Identification of Sarcocystis spp. in One-humped Camels (Camelus dromedarius) from Riyadh and Dammam, Saudi Arabia, via Histological and Phylogenetic Approaches
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Macroscopic Examination
2.3. Microscopic Examination
2.4. Digestion Method
2.5. Histopathological Examination
2.6. Transmission Electron Microscopy (TEM)
2.7. Molecular Analysis
2.7.1. DNA Extraction and PCR Amplification
2.7.2. DNA Sequencing
2.8. Statistical Analysis
3. Results
3.1. Macroscopic Examination
3.2. Microscopic Examination
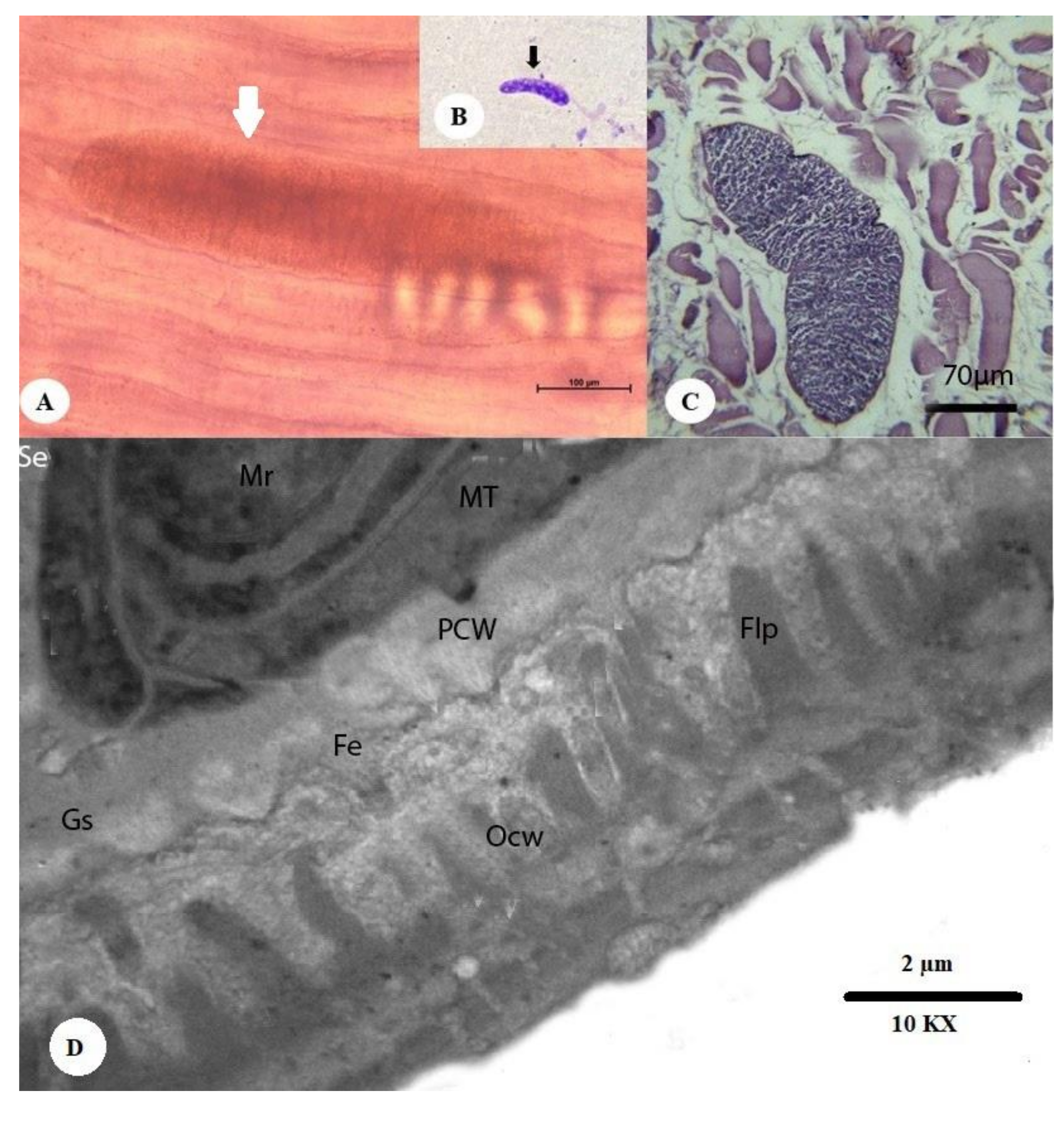
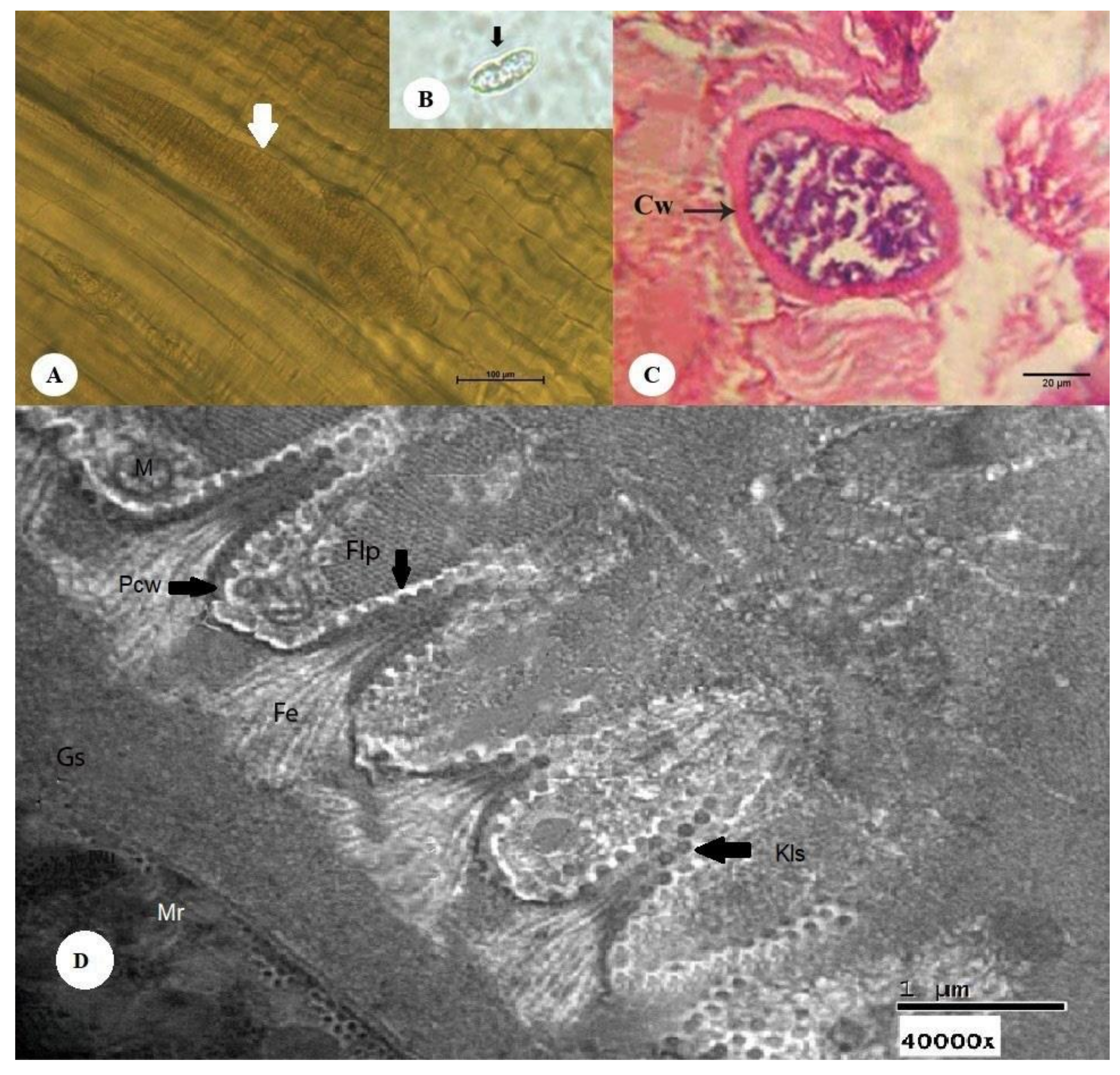
3.2.1. Thin-Walled Microscopic Sarcocysts
3.2.2. Thick-walled Microscopic Sarcocysts
3.3. Genetic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miescher, F. Über eigenthümliche Schläuche in den Muskeln einer Hausmaus. Ber. Verhandl Nat. Gesell Basel 1843, 5, 198–202. [Google Scholar]
- Tenter, A.M. Current research on Sarcocystis species of domestic animals. Int. J. Parasitol. 1995, 25, 1311–1330. [Google Scholar] [CrossRef]
- Dubey, J. Foodborne and waterborne zoonotic sarcocystosis. Food Waterborne Parasitol. 2015, 1, 2–11. [Google Scholar] [CrossRef]
- Fayer, R. Sarcocystis spp. Human Infections. Clin. Microbiol. Rev. 2004, 17, 894–902. [Google Scholar] [CrossRef]
- Hidron, A.; Vogenthaler, N.; Santos-Preciado, J.I.; Rodriguez-Morales, A.J.; Franco-Paredes, C.; Rassi, A. Cardiac Involvement with Parasitic Infections. Clin. Microbiol. Rev. 2010, 23, 324–349. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Miller, M.; Barr, B.; Nordhausen, R.; James, E.; Magargal, S.; Murray, M.; Conrad, P.; Toy-Choutka, S.; Jessup, D.; Grigg, M. Ultrastructural and molecular confirmation of the development of Sarcocystis neurona tissue cysts in the central nervous system of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 2009, 39, 1363–1372. [Google Scholar] [CrossRef]
