Effect of Lipopolysaccharide and Muramyl Dipeptide on Apoptosis of Bovine Mammary Gland Lymphocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Induction of Inflammatory Response
2.4. Flow Cytometry Analysis of Apoptosis and CD44
2.5. Statistical Analysis
3. Results
3.1. The inflammatory Response of the Mammary Gland to LPS and MDP
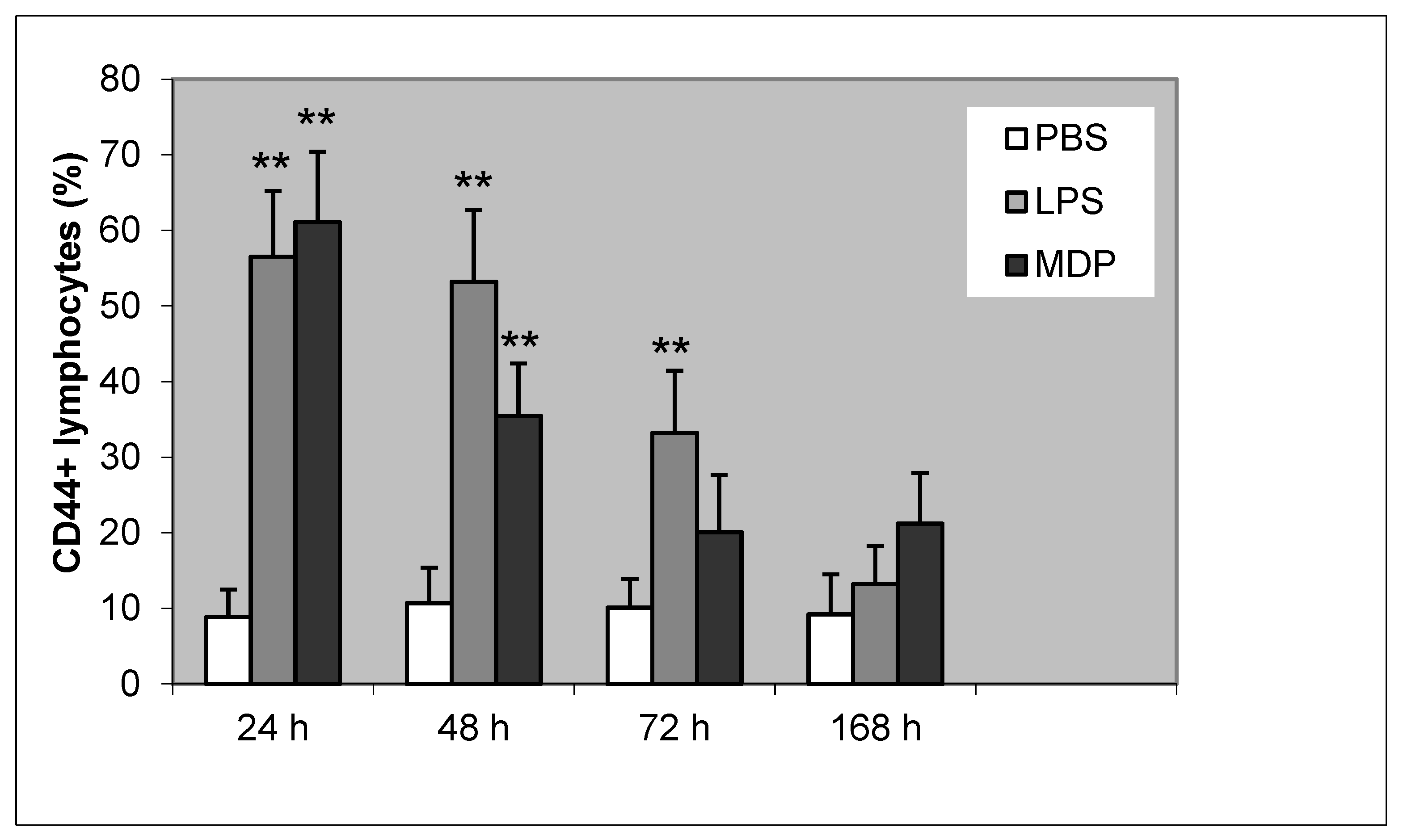
3.2. The Dynamics of Lymphocyte Apoptosis and CD44 Expression on Lymphocytes During LPS and MDP Infection
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sadeghi-Sefidmazgi, A.; Moradi-Shahrbabak, M.; Nejati-Javaremi, A.; Miraei-Ashtiani, S.R.; Amer, P.R. Estimation of economic values and financial losses associated with clinical mastitis and somatic cell score in Holstein dairy cattle. Animal 2011, 5, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kvapilik, J.; Hanus, O.; Barton, L.; Klimesova, M.V.; Roubal, P. Mastitis of dairy cows and financial losses: An economic meta-analysis and model calculation. Bulg. J. Anim. Sci. 2015, 21, 1092–1105. [Google Scholar]
- Romero, J.; Benavides, E.; Meza, C. Assessing financial impacts of subclinical mastitis on Colombian Dairy farms. Front. Vet. Sci. 2018, 5, 273. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 8th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 52–63. [Google Scholar]
- Liu, G.; Ding, L.; Han, B.; Piepers, S.; Naqvi, S.A.; Barkema, H.W.; Ali, T.; de Vliegher, S.; Xu, S.; Gao, J. Characteristics of Escherichia coli isolated from bovine mastitis exposed to subminimum inhibitory concentrations of cefalotin or ceftazidime. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, R.N.; Middleton, J.R.; McDougall, S.; Katholm, J.; Schukken, Y.H. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J. Mammary Gland Biol. Neoplasia 2011, 16, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K. Staphylococcus aureus intracellular survival: A closer look in the process. Virulence 2017, 8, 1506–1507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almeida, R.A.; Luther, D.A.; Park, H.M.; Oliver, S.P. Identification, isolation, and partial characterization of a novel Streptococcus uberis adhesion molecule (SUAM). Vet. Microbiol. 2006, 115, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.; Dunlap, J.R.; Oliver, S.P. Binding of host factors influences internalization and intracellular trafficking of Streptococcus uberis in bovine mammary epithelial cells. Vet. Med. Int. 2010. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.; Kerro-Dego, O.; Headrick, S.I.; Lewis, M.J.; Oliver, S.P. Role of Streptococcus uberis adhesion molecule in the pathogenesis of Streptococcus uberis mastitis. Vet. Microbiol. 2015, 179, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.; Kerro-Dego, O.; Prado, M.E.; Headrick, S.I.; Lewis, M.J.; Siebert, L.J.; Pighetti, G.M.; Oliver, S.P. Protective effect of anti-SUAM antibodies on Streptococcus uberis mastitis. Vet. Res. 2015, 46, 133. [Google Scholar] [CrossRef] [PubMed]
- Reinoso, E.B. Bovine mastitis caused by Streptococcus uberis: Virulence factors and biofilm. J. Microb. Biochem. Technol. 2017, 9, 5. [Google Scholar]
