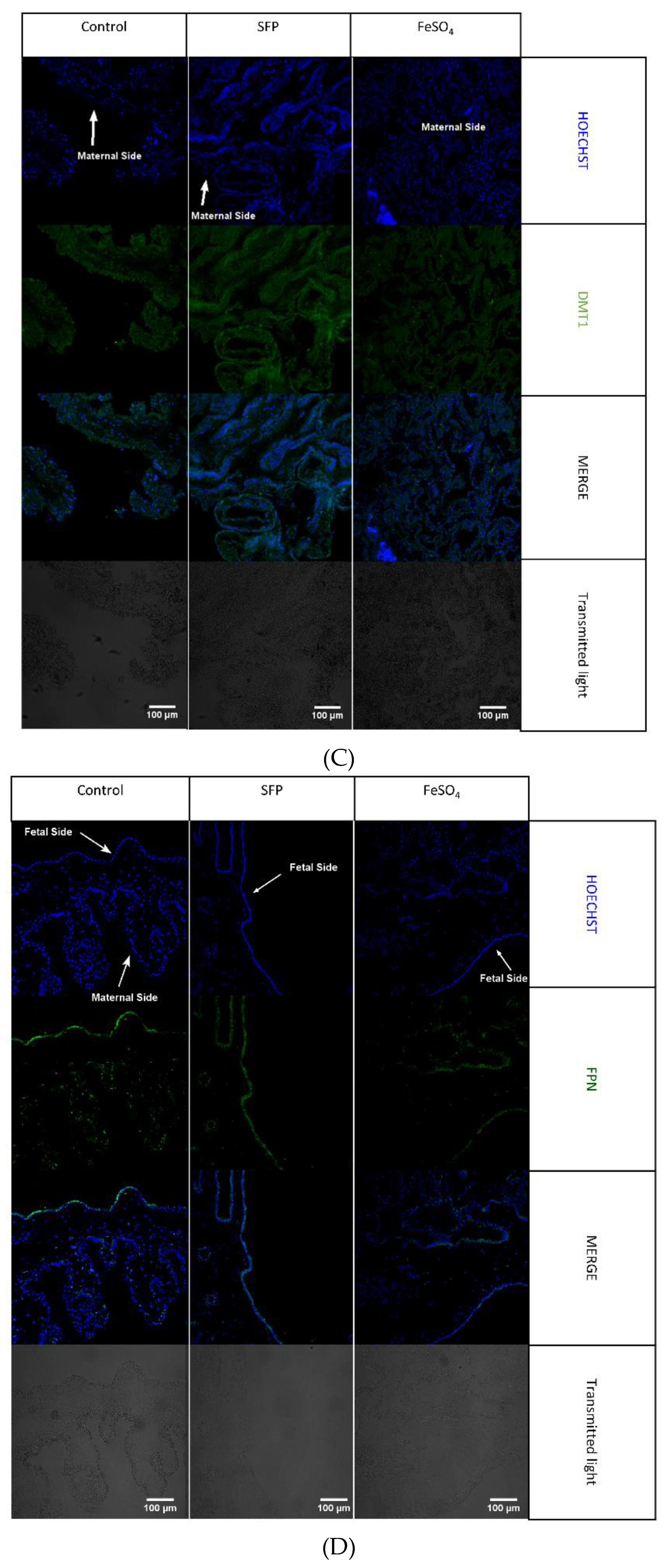
3.6. The Effect of Oral Iron Supplementation on Placental Morphology and Expression of Iron Transporters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koenig, M.D.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef]
- Bothwell, T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000, 72, 257S–264S. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.F.; Newcombe, R.G.; O’Riordan, J.; Coles, E.C.; Pearson, J.F. Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet 1986, 327, 992–995. [Google Scholar] [CrossRef]
- Scholl, T.O.; Hediger, M.L. Anemia and iron-deficiency anemia: Compilation of data on pregnancy outcome. Am. J. Clin. Nutr. 1994, 59, 492s–501s. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Fong, Y.F.; Arulkumaran, S. Anaemia in pregnancy—A cross-sectional study in Singapore. Eur. J. Clin. Nutr. 1998, 52, 65–70. [Google Scholar] [CrossRef]
- Yip, R. Significance of an abnormally low or high hemoglobin concentration during pregnancy: Special consideration of iron nutrition. Am. J. Clin. Nutr. 2000, 72, 272–279. [Google Scholar] [CrossRef]
- Oliver, M.H.; Jaquiery, A.L.; Bloomfield, F.H.; Harding, J.E. The effects of maternal nutrition around the time of conception on the health of the offspring. Soc. Reprod. Fertil. Suppl. 2007, 64, 397–410. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Daily Iron and Folic Acid Supplementation During Pregnancy; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Peña-Rosas, J.P.; Viteri, F.E. Effects and safety of preventive oral iron or iron + folic acid supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2009, 4. [Google Scholar] [CrossRef]
- Apgar, V. A proposal for a new method of evaluation of the newborn infant. Anesth. Analg. 2015, 120, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Koopmans, S.J.; Lallès, J.P.; Le Huerou-Luron, I.; De Jager, N.; Schuurman, T.; Val-Laillet, D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.L.; Ji, F.; Wu, G.; Blanton, J.R.; Kim, S.W. Growth and compositional changes of fetal tissues in pigs. J. Anim. Sci. 2004, 82, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.K.; da Silva, C.A.; Silva dos Santos, R.D.K.; Dias, C.P.; Callegari, M.A.; de Oliveira, E.R. The effect of supplementing sow and piglet diets with different forms of iron. Rev. Bras. Zootec. 2016, 45, 615–621. [Google Scholar] [CrossRef]
- Zhao, P.; Upadhaya, S.D.; Li, J.; Kim, I. Comparison effects of dietary iron dextran and bacterial-iron supplementation on growth performance, fecal microbial flora, and blood profiles in sows and their litters. Anim. Sci. J. 2015, 86, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Buffler, M.; Becker, C.; Windisch, W.M. Effects of different iron supply to pregnant sows (Sus scrofa domestica L.) on reproductive performance as well as iron status of new-born piglets. Arch. Anim. Nutr. 2017, 71, 219–230. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Dong, D.; Jiang, S.; Yang, Z.; Wang, Y. Effect of different sources and levels of iron in the diet of sows on iron status in neonatal pigs. Anim. Nutr. 2018, 4, 197–202. [Google Scholar] [CrossRef]