- Kadim, I.; Mahgoub, O.; Purchas, R. A review of the growth, and of the carcass and meat quality characteristics of the one-humped camel (Camelus dromedaries). Meat Sci. 2008, 80, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Kadim, I.T.; Mahgoub, O.; Mbaga, M. Potential of camel meat as a non-traditional high quality source of protein for human consumption. Anim. Front. 2014, 4, 13–17. [Google Scholar] [CrossRef]
- Mandour, A.M.; Rabie, S.A.; Mohammed, N.; Hussein, N.M. On the presence of Sarcocystis miescheri sp. nov. in camels of Qena Governorate. Egypt. Acad. J. Boil. Sci. E. Med Èntomol. Parasitol. 2011, 3, 1–7. [Google Scholar] [CrossRef]
- Hamidinejat, H.; Hekmatimoghaddam, S.; Jafari, H.; Sazmand, A.; Molayan, P.H.; Derakhshan, L.; Mirabdollahi, S. Prevalence and distribution patterns of Sarcocystis in camels (Camelus dromedarius) in Yazd province, Iran. J. Parasit. Dis. 2012, 37, 163–165. [Google Scholar] [CrossRef]
- Omer, S.A.; Alzuraiq, A.A.; Mohammed, O.B. Prevalence and molecular detection of Sarcocystis spp. infection in the dromedary camel (Camelus dromedarius) in Riyadh city, Saudi Arabia. Biomed. Res. 2017, 28, 4962–4965. [Google Scholar]
- Oryan, A.; Moghaddar, N.; Gaur, S.N. The distribution pattern of Sarcocystis species, their transmission and pathogenesis in sheep in Fars Province of Iran. Veter. Res. Commun. 1996, 20, 243–253. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A.; Fayer, R.; Livingston, C.W. Sarcocystis arieticanis and Other Sarcocystis Species in Sheep in the United States. J. Parasitol. 1988, 74, 1033. [Google Scholar] [CrossRef]
