First Evaluations and Cryopreservation of Semen Samples from Sunda Clouded Leopards (Neofelis diardi)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Anesthesia
2.3. Examination of Reproductive Tracts
2.4. Electro-Ejaculation
2.5. Semen Evaluation
2.6. Cryopreservation
2.7. Thawing Procedure and Post-Thaw Analysis
2.8. Statistical Analyses
3. Results
3.1. Testicular Biometry
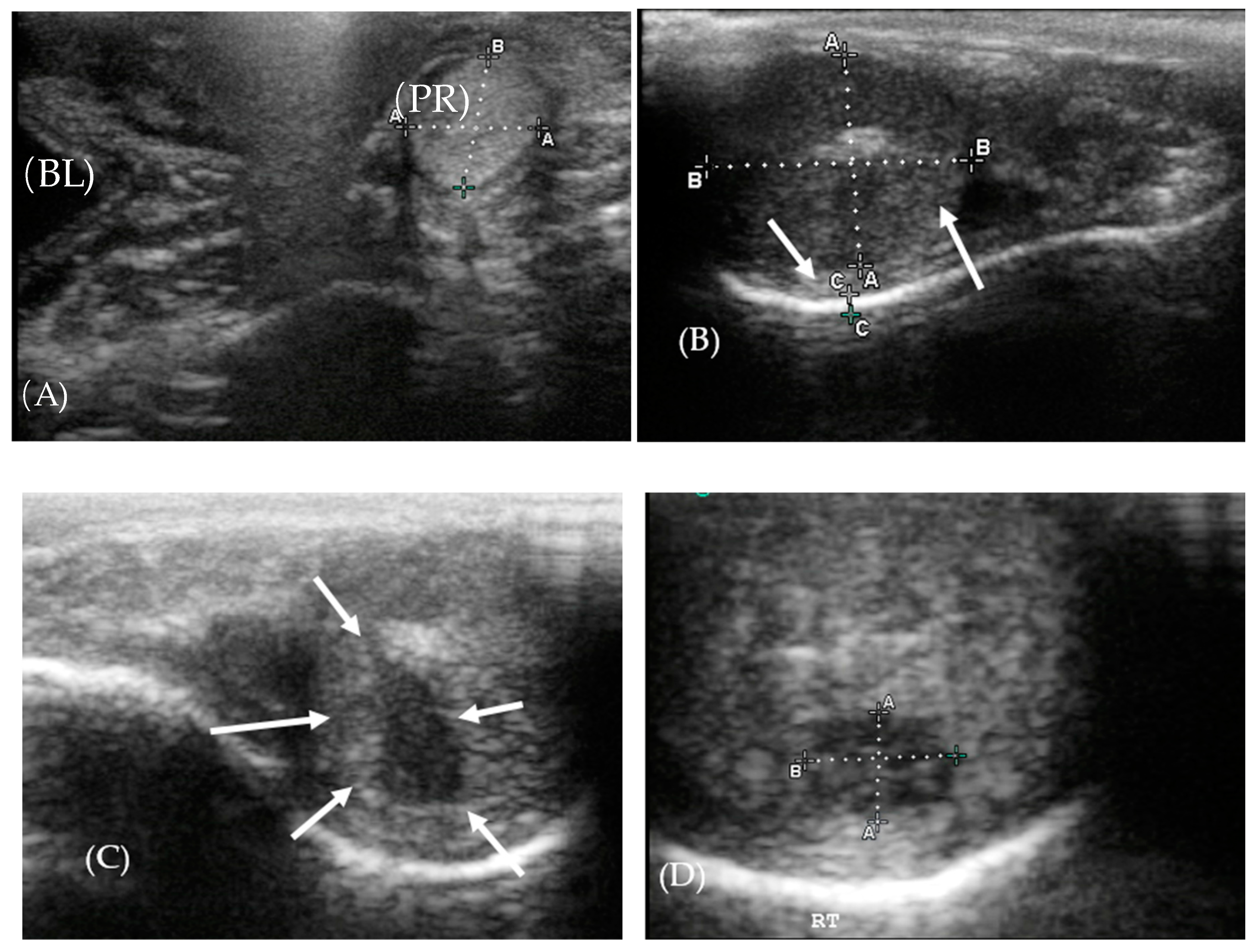
3.2. Ultrasonography of the Reproductive Tract
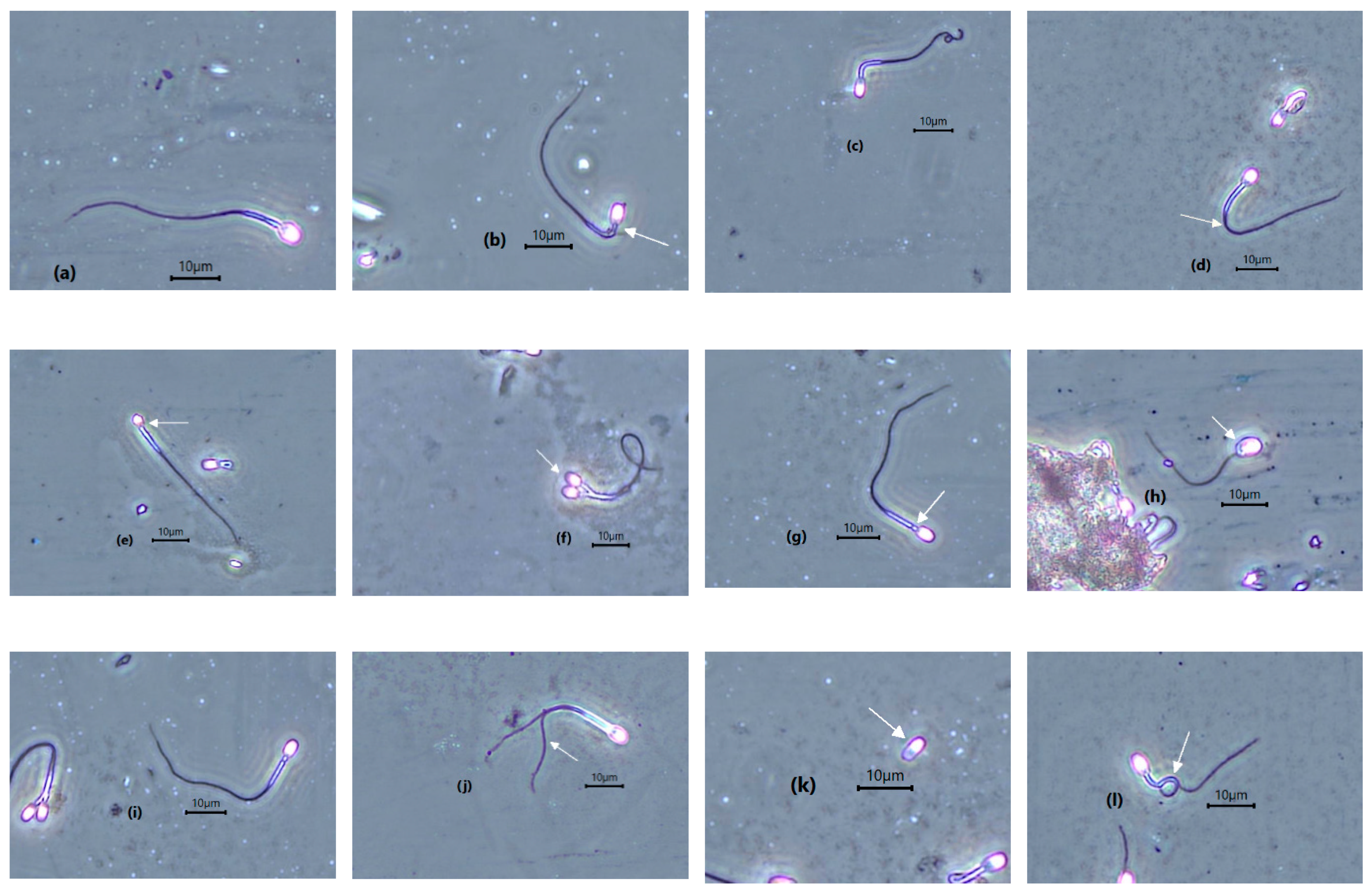
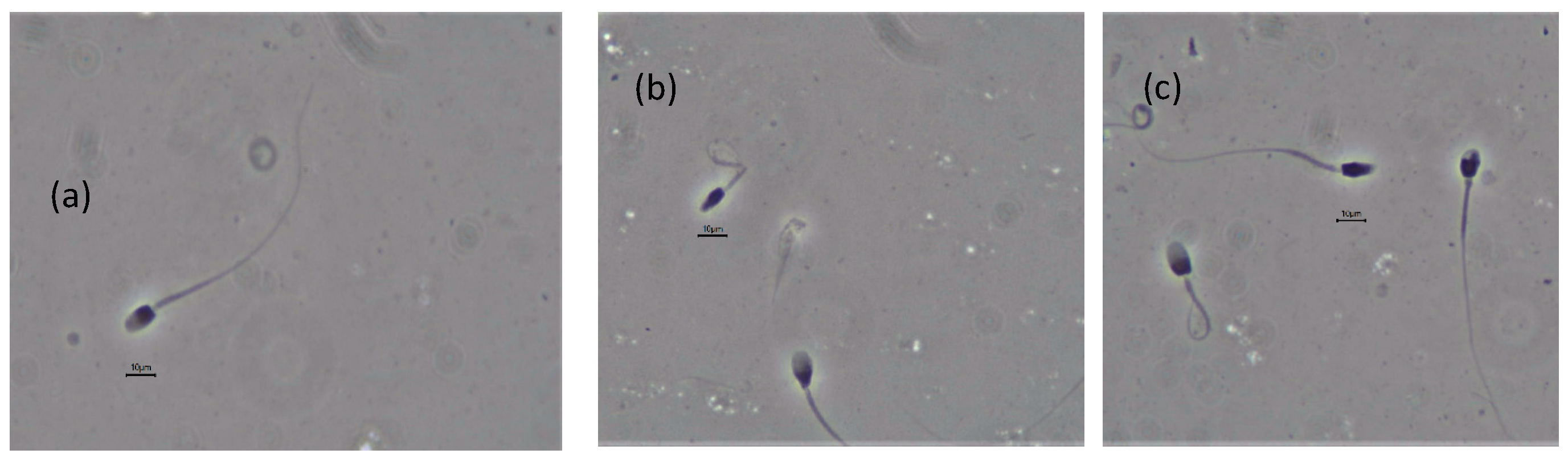
3.3. Semen Evaluation
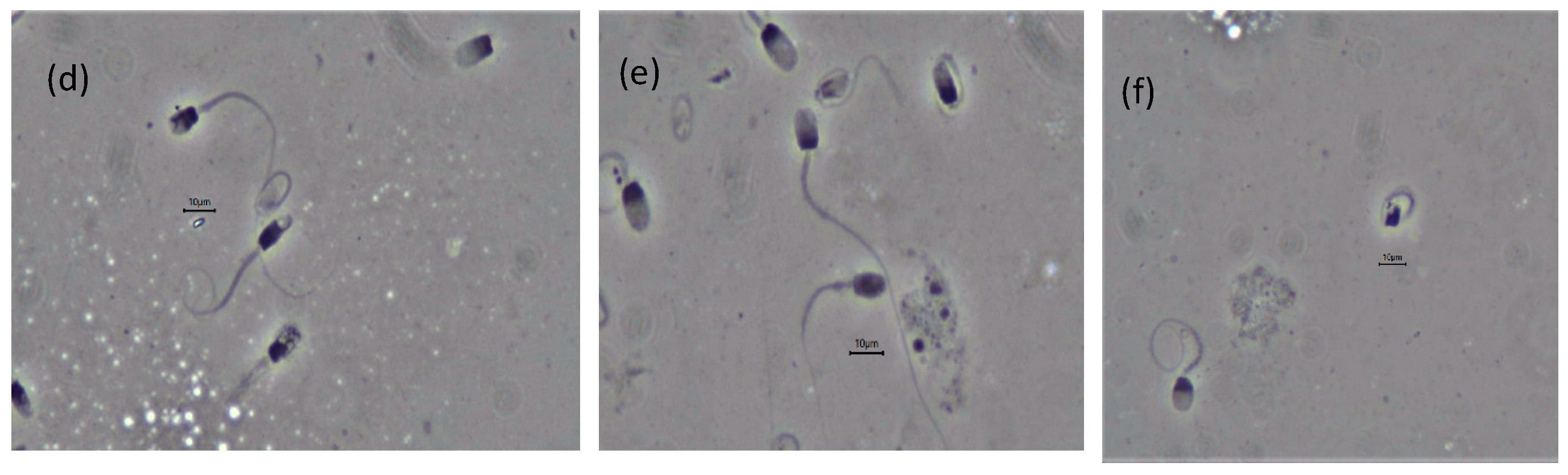
3.4. Post-Thaw Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hearn, A.J.; Ross, J.; Bernard, H.; Bakar, S.A.; Goossens, B.; Hunter, L.T.B.; Macdonald, D.W. Response of Sunda clouded leopard Neofelis diardi population density to anthropogenic disturbance: Refining estimates of its conservation status in Sabah. Oryx 2019, 53, 643–653. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Version. Available online: https://www.iucnredlist.org (accessed on 22 May 2020).
- Neofelis Nebulosa. Animal Diversity Web. University of Michigan. Available online: http://animaldiversity.org/Neofelis_nebulosa/ (accessed on 22 May 2020).
- Tipkantha, W.; Thuwanut, P.; Morrell, J.; Comizzoli, P.; Chatdarong, K. Influence of living status (single vs. paired) and centrifugation with colloids on the sperm morphology and functionality in the clouded leopard (Neofelis nebulosa). Theriogenology 2016, 86, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.K.; Durrant, B.S. Reproductive parameters of clouded leopards (Neofelis nebulosa). Zoo Biol. 1997, 8, 1059–1068. [Google Scholar] [CrossRef]
- Thongphakdee, A.; Sukparangsi, W.; Comizzoli, P.; Chatdarong, K. Reproductive biology and biotechnologies in wild felids. Theriogenology 2020. [Google Scholar] [CrossRef]
- Comizzoli, P. Biobanking efforts and new advances in male fertility preservation for rare and endangered species. Asian. J. Androl. 2015, 17, 640–645. [Google Scholar] [CrossRef]
- Tipkantha, W.; Thuwanut, P.; Siriaroonrat, B.; Comizzoli, P.; Chatdarong, K. Mitigation of sperm tail abnormalities using demembranation approach in the clouded leopard (Neofelis nebulosa). Reprod. Domest. Anim. 2017, 2, 214–218. [Google Scholar] [CrossRef]
- Etim, N.N. Testicular and epididymal morphometrics characteristics: Viable indicators of reproductive ability of farm animals. Am. J. Biomed. Eng. 2015, 1, 39–44. [Google Scholar]
- Paltiel, H.J.; Daimond, D.A.; Canzio, J.D.; Zurakowski, D.; Borer, J.G.; Atala, A. Testicular volume: Comparison of orchidometer and US measurements in dogs. Radiology 2002, 222, 114–119. [Google Scholar] [CrossRef]
- Howard, J.G. Semen collection and analysis in carnivores. In Zoo and wild animal medicine: Current therapy III; WB Saunders Co: Square West, PA, USA, 1993; pp. 390–399. [Google Scholar]
- Oliveira, K.G.; Leao, D.L.; Almeida, D.V.C.; Santos, R.R. Seminal characteristics and cryopreservation of sperm from the squirrel monkey, Saimiri collinsi. Theriogenology 2015, 84, 743–749. [Google Scholar] [CrossRef]
- Semen Collection and Breeding Soundness Examination in the Dog. World Small Animal Veterinary Association World Congress Proceedings. Available online: https://wsava.org/ (accessed on 22 May 2020).