- Barros, C.A.; Pascoal, L.A.F.; Watanabe, P.H.; Martins, T.D.D.; Andrade, T.S.; Ribeiro, J.E.S. Dietary iron chelate for sows and effects on iron supplementation in piglet. An. Acad. Bras. Cienc. 2019, 91, 1–9. [Google Scholar] [CrossRef]
- Wan, D.; Zhang, Y.M.; Wu, X.; Lin, X.; Shu, X.G.; Zhou, X.H.; Du, H.T.; Xing, W.G.; Liu, H.N.; Li, L.; et al. Maternal dietary supplementation with ferrous N-carbamylglycinate chelate affects sow reproductive performance and iron status of neonatal piglets. Animal 2018, 12, 1372–1379. [Google Scholar] [CrossRef]
- Bhattarai, S.; Framstad, T.; Nielsen, J.P. Iron treatment of pregnant sows in a Danish herd without iron deficiency anemia did not improve sow and piglet hematology or stillbirth rate. Acta Vet. Scand. 2019, 61, 1–9. [Google Scholar] [CrossRef]
- Szudzik, M.; Starzyński, R.R.; Jończy, A.; Mazgaj, R.; Lenartowicz, M.; Lipiński, P. Iron supplementation in suckling piglets: An ostensibly easy therapy of neonatal iron deficiency anemia. Pharmaceuticals 2018, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Asperti, M.; Gryzik, M.; Brilli, E.; Castagna, A.; Corbella, M.; Gottardo, R.; Girelli, D.; Tarantino, G.; Arosio, P.; Poli, M. Sucrosomial® iron supplementation in mice: Effects on blood parameters, hepcidin, and inflammation. Nutrients 2018, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Brilli, E.; Mattii, L.; Testai, L.; Moscato, S.; Citi, V.; Tarantino, G.; Zambito, Y. Ex vivo and in vivo study of sucrosomial® iron intestinal absorption and bioavailability. Int. J. Mol. Sci. 2018, 19, 2722. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Berti, C.; Mandò, C.; Martinelli, A.; Mazzali, C.; Cetin, I. Effects of different regimens of iron prophylaxis on maternal iron status and pregnancy outcome: A randomized control trial. J. Matern. Neonatal Med. 2017, 30, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Szudzik, M.; Lipiński, P.; Jończy, A.; Mazgaj, R.; Pieszka, M.; Kamyczek, M.; Smuda, E.; Starzyński, R.R. Long-term effect of split iron dextran/hemoglobin supplementation on erythrocyte and iron status, growth performance, carcass parameters, and meat quality of Polish Large White and 990 Line Pigs. Biol. Trace. Elem. Res. 2020, 196, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Nutrient Requirements of Swine; The National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-48903-4. [Google Scholar]
- Torrance, J.D.; Bothwell, T.H.; Cook, J.D. Tissue iron stores. Iron Methods Hematol. 1980, 1, 90–115. [Google Scholar]
- Starzyński, R.R.; Laarakkers, C.M.M.; Tjalsma, H.; Swinkels, D.W.; Pieszka, M.; Styś, A.; Mickiewicz, M.; Lipiński, P. Iron supplementation in suckling piglets: How to correct iron deficiency anemia without affecting plasma hepcidin levels. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Staroń, R.; Van Swelm, R.P.L.; Lipiński, P.; Gajowiak, A.; Lenartowicz, M.; Bednarz, A.; Gajewska, M.; Pieszka, M.; Laarakkers, C.M.M.; Swinkels, D.W.; et al. Urinary hepcidin levels in iron-deficient and iron-supplemented piglets correlate with hepcidin hepatic mRNA and serum levels and with body iron status. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Starzynski, R.R.; Canonne-Hergaux, F.; Lenartowicz, M.; Krzeptowski, W.; Willemetz, A.; Stys, A.; Biera, J.; Pietrzak, P.; Dziaman, T.; Lipínski, P. Ferroportin expression in haem oxygenase 1-deficient mice. Biochem. J. 2013, 449, 69–78. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. An. Int. Rev. J. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, C.; Nicolas, G.; Vaulont, S. Hepcidin, a key regulator of iron metabolism. Hematologie 2003, 9, 27–36. [Google Scholar]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef]