- Hotchkiss, R.S.; Coopersmith, C.M.; Karl, I.E. Prevention of lymphocyte apoptosis—A potential treatment of sepsis? Clin. Infect. Dis. 2005, 41 (Suppl. S7), S465–S469. [Google Scholar] [CrossRef]
- Carol, M.; Borruel, N.; Antolin, M.; Llopis, M.; Casellas, F.; Guarner, F.; Malagelada, J.R. Modulation of Apoptosis in Intestinal Lymphocytes by a Probiotic Bacteria in Crohn’s Disease. J. Leukoc. Biol. 2006, 79, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Slama, P.; Sladek, Z.; Rysanek, D.; Langrova, T. Effect of Staphylococcus aureus and Streptococcus uberis on apoptosis of bovine mammary gland lymphocytes. Res. Vet. Sci. 2009, 87, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Slama, P.; Zavadilova, T.; Kratochvilova, L.; Kharkevich, K.; Uhrincat, M.; Tancin, V. Effect of peptidoglycan of Staphylococcus Aureus on apoptosis of bovine mammary gland lymphocytes. J. Microbiol. Biotech. Food Sci. 2019, 9(S), 445–446. [Google Scholar] [CrossRef]
- Leitner, G.; Chaffer, M.; Krifucks, O.; Glickman, A.; Ezra, E.; Saran, A. Milk leucocyte populations in heifers free of udder infection. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Faldyna, M.; Leva, L.; Sladek, Z.; Rysanek, D.; Toman, M. γδ-TCR+CD2– lymphocytes are recruited into bovine mammary gland after stimulation. Vet. Med. Czech 2006, 51, 258–264. [Google Scholar] [CrossRef]
- Sustrova, T.; Slama, P. The effect of Staphylococcus aureus bacteria to proportion of gamma delta T-lymphocytes from bovine mammary gland. In MENDELNET 2013, Proceedings of the 20th International PhD Students Conference MENDELNET 2013, Brno, Czech Republic, 20–21 November 2013; Skarpa, P., Ryant, P., Cerkal, R., Polak, O., Kovarnik, J., Eds.; Mendel University in Brno: Brno, Czech Republic, 2013; pp. 788–792. [Google Scholar]
- Slama, P.; Sladek, Z.; Kabourkova, E.; Havlicek, Z.; Kwak, J.Y. Apoptosis of gamma delta T cells during inflammatory response of bovine mammary gland induced by Staphylococcus aureus. Eur. J. Immunol. 2016, 46 (Suppl. S1), 495. [Google Scholar]
- Zouharova, M.; Rysanek, D. Multiplex PCR and RPLA identification of Staphylococcus aureus enterotoxigenic strains from bulk tank milk. Zoonoses Public Health 2008, 55, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Haslinger, B.; Strangfeld, K.; Peters, G.; Schulze-Osthoff, K.; Sinha, B. Staphylococcus aureus alpha-toxin induces apoptosis in peripheral blood mononuclear cells: Role of endogenous tumour necrosis factor-alpha and the mitochondrial death pathway. Cell. Microbiol. 2003, 5, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Lee, S.U.; Ferens, W.A.; Samuels, S.; Davis, W.C.; Fox, L.K.; Ahn, J.S.; Seo, K.S.; Chang, B.S.; Hwang, S.Y.; et al. Unique features of bovine lymphocytes exposed to a staphylococcal enterotoxin. J. Vet. Sci. 2006, 7, 233–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lesley, J.; Hyman, R. CD44 structure and function. Front. Biosci. 1998, 3, D616–D630. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.P.; Lokeshwar, V.B. The role of CD44 in disease pathophysiology and targeted treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.G.; Wu, K.M. The role of CD44 in epithelial-mesenchymal transition and cancer development. OncoTargets Ther. 2015, 16, 3783–3792. [Google Scholar]