- Dong, Q.; Rodenburg, S.E.; Huang, C.; VandeVoort, C.A. Effect of pre-freezing conditions on semen cryopreservation of rhesus monkey. Theriogenology 2008, 70, 61–69. [Google Scholar] [CrossRef]
- Dong, Q.; Correa, L.M.; VandeVoort, C.A. Rhesus monkey sperm cryopreservation with TEST-yolk extender in the absence of permeable cryoprotectant. Cryobiology 2009, 58, 20–27. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singh, P.; Singh, N. Effect of testicular consistency on semen quality of Murrah buffalo breeding bulls. Indian J. Dairy. Sci. 2019, 72, 93–96. [Google Scholar]
- Axner, E.; Strom, B.; Linde-Forsberg, C.; Gustavsson, I.; Lindblad, K.; Wallgent, M. Reproductive disorders in 10 domestic male cats. J. Small. Anim. Pract. 1996, 37, 394–401. [Google Scholar] [CrossRef]
- Englar, R.E. Common Clinical Presentations in Dogs and Cats, 1st ed.; John Wiley & Sons, Inc: Rio de Jeneiro, Brazil, 2019; pp. 719–728. [Google Scholar]
- Wildt, D.E.; Howard, J.G.; Hall, L.L.; Bush, M. Reproductive physiology of the Clouded Leopard: 1, Electroejaculates contain high proportions of pleiomorphic spermatozoa throughout the year. Biol. Reprod. 1986, 34, 937–947. [Google Scholar] [CrossRef]
- Bull Reproductive Soundness: Brahman News. Available online: http://www.brahman.com.au/technical_information/selection/reproductiveSoundness.html (accessed on 22 May 2020).
- Câmara, L.B.R.M.; Câmara, D.R.; Maiorino, F.C.; Silva, Júnior, V.A.; Guerra, M.M.P. Canine testicular disorders and their influence on sperm morphology. Anim. Reprod. 2014, 11, 32–36. [Google Scholar]
- Finch, C.E.; Schneider, E.L. The Biology of Aging; CE Finch & EL Schneider: New York, NY, USA, 1985; pp. 478–510. [Google Scholar]
- Robert, D.H.; Christine, C.; Michael, P.; Alan, S. Prevalence and significance of heterogenous testes revealed on sonography: Ex vivo sonographic-pathologic correlation. AJR 2000, 175, 347–352. [Google Scholar]
- Meites, J. The neuroendocrinology of hypothalamic aging. Neuroendocrine. Perspectives. 1986, 5, 179–189. [Google Scholar]
- England, G.C.W.; Bright, L.; Pritchard, B.; Bowen, I.M.; De Souza, M.B.; Silva, L.D.M.; Moxon, R. Canine reproductive ultrasound examination for predicting future sperm quality. Reprod. Dom. Anim. 2017, 52, 202–207. [Google Scholar] [CrossRef]
- Andreas, G.W.; Hebert, A.V. Diseases of Abdomen and Pelvis. Available online: https://fotheme.xyz/diseases-of-the-abdomen-and-pelvis-pdf/ (accessed on 17 June 2020).