- Rishi, G.; Subramaniam, V.N. The liver in regulation of iron homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G157–G165. [Google Scholar] [CrossRef]
- Anderson, E.R.; Shah, Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013, 3, 315–330. [Google Scholar]
- Cao, C.; Fleming, M.D. The placenta: The forgotten essential organ of iron transport. Nutr. Rev. 2016, 74, 421–431. [Google Scholar] [CrossRef]
- Merle, U.; Fein, E.; Gehrke, S.G.; Stremmel, W.; Kulaksiz, H. The iron regulatory peptide hepcidin is expressed in the heart and regulated by hypoxia and inflammation. Endocrinology 2007, 148, 2663–2668. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Placental iron transport: The mechanism and regulatory circuits. Free Radic. Biol. Med. 2019, 133, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, S.; Todros, T.; Nuzzo, A.M.; Rolfo, A. Maternal serum levels and placental expression of hepcidin in preeclampsia. Pregnancy Hypertens. 2018, 11, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Duck, K.A.; Connor, J.R. Iron uptake and transport across physiological barriers. BioMetals 2016, 29, 573–591. [Google Scholar] [CrossRef]
- Lipiński, P.; Styś, A.; Starzyński, R.R. Molecular insights into the regulation of iron metabolism during the prenatal and early postnatal periods. Cell. Mol. Life Sci. 2013, 70, 23–38. [Google Scholar] [CrossRef]
- Fisher, A.L.; Nemeth, E. Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 2017, 106, 1567S–1574S. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Cindrova-Davies, T.; Muttukrishna, S.; Burton, G.J.; Porter, J.; Jauniaux, E. Hepcidin and iron species distribution inside the first-trimester human gestational sac. Mol. Hum. Reprod. 2011, 17, 227–232. [Google Scholar] [CrossRef]
- Zlatanova, I.; Pinto, C.; Bonnin, P.; Mathieu, J.R.R.; Bakker, W.; Vilar, J.; Lemitre, M.; Voehringer, D.; Vaulont, S.; Peyssonnaux, C.; et al. Iron regulator hepcidin impairs macrophage-dependent cardiac repair after injury. Circulation 2019, 139, 1530–1547. [Google Scholar] [CrossRef]
- Canali, S.; Zumbrennen-Bullough, K.B.; Core, A.B.; Wang, C.Y.; Nairz, M.; Bouley, R.; Swirski, F.K.; Babitt, J.L. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood 2017, 129, 405–414. [Google Scholar] [CrossRef]
- Bastin, J.; Drakesmith, H.; Rees, M.; Sargent, I.; Townsend, A. Localisation of proteins of iron metabolism in the human placenta and liver. Br. J. Haematol. 2006, 134, 532–543. [Google Scholar] [CrossRef]
- Elli, L.; Ferretti, F.; Branchi, F.; Tomba, C.; Lombardo, V.; Scricciolo, A.; Doneda, L.; Roncoroni, L. Sucrosomial iron supplementation in anemic patients with celiac disease not tolerating oral ferrous sulfate: A prospective study. Nutrients 2018, 10, 330. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Muñoz, M. Sucrosomial® iron: A new generation iron for improving oral supplementation. Pharmaceuticals 2018, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.L.; Lasley, E.; Bolin, D.W. Anemia in Suckling Pigs; Agricultural Experiment Station, North Dakota State University: Fargo, ND, USA, 1949; Volume 11, p. 106. [Google Scholar]