- Fayer, R. Economic losses to Sarcocystis. Natl. Wool. Grow. 1976, 66, 22–28. [Google Scholar]
- Munday, B. The effect of Sarcocystis ovicanis on growth rate and haematocrit in lambs. Veter. Parasitol. 1979, 5, 129–135. [Google Scholar] [CrossRef]
- Munday, B. Effects of different doses of dog-derived Sarcocystis sporocysts on growth rate and haematocrit in lambs. Veter. Parasitol. 1986, 21, 21–24. [Google Scholar] [CrossRef]
- Valinezhad, A.; Oryan, A.; Ahmadi, N. Sarcocystis and Its Complications in Camels (Camelus dromedarius) of Eastern Provinces of Iran. Korean J. Parasitol. 2008, 46, 229–234. [Google Scholar] [CrossRef]
- Abdel-Ghaffar, F.; Mehlhorn, H.; Bashtar, A.-R.; Al-Rasheid, K.; Sakran, T.; El-Fayoumi, H. Life cycle of Sarcocystis camelicanis infecting the camel (Camelus dromedarius) and the dog (Canis familiaris), light and electron microscopic study. Parasitol. Res. 2009, 106, 189–195. [Google Scholar] [CrossRef]
- Entzeroth, R.; A Ghaffar, F.; Chobotar, B.; Scholtyseck, E. Fine structural study of Sarcocystis sp. from Egyptian camels (Camelus dromed arius). Acta Veter. Acad. Sci. Hung. 1981, 29, 335–339. [Google Scholar]
- Motamedi, G.R.; Dalimi, A.; Nouri, A.; Aghaeipour, K. Ultrastructural and molecular characterization of Sarcocystis isolated from camel (Camelus dromedarius) in Iran. Parasitol. Res. 2010, 108, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Eslampanah, M.; Motamedi, G.R.; Dalimi, A.; Noori, A.; Habibi, G.R.; Aghaeepour, K.; Niroumand, M. Study of camel and goat Sarcocystis by electron microscopic and PCR-RFLP. Vet. Res. Biol. Prod. 2016, 29, 77–84. [Google Scholar]