- Peters, M.A.; De Rooij, D.G.; Teerds, K.J.; Van Der Gaag, I.; Van Sluijs, F.J. Spermatogenesis and testicular tumours in ageing dogs. J. Reprod. Fertil. 2000, 120, 443–452. [Google Scholar] [CrossRef]
- Bhanmeechao, C.; Srisuwatanasagul, S.; Ponglowhapan, S. Age-related changes in interstitial fibrosis and germ cell degeneration of the canine testis. Reprod. Domest. Anim. 2018, 53, 37–43. [Google Scholar] [CrossRef]
- Elcock, L.H.; Schoning, P. Age-related changes in the cat testis and epididymis. Am. J. Vet. Res. 1984, 45, 2380–2384. [Google Scholar]
- Seawright, A.A.; English, P.B.; Gartner, R.J. Hypervitaminosis A of the cat. Adv. Vet. Sci. Comp. Med. 1970, 14, 1–27. [Google Scholar]
- Neaves, W.B.; Johnson, L.; Porter, J.C., Jr.; Petty, C.S. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J. Clin. Endocrinol. Metab 1984, 59, 756–763. [Google Scholar] [CrossRef]
- Kaiser, F.E.; Morley, J.E. Gonadotropins, testosterone, and the aging male. Neurobiol. Aging. 1994, 15, 559–563. [Google Scholar] [CrossRef]
- Edwards, K.L.; Edes, A.N.; Brown, J.L. Stress, well-being and reproductive success. In Reproductive Sciences in Animal Conservation, 2nd ed.; Comizzoli, P., Brown, J.L., Holt, W.V., Eds.; Springer Nature: Brigitte, Switzerland, 2019; pp. 91–162. [Google Scholar]
- Allen, M.L.; Wittmer, H.U.; Setiawan, E.; Jaffe, S.; Marshall, A.J. Scent marking in Sunda clouded leopard (Neofelis diardi): Novel observations close a key gap in understanding felid communication behaviours. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- Howard, J.G.; Allen, M.E. Nutritional factors affecting semen quality in felids. In Zoo and Wild Animal Medicine Current Therapy; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 272–283. [Google Scholar]
- Pukazhenti, B.S.; Laroe, D.; Crosier, A.; Bush, L.M.; Spindler, R.; Pelican, K.M. Challenges in cryopreservation of clouded leopard (Neofelis nebulosa) spermatozoa. Theriogenology 2006, 66, 1790–1796. [Google Scholar] [CrossRef]

| Date | Leopard 1 | Leopard 2 |
|---|---|---|
| 15 January 2018 | Anesthesia and electro-ejaculation | Anesthesia and electro-ejaculation |
| 28 February 2018 | Anesthesia and electro-ejaculation | Anesthesia and electro-ejaculation |
| 18 April 2018 | Anesthesia and electro-ejaculation | Anesthesia and electro-ejaculation |
| 22 May 2018 | Anesthesia and electro-ejaculation | Anesthesia and electro-ejaculation |
| 22 June 2018 | Anesthesia and electro-ejaculation | No collection |
| 9 August 2018 | anesthesia and electro-ejaculation | Anesthesia and electro-ejaculation |
| 20 September 2018 | anesthesia and electro-ejaculation | No collection |
| 9 October 2018 | anesthesia and electro-ejaculation | Anesthesia and electro-ejaculation |
| 20 November 2018 | anesthesia and electro-ejaculation | Anesthesia and electro-ejaculation |
| 3 December 2019 | anesthesia and electro-ejaculation | No collection |
| Leopard | Testis | Testicular Consistency Score | Length (cm) | Width (cm) | Circumference of Testis (cm) | Volume (cm3) | Total Volume (cm3) |
|---|---|---|---|---|---|---|---|