- Lipiński, P.; Starzyński, R.R.; Canonne-Hergaux, F.; Tudek, B.; Oliński, R.; Kowalczyk, P.; Dziaman, T.; Thibaudeau, O.; Gralak, M.A.; Smuda, E.; et al. Benefits and risks of iron supplementation in anemic neonatal pigs. Am. J. Pathol. 2010, 177, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.C.; Mahan, D.C. Effects of neonatal iron status, iron injections at birth, and weaning in young pigs from sows fed either organic or inorganic trace minerals. J. Anim. Sci. 2008, 86, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.M.; Miller, E.R.; Whetter, P.A.; Ullrey, D.E. Concentration of minerals in tissues of pigs from dams fed different levels of dietary zinc. J. Anim. Sci. 1983, 57, 130–138. [Google Scholar] [CrossRef]
- Svoboda, M.; Píšťková, K. Oral iron administration in suckling piglets—A review. Acta Vet. Brno 2018, 87, 77–83. [Google Scholar] [CrossRef]
- Collard, K.J. Iron homeostasis in the neonate. Pediatrics 2009, 123, 1208–1216. [Google Scholar] [CrossRef]
- John, B.Y.; Mcgowan, P.; Crichton, A. Iron deficiency in pigs. Biochem. J. 1924, 18, 265–272. [Google Scholar]
- Szabo, P.; Bilkei, G. Iron deficiency in outdoor pig production. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2002, 49, 390–391. [Google Scholar] [CrossRef]
- Venn, J.A.J.; Mccance, R.A.; Widdowson, E.M. Iron metabolism in piglet anaemia. J. Comp. Pathol. Ther. 1947, 57, 314–325. [Google Scholar] [CrossRef]
- Csapó, J.; Martin, T.G.; Csapó-Kiss, Z.S.; Házas, Z. Protein, fats, vitamin and mineral concentrations in porcine colostrum and milk from parturition to 60 days. Int. Dairy J. 1996, 6, 881–902. [Google Scholar] [CrossRef]
- Rauw, W.M.; Kanis, E.; Noordhuizen-Stassen, E.N.; Grommers, F.J. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Nash, C.M.; Allen, V.M. The use of parenteral iron therapy for the treatment of postpartum anemia. J. Obstet. Gynaecol. Can. 2015, 37, 439–442. [Google Scholar] [CrossRef]
- Pond, W.G.; Lowrey, R.S.; Maner, J.H.; Loosli, J.K. Parenteral iron administration to sows during gestation or lactation. J. Anim. Sci. 1961, 20, 747–750. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Malave, H.G.; Flores-Urrutia, M.C.; Dowswell, T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Jahan, M.; Kracht, S.; Ho, Y.; Haque, Z.; Bhattachatyya, B.N.; Wynn, P.C.; Wang, B. Dietary lactoferrin supplementation to gilts during gestation and lactation improves pig production and immunity. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Chaney, C.H.; Barnhart, C.E. The effect of iron supplementation on the prevention of anemia in baby. Am. J. Vet. Res. 1964, 25, 420–423. [Google Scholar]
- Nath, M.K.; Mahanta, P.N.; Nath, D.R. Prevention and control of piglet anaemia by oral supplementation of ferrocom in sows and their piglets. AARJMD Asian Acad. Res. J. Multidiscip. 2015, 1. [Google Scholar] [CrossRef]