- Bittencourt, M.V.; Meneses, I.D.S.; Ribeiro-Andrade, M.; De Jesus, R.F.; De Araújo, F.R.; Gondim, L.F. Sarcocystis spp. in sheep and goats: Frequency of infection and species identification by morphological, ultrastructural, and molecular tests in Bahia, Brazil. Parasitol. Res. 2016, 115, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Odening, K.; Stolte, M.; Bockhardt, I. On the diagnostics of Sarcocystis in cattle: Sarcocysts of a species unusual for Bos taurus in a dwarf zebu. Veter. Parasitol. 1996, 66, 19–24. [Google Scholar] [CrossRef]
- Dubey, J.; Speer, C.; Charleston, W. Ultrastructural differentiation between sarcocysts of Sarcocystis hirsuta and Sarcocystis hominis. Veter. Parasitol. 1989, 34, 153–157. [Google Scholar] [CrossRef]
- Hamidinejat, H.; Moetamedi, H.; Alborzi, A.; Hatami, A. Molecular detection of Sarcocystis species in slaughtered sheep by PCR–RFLP from south-western of Iran. J. Parasit. Dis. 2013, 38, 233–237. [Google Scholar] [CrossRef][Green Version]
- Carleton, H.M. Histological Technique for Normal and Pathological Tissues and the Identification of Parasites, 3rd ed.; Oxford University Press: London, UK, 1957. [Google Scholar]
- Gjerde, B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int. J. Parasitol. 2013, 43, 579–591. [Google Scholar] [CrossRef]
- Gjerde, B. Sarcocystis species in red deer revisited: With a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitology 2013, 141, 441–452. [Google Scholar] [CrossRef]
- Metwally, D.M.; Al-Damigh, M.A.; Al-Turaiki, I.; El-Khadragy, M.F. Molecular Characterization of Sarcocystis Species Isolated from Sheep and Goats in Riyadh, Saudi Arabia. Animals 2019, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Geneious Prime. Available online: http://www.geneious.com/ (accessed on 7 April 2020).