- Naor, D. Interaction Between Hyaluronic acid and its receptors (Cd44, rHaMM) regulates the activity of inflammation and Cancer. Front. Immunol. 2016, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Schrager, H.M.; Alberti, S.; Cywes, C.; Dougherty, G.J.; Wessels, M.R. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J. Clin. Investig. 1998, 101, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Langrova, T.; Sladek, Z.; Rysanek, D. Expression of CD14 and CD44 on bovine polymorphonuclear leukocytes during resolution of mammary inflammatory response induced by muramyldipeptide and lipopolysaccharide. Vet. Med. Czech 2008, 53, 1–11. [Google Scholar] [CrossRef]
- Sladek, Z.; Rysanek, D. Expression of macrophage CD44 receptor in the course of experimental inflammatory response of bovine mammary gland induced by lipopolysaccharide and muramyl dipeptide. Res. Vet. Sci. 2009, 86, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zavadilova, T.; Sladek, Z.; Kratochvilova, L.; Slama, P.; Rysanek, D. The effect of muramyl dipeptide and lipopolysaccharide on expression of CD14 and CD44 by bovine mammary gland neutrophils in vitro. J. Microbiol. Biotech. Food Sci. 2019, 9(S), 467–472. [Google Scholar] [CrossRef]
- Sladek, Z.; Rysanek, D.; Faldyna, M. Activation of phagocytes during initiation and resolution of mammary gland injury induced by lipopolysaccharide in heifers. Vet. Res. 2002, 33, 191–204. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Sladek, Z.; Rysanek, D. Expression of macrophage CD14 receptor in the course of experimental inflammatory responses induced by lipopolysaccharide and muramyl dipeptide. Vet. Med. Czech 2008, 53, 347–357. [Google Scholar] [CrossRef]
- Slama, P.; Zavadilova, T.; Kratochvilova, L.; Kharkevich, K. Effect of peptidoglycan on expression of CD44 on bovine mammary gland lymphocytes. J. Microbiol. Biotech. Food Sci. 2019, 9(S), 447–448. [Google Scholar] [CrossRef]
- Pilon-Thomas, S.; Verhaegen, M.; Kuhn, L.; Riker, A.; Mule, J.J. Induction of anti-tumor immunity by vaccination with dendritic cells pulsed with anti-CD44 IgG opsonized tumor cells. Cancer Immunol. Immunother. 2006, 55, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Rajasagi, M.; von Au, A.; Singh, R.; Hartmann, N.; Zoller, M.; Marhaba, R. Anti-CD44 induces apoptosis in T lymphoma via mitochondrial depolarization. J. Cell Mol. Med. 2010, 14, 1453–1467. [Google Scholar] [CrossRef] [PubMed]
- Baaten, B.J.G.; Li, C.R.; Bradley, L.M. Multifaceted regulation of T cells by CD44. Commun. Integr. Biol. 2010, 3, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.M.; Kitada, S.; Leoni, L.M.; Zapata, J.M.; Karras, J.G.; Tsukada, N.; Kipps, T.J.; Choi, Y.S.; Bennett, F.; Reed, J.C. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood 2002, 100, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Gibellini, F.; Njuguna, N.; Hazan-Halevy, I.; Farooqui, M.; Bern, S.; Keyvanfar, K.; Lee, E.; Wilson, W.; Wiestner, A. Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk. Lymphoma 2011, 52, 1758–1769. [Google Scholar] [CrossRef]