- Loudenslager, M.J.; Ku, P.K.; Whetter, P.A.; Ullrey, D.E.; Whitehair, C.K.; Stowe, H.D.; Miller, E.R. Importance of diet of dam and colostrum to the biological antioxidant status and parenteral iron tolerance of the pig. J. Anim. Sci. 1986, 63, 1905–1914. [Google Scholar] [CrossRef]
- Bertechini, A.G.; Fassani, E.J.; de Brito, J.Á.G.; Barrios, P.R. Effects of dietary mineral bioplex in pregnant and lactating sow diets on piglet performance and physiological characteristics. Rev. Bras. Zootec. 2012, 41, 624–629. [Google Scholar] [CrossRef]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef]
- Choi, J.W.; Pai, S.H. Change in erythropoiesis with gestational age during pregnancy. Ann. Hematol. 2001, 80, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Cavill, I. Iron and erythropoiesis in normal subjects and in pregnancy. J. Perinat. Med. 1995, 23, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Letsky, E.A. Erythropoiesis in pregnancy. J. Perinat. Med. 1995, 23, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, S.; Kroot, J.J.C.; Zijderveld, G.; Wiegerinck, E.T.; Spaanderman, M.E.A.; Swinkels, D.W. The iron regulatory hormone hepcidin is decreased in pregnancy: A prospective longitudinal study. Clin. Chem. Lab. Med. 2013, 51, 1395–1401. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Wong, S.; Koenig, M.D.; Tussing-Humphreys, L.; Chu, A.; Lelić, M.; Ganz, T.; Nemeth, E. Effects of maternal iron status on placental and fetal iron homeostasis. J. Clin. Invest. 2020, 130, 625–640. [Google Scholar] [CrossRef]
- Rehu, M.; Punnonen, K.; Ostland, V.; Heinonen, S.; Westerman, M.; Pulkki, K.; Sankilampi, U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur. J. Haematol. 2010, 85, 345–352. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Gayet, T.; Devillard, S.; Gamelon, M.; Brandt, S.; Say, L.; Baubet, E. On the evolutionary consequences of increasing litter size with multiple paternity in wild boar (Sus scrofa scrofa). Evolution 2016, 70, 1386–1397. [Google Scholar] [CrossRef]
- Nebert, D.W.; Gálvez-Peralta, M.; Hay, E.B.; Li, H.; Johansson, E.; Yin, C.; Wang, B.; He, L.; Soleimani, M. ZIP14 and ZIP8 zinc/bicarbonate symporters in Xenopus oocytes: Characterization of metal uptake and inhibition. Metallomics 2012, 4, 1218–1225. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Young, M.F.; Essley, B.V.; McNanley, T.J.; Cooper, E.M.; Pressman, E.K.; McIntyre, A.W.; Orlando, M.S.; Abkowitz, J.L.; Guillet, R.; et al. Placental expression of the heme transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J. Nutr. 2011, 141, 1267–1272. [Google Scholar] [CrossRef]
- Baron, J.; Ben-David, G.; Hallak, M. Changes in non-transferrin-bound iron (NTBI) in pregnant women on iron supplements. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 281–282. [Google Scholar] [CrossRef] [PubMed]

| Experimental Groups | Gestational Days | Supplementation | Time | Supplementation * Time | p-Value 80 vs. 114 Day | ||||
|---|---|---|---|---|---|---|---|---|---|
| Day 80 | Day 114 | ||||||||
| F | p-Value | F | p-Value | F | p-Value | ||||
| Hb (g/dL) | |||||||||
| Control | 12.2 ± 0.8 | 11.7 ± 1.9 | 6.539 | 0.0311* | 0.1384 | 0.7227 | 0.5869 | 0.5851 | 0.9519 |
| SFP | 9.8 ± 2.9 | 11.0 ± 0.6 | 0.9993 | ||||||
| FeSO4 | 11.1 ± 0.88 | 10.2 ± 2.24 | 0.7189 | ||||||
| RBC (mln/mm3) | |||||||||