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; López, R.; McWilliam, H.; Remmert, M.; Soeding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Boil. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O.; Dufayard, J.-F. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Boil. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Wahba, A.A. Morphobiological Studies on Cryptosporidia and Sarcocystis in Animals and Their Impact on Their Health. Ph.D. Thesis, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt, 1994. [Google Scholar]
- Fatani, A.; Hilali, M.A.; Al-Atiya, S.; Al-Shami, S. Prevalence of Sarcocystis in camels (Camelus dromedarius) from Al-Ahsa, Saudi Arabia. Veter. Parasitol. 1996, 62, 241–245. [Google Scholar] [CrossRef]
- Al-Quraishy, S.A.; Bashtar, A.R.; Al-Rasheid, K.; Abdel-Ghaffar, F. Prevalence and ultrastructure of Sarcocystis species infecting camels (Camelus dromedarius) slaughtered in Riyadh city Saudi Arabia. Saudi J. Biol. Sci. 2004, 11, 135–141. [Google Scholar]
- Woldemeskel, M.; Gumi, B. Prevalence of sarcocysts in one-humped camel (Camelus dromedarius) from Southern Ethiopia. J. Vet. Med. Ser. B 2001, 48, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Hilali, M.; Mohamed, A. The dog (Canis familiaris) as the final host of Sarcocystis cameli (Mason, 1910). Trop. Parasitol. 1980, 31, 213–214. [Google Scholar]
- Abd-Elmalek, B.S.; Abed, G.H.; Mandour, A.M. Study on Sarcosystis sp. By Light and Electron Microscopy in Camel Muscles at Assiut Governorate. J. Veter. Sci. Technol. 2015, 6. [Google Scholar] [CrossRef]
- Mason, F.E. Sarcocysts in the camel in Egypt. J. Comp. Pathol. Ther. 1910, 23, 168–176. [Google Scholar] [CrossRef]
- Moré, G.; Regensburger, C.; Gos, M.L.; Pardini, L.; Verma, S.K.; Ctibor, J.; Serrano-Martínez, E.; Dubey, J.P.; Venturini, M.C. Sarcocystis masoni, n sp. (Apicomplexa: Sarcocystidae), and redescription of Sarcocystis aucheniae from llama (Lama glama), guanaco (Lama guanicoe) and alpaca (Vicugna pacos). Parasitology 2016, 143, 617–626. [Google Scholar]
- Singh, D.; Kumar, B. UP Pandit Deen Dayal Upadhyaya Pashu-Chikitsa Vigyan Vishwavidyalaya Evam Go Anusandhan Sansthan. Ph.D. Thesis, Mathura, Mathura, India, 2018. [Google Scholar]
- Gjerde, B.; Luzón, M.; Alunda, J.M.; De La Fuente, C. Morphological and molecular characteristics of six Sarcocystis spp. from red deer (Cervus elaphus) in Spain, including Sarcocystis cervicanis and three new species. Parasitol. Res. 2017, 116, 2795–2811. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Gjerde, B.; Villa, L.; Minazzi, S.; Zanzani, S.A.; Riccaboni, P.; Sironi, G.; Manfredi, M.T. Prevalence and molecular characterisation of Sarcocystis miescheriana and Sarcocystis suihominis in wild boars (Sus scrofa) in Italy. Parasitol. Res. 2019, 118, 1271–1287. [Google Scholar] [CrossRef]
- Murata, R.; Suzuki, J.; Hyuga, A.; Shinkai, T.; Sadamasu, K. Molecular identification and characterization of Sarcocystis spp. in horsemeat and beef marketed in Japan. Parasite 2018, 25, 27. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metwally, D.M.; Al-Otaibi, T.T.; Al-Turaiki, I.M.; El-Khadragy, M.F.; Alajmi, R.A. Identification of Sarcocystis spp. in One-humped Camels (Camelus dromedarius) from Riyadh and Dammam, Saudi Arabia, via Histological and Phylogenetic Approaches. Animals 2020, 10, 1108. https://doi.org/10.3390/ani10071108
Metwally DM, Al-Otaibi TT, Al-Turaiki IM, El-Khadragy MF, Alajmi RA. Identification of Sarcocystis spp. in One-humped Camels (Camelus dromedarius) from Riyadh and Dammam, Saudi Arabia, via Histological and Phylogenetic Approaches. Animals. 2020; 10(7):1108. https://doi.org/10.3390/ani10071108
Chicago/Turabian StyleMetwally, Dina M., Tahani T. Al-Otaibi, Isra M. Al-Turaiki, Manal F. El-Khadragy, and Reem A. Alajmi. 2020. "Identification of Sarcocystis spp. in One-humped Camels (Camelus dromedarius) from Riyadh and Dammam, Saudi Arabia, via Histological and Phylogenetic Approaches" Animals 10, no. 7: 1108. https://doi.org/10.3390/ani10071108
APA StyleMetwally, D. M., Al-Otaibi, T. T., Al-Turaiki, I. M., El-Khadragy, M. F., & Alajmi, R. A. (2020). Identification of Sarcocystis spp. in One-humped Camels (Camelus dromedarius) from Riyadh and Dammam, Saudi Arabia, via Histological and Phylogenetic Approaches. Animals, 10(7), 1108. https://doi.org/10.3390/ani10071108