- Fedorchenko, O.; Stiefelhagen, M.; Peer-Zada, A.A.; Barthel, R.; Mayer, P.; Eckei, L.; Breuer, A.; Crispatzu, G.; Rosen, N.; Landwehr, T.; et al. CD44 regulates the apoptotic response and promotes disease development in chronic lymphocytic leukemia. Blood 2013, 121, 4126–4136. [Google Scholar] [CrossRef]
- Zhang, S.P.; Wum, C.; Farrah-Fecteau, J.; Cul, B.; Chen, L.; Zhang, L.; Wu, R.; Rassenti, L.; Lao, F.; Weigand, S.; et al. Targeting chronic lymphocytic leukemia cells with a humanized monoclonal antibody specific for CD44. Proc. Natl. Acad. Sci. USA 2013, 110, 6127–6132. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, J.C.; Greil, R.; Hartmann, T.N. The role of CD44 in the pathophysiology of chronic lymphocytic leukemia. Front. Immunol. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Yang-Yen, H.F. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J. Biol. Chem. 2001, 276, 46024–46030. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Saito, K.; Mine, S.; Matsushita, S.; Tanaka, Y. Engagement of CD44 upregulates Fas Ligand expression on T cells leading to activation-induced cell death. Apoptosis 2007, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Johnson, P. Hyaluronan induces cell death in activated T cells through CD44. J. Immunol. 2008, 181, 7044–7054. [Google Scholar] [CrossRef] [PubMed]
- Mielgo, A.; van Driel, M.; Bloem, A.; Landmann, L.; Gunthert, U. A novel antiapoptotic mechanism based on interference of fas signaling by CD44 variant isoforms. Cell Death Differ. 2006, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Foger, N.; Marhaba, R.; Zoller, M. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur. J. Immunol. 2000, 30, 2888–2899. [Google Scholar] [CrossRef]
- Assayag-Asherie, N.; Sever, D.; Bogdani, M.; Johnson, P.; Weiss, T.; Ginzberg, A.; Perles, S.; Weiss, L.; Sebban, L.E.; Turley, E.A.; et al. Can CD44 be a mediator of cell destruction? The challenge of type 1 diabetes. PLoS ONE 2015, 10, e0143589. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.S.; Shapiro, P.S.; Ahn, N.G. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 1998, 74, 49. [Google Scholar] [PubMed]
- Gee, L.; Lim, W.; Ma, W.; Nandan, D.; Diaz-Mitoma, F.; Kozlowski, M.; Kumar, A. Differential regulation of CD44 expression by Lipopolysaccharide (LPS) and TNF-α in human monocytic cells: Distinct involvement of c-Jun N-Terminal kinase in LPS-Induced CD44 expression. J. Immunol. 2002, 169, 5660–5672. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.J.; Paape, M.J.; Connor, E.E.; Bannerman, D.D.; Fetterer, R.H.; Peters, R.R. Bacterial lipopolysaccharide stimulates bovine neutrophil production of TNF-alpha, IL-1beta, IL-12 and IFN-gamma. Vet. Res. 2007, 38, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Kratochvilova, L.; Kharkevich, K.; Slama, P. TNF-alpha and IL-10 are produced by leukocytes during the experimental inflammatory response of bovine mammary gland induced by peptidoglycan. In MENDELNET 2018, Proceedings of the 25th International PhD Students Conference MENDELNET 2018, Brno, Czech Republic, 7–8 November 2018; Cerkal, R., Belcredi, N.B., Prokesova, L., Eds.; Mendel University in Brno: Brno, Czech Republic, 2018; pp. 376–379. [Google Scholar]