| 1 | Right | 1.8 ± 0.7 | 3.3 ± 0.3 | 2.3 ± 0.2 | 7.2 ± 0.6 a | 9.5 ± 1.4 a | 18.9 ± 1.7 a |
| Left | 1.8 ± 0.7 | 3.5 ± 0.4 | 2.3 ± 0.2 | 7.2 ± 0.6 a | 9.5 ± 1.0 a | ||
| 2 | Right | 3.0 ± 1.7 | 3.6 ± 1.0 | 2.7 ± 0.4 | 8.7 ± 1.1 b | 13.8 ± 2.7 b | 24.8 ± 4.6 b |
| Left | 2.8 ± 1.5 | 3.4 ± 0.6 | 2.5 ± 0.3 | 7.5 ± 0.4 ab | 11.0 ± 3.3 ab |
| Semen Characteristic | Leopard 1 (10 Samples) | Range (10 Samples) | Leopard 2 (3 Samples) | Range (3 Samples) |
|---|---|---|---|---|
| pH | 7.9 ± 0.9 a | 5.5–9.0 | 6.8 ± 1.1 b* | 5.0–9.0 |
| Volume (µl) | 402 ± 92 | 230–560 | 326 ± 197 ** | 115–700 |
| Motility (%) | 54.5 ± 24.2 a | 10–85 | 2.4 ± 3.5 b | 0–10 |
| Progressive motility (%) | 43.2 ± 23.5 a | 7–80 | 1.0 ± 1.7 b | 1–5 |
| Sperm motility index | 54.3 ± 24.9 a | 22.5–82.5 | 12.8 ± 1.65 b | 11–15 |
| Sperm concentration (×106/mL) | 122.4 ± 84.7 a | 51.3–304.4 | 6.8 ± 3.38 b | 3.3–11.2 |
| Total sperm (×106) | 44.6 ± 24.8 a | 13.7–97.4 | 1.8 ± 0.42 b | 1.3–2.3 |
| Viability (%) | 55.2 ± 18.2 | 28.7–73.9 | 52.1 ± 14.1 | 35.6–70 |
| Normal morphology (%) | 23.9 ± 12.3 | 8.7–47.6 | 31.2 ± 9.1 | 23.3–44 |
| Morphology (%) | Leopard 1 (10 Samples) | Range | Leopard 2 (3 Samples) | Range |
|---|---|---|---|---|
| Abnormal sperm | 75.8 ± 12.0 | 52.4–91.3 | 68.8 ± 9.1 | 56–76.7 |
| Macrocephalic | 0.6 ± 0.9 | 0.0–3.0 | 0.3 ± 0.2 | 0.0–0.5 |
| Microcephalic | 2.6 ± 4.1 | 0.0–14 | 4.0 ± 1.7 | 1.7–5.4 |
| Bicephalic | 0.17 ± 0.3 | 0.0–0.9 | 0.3 ± 0.2 | 0.0–0.5 |
| Mid piece defect | 27.9 ± 7.3 | 15.8–39.13 | 28.7 ± 3.5 | 24–32.3 |
| Bent tail | 15.1 ± 7.6 | 4.4–32.6 | 16.4 ± 4.7 | 10–21.3 |
| Coiled tail | 6.2 ± 10.8 | 0.0–38.3 | 6.8 ± 3.4 | 1.3–8.5 |
| Tightly coiled tail | 21.8 ± 14.9 | 0.0–45 | 14.9 ± 3.2 | 11–18.8 |
| Semen Characteristics (%) | Sample 1 | Sample 2 |
|---|---|---|
| General motility | 10 | 5 |
| Progressive motility | 3 | 5 |
| Sperm viability | 13.6 | 11.6 |
| Normal spermatozoa | 21.5 | 40.2 |
| Normal acrosome | 42.7 | 65.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainuddin, Z.Z.; Mohamed Tarmizi, M.R.; Yap, K.C.; Comizzoli, P.; Sipangkui, S. First Evaluations and Cryopreservation of Semen Samples from Sunda Clouded Leopards (Neofelis diardi). Animals 2020, 10, 1072. https://doi.org/10.3390/ani10061072
Zainuddin ZZ, Mohamed Tarmizi MR, Yap KC, Comizzoli P, Sipangkui S. First Evaluations and Cryopreservation of Semen Samples from Sunda Clouded Leopards (Neofelis diardi). Animals. 2020; 10(6):1072. https://doi.org/10.3390/ani10061072
Chicago/Turabian StyleZainuddin, Zainal Zahari, Mohamed Reza Mohamed Tarmizi, Keng Chee Yap, Pierre Comizzoli, and Symphorosa Sipangkui. 2020. "First Evaluations and Cryopreservation of Semen Samples from Sunda Clouded Leopards (Neofelis diardi)" Animals 10, no. 6: 1072. https://doi.org/10.3390/ani10061072
APA StyleZainuddin, Z. Z., Mohamed Tarmizi, M. R., Yap, K. C., Comizzoli, P., & Sipangkui, S. (2020). First Evaluations and Cryopreservation of Semen Samples from Sunda Clouded Leopards (Neofelis diardi). Animals, 10(6), 1072. https://doi.org/10.3390/ani10061072