| Control | 6.2 ± 0.6 | 5.8 ± 1.1 | 6.480 | 0.0317* | 1.504 | 0.2660 | 0.5245 | 0.6167 | >0.9999 |
| SFP | 5.3 ± 1.7 | 5.6 ± 0.3 | 0.8496 | ||||||
| FeSO4 | 5.7 ± 0.6 | 6.9 ± 4.6 | 0.5106 | ||||||
| MCV (µm3) | |||||||||
| Control | 61.2 ± 3.1 | 63.2 ± 2.7 | 0.2633 | 0.7769 | 38.23 | 0.0008 ** | 1.409 | 0.3150 | 0.192 |
| SFP | 58.4 ± 4.9 | 62.8 ± 3.5 | 0.0132* | ||||||
| FeSO4 | 62.4 ± 4.9 | 64.5 ± 4.5 | 0.0198* | ||||||
| Plasma iron level (µmol/L) | |||||||||
| Control | 15.6 ± 3 | 12.3 ± 4.3 | 0.02066 | 0.9796 | 4.767 | 0.0717 | 0.1594 | 0.8562 | 0.8294 |
| SFP | 14.4 ± 4.7 | 12.8 ± 2.8 | 0.4135 | ||||||
| FeSO4 | 15.3 ± 1.8 | 12.8 ± 1.3 | 0.5222 | ||||||
| Plasma ferritin concentration (ng/mL) | |||||||||
| Control | 514.3 ± 117.4 | 199.8 ± 57 | 0.8294 | 0.4808 | 29.20 | 0.0017** | 0.7431 | 0.5148 | 0.0597 |
| SFP | 557.9 ± 67.5 | 231.1 ± 56.4 | 0.0218* | ||||||
| FeSO4 | 402.7 ± 180.4 | 176.4 ± 85.3 | 0.1831 | ||||||
| Plasma hepcidin level (nM) | |||||||||
| Control | 2.33 ± 0.8 | 0.8 ± 0.42 | 3.979 | 0.0794 | 41.62 | 0.0007 ** | 4.506 | 0.0638 | 0.5571 |
| SFP | 6.3 ± 1.58 | 0.96 ± 0.36 | 0.0115 * | ||||||
| FeSO4 | 7.2 ± 2.43 | 1 ± 0.45 | 0.0055 ** | ||||||
| Blood Parameters | Control | SFP | FeSO4 | p-Value | |
|---|---|---|---|---|---|
| Control vs. …. | |||||
| Hb (g/dL) | 9.4 ± 1.4 | 9.8 ± 1 | 9 ± 1.3 | SFP 0.6906 | FeSO4 0.7161 |
| RBC mln/mm3 | 4.9 ± 0.7 | 5.1 ± 0.9 | 4.69 ± 0.6 | SFP 0.8252 | FeSO4 0.7332 |
| MCV (µm3) | 60 ± 2.7 | 63.1 ± 3.7 | 61.6 ± 4 | SFP 0.1301 | FeSO4 0.5538 |
| Serum Iron (µmol/L) | 14.8 ± 9.9 | 10.5 ± 8.4 | 18 ± 8.1 | SFP 0.2284 | FeSO4 0.9994 |
| Serum Ferritin (ng/mL) | 823.5 ± 161.4 | 906.2 ± 75.1 | 772.7 ± 137.6 | SFP 0.3656 | FeSO4 0.6631 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazgaj, R.; Szudzik, M.; Lipiński, P.; Jończy, A.; Smuda, E.; Kamyczek, M.; Cieślak, B.; Swinkels, D.; Lenartowicz, M.; Starzyński, R.R. Effect of Oral Supplementation of Healthy Pregnant Sows with Sucrosomial Ferric Pyrophosphate on Maternal Iron Status and Hepatic Iron Stores in Newborn Piglets. Animals 2020, 10, 1113. https://doi.org/10.3390/ani10071113
Mazgaj R, Szudzik M, Lipiński P, Jończy A, Smuda E, Kamyczek M, Cieślak B, Swinkels D, Lenartowicz M, Starzyński RR. Effect of Oral Supplementation of Healthy Pregnant Sows with Sucrosomial Ferric Pyrophosphate on Maternal Iron Status and Hepatic Iron Stores in Newborn Piglets. Animals. 2020; 10(7):1113. https://doi.org/10.3390/ani10071113
Chicago/Turabian StyleMazgaj, Rafał, Mateusz Szudzik, Paweł Lipiński, Aneta Jończy, Ewa Smuda, Marian Kamyczek, Beata Cieślak, Dorine Swinkels, Małgorzata Lenartowicz, and Rafał R. Starzyński. 2020. "Effect of Oral Supplementation of Healthy Pregnant Sows with Sucrosomial Ferric Pyrophosphate on Maternal Iron Status and Hepatic Iron Stores in Newborn Piglets" Animals 10, no. 7: 1113. https://doi.org/10.3390/ani10071113
APA StyleMazgaj, R., Szudzik, M., Lipiński, P., Jończy, A., Smuda, E., Kamyczek, M., Cieślak, B., Swinkels, D., Lenartowicz, M., & Starzyński, R. R. (2020). Effect of Oral Supplementation of Healthy Pregnant Sows with Sucrosomial Ferric Pyrophosphate on Maternal Iron Status and Hepatic Iron Stores in Newborn Piglets. Animals, 10(7), 1113. https://doi.org/10.3390/ani10071113