- Salguero, F.J.; Sanchez-Cordon, P.J.; Nunez, A.; de Marco, M.F.; Gomez-Villamandos, J.C. Proinflammatory cytokines induce lymphocyte apoptosis in acute african swine fever infection. J. Comp. Path. 2005, 132, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Normanton, M.; Alvarenga, H.; Hamerschlak, N.; Ribeiro, A.; Kondo, A.; Rizzo, L.V.; Marti, L.C. Interleukin 7 plays a role in T lymphocyte apoptosis inhibition driven by mesenchymal stem cell without favoring proliferation and cytokines secretion. PLoS ONE 2014, 9, e106673. [Google Scholar] [CrossRef] [PubMed]

| Agent | 24 h | 48 h | 72 h | 168 h |
|---|---|---|---|---|
| PBS | 12.1 ± 2.9 | 5.1 ± 1.9 | 3.2 ± 1.5 | 1.3 ± 0.7 |
| LPS | 45.3 ± 9.8 ** | 18.2 ± 9.0 * | 7.2 ± 3.1 * | 1.8 ± 0.6 |
| MDP | 31.5 ± 5.9 * | 11.9 ± 5.8 | 4.5 ± 3.0 | 1.5 ± 0.5 |
| Agent | Cells | 24 h | 48 h | 72 h | 168 h |
|---|---|---|---|---|---|
| PBS | Lymphocytes | 7.5 ± 4.2 | 18.7 ± 12.9 | 17.2 ± 10.5 | 9.3 ± 4.7 |
| Macrophages | 30.1 ± 9.8 | 36.1 ± 7.3 | 55.3 ± 11.6 | 80.3 ± 12.0 | |
| Neutrophils | 62.4 ± 7.2 | 45.2 ± 13.2 | 27.5 ± 8.6 | 10.4 ± 4.2 | |
| LPS | Lymphocytes | 2.1 ± 0.7 ** | 6.3 ± 2.7 ** | 16.2 ± 7.4 | 24.7 ± 10.6 ** |
| Macrophages | 12.7 ± 3.1 ** | 13.2 ± 5.6 ** | 32.7 ± 13.3 * | 50.4 ± 9.8 * | |
| Neutrophils | 85.2 ± 8.8 ** | 80.5 ± 12.3 ** | 51.1 ± 14.4 ** | 24.9 ± 9.3 ** | |
| MDP | Lymphocytes | 6.7 ± 3.8 | 18.9 ± 6.8 | 24.5 ± 8.0 | 21.4 ± 9.5 |
| Macrophages | 11.2 ± 4.3 ** | 25.6 ± 7.6 | 50.2 ± 12.3 | 62.8 ± 12.9 * | |
| Neutrophils | 82.1 ± 9.6 ** | 55.5 ± 10.7 | 25.3 ± 8.2 | 15.8 ± 6.2 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slama, P.; Kabourkova, E.; Sladek, Z.; Zavadilova, T.; Kratochvilova, L.; Kharkevich, K.; Roychoudhury, S.; Pavlik, A.; Roztocilova, A.; Uhrincat, M.; et al. Effect of Lipopolysaccharide and Muramyl Dipeptide on Apoptosis of Bovine Mammary Gland Lymphocytes. Animals 2020, 10, 990. https://doi.org/10.3390/ani10060990
Slama P, Kabourkova E, Sladek Z, Zavadilova T, Kratochvilova L, Kharkevich K, Roychoudhury S, Pavlik A, Roztocilova A, Uhrincat M, et al. Effect of Lipopolysaccharide and Muramyl Dipeptide on Apoptosis of Bovine Mammary Gland Lymphocytes. Animals. 2020; 10(6):990. https://doi.org/10.3390/ani10060990
Chicago/Turabian StyleSlama, Petr, Eliska Kabourkova, Zbysek Sladek, Terezie Zavadilova, Lucie Kratochvilova, Kristina Kharkevich, Shubhadeep Roychoudhury, Ales Pavlik, Andrea Roztocilova, Michal Uhrincat, and et al. 2020. "Effect of Lipopolysaccharide and Muramyl Dipeptide on Apoptosis of Bovine Mammary Gland Lymphocytes" Animals 10, no. 6: 990. https://doi.org/10.3390/ani10060990
APA StyleSlama, P., Kabourkova, E., Sladek, Z., Zavadilova, T., Kratochvilova, L., Kharkevich, K., Roychoudhury, S., Pavlik, A., Roztocilova, A., Uhrincat, M., Tancin, V., Kimura, K., Konecny, R., Kiku, Y., Watanabe, A., Kwak, J.-Y., & Zouharova, M. (2020). Effect of Lipopolysaccharide and Muramyl Dipeptide on Apoptosis of Bovine Mammary Gland Lymphocytes. Animals, 10(6), 990. https://doi.org/10.3390/ani10060990







