Comparison of the Glucocorticoid Concentrations between Three Species of Lemuridae Kept in a Temporary Housing Facility
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Subjects and Housing
2.3. Faecal Sampling and Analysis
2.4. Statistical Analysis
3. Results
3.1. Ring-Tailed Lemurs
3.2. White-Headed Lemurs
3.3. Collared Brown Lemurs
3.4. Species Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.H.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Pribbenow, S.; Jewgenow, K.; Vargas, A.; Rodrigo, S.; Naidenko, S.; Dehnhard, M. Validation of an enzyme immunoassay for the measurement of faecal glucocorticoid metabolites in Eurasian (Lynx lynx) and Iberian lynx (Lynx pardinus). Gen. Comp. Endocrinol. 2014, 206, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Bashaw, M.J.; Sicks, F.; Palme, R.; Schwarzenberger, F.; Tordiffe, A.S.W.; Ganswindt, A. Non-invasive assessment of adrenocortical activity as a measure of stress in giraffe (Giraffa camelopardalis). BMC Vet. Res. 2016, 235, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Möstl, E. Stress hormones in mammals and birds: Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann. NY Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Touma, C.; Palme, R. Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Ann. NY Acad. Sci. 2005, 1046, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Touma, C.; Palme, R.; Sachser, N. Analyzing corticosterone metabolites in fecal samples of mice: A noninvasive technique to monitor stress hormones. Horm. Behav. 2004, 45, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Palme, R. Measuring fecal steroids: Guidelines for practical application. Ann. NY Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Millspaugh, J.J.; Washburn, B.E. Use of fecal glucocorticoid metabolite measures in conservation biology research: Considerations for application and interpretation. Gen. Comp. Endocrinol. 2004, 138, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.; Mendl, M. Why is there no simple way of measuring animal welfare? Anim. Welf. 1993, 2, 301–319. [Google Scholar]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Weingrill, T.; Willems, E.P.; Zimmermann, N.; Steinmetz, H.; Heistermann, M.M. Species-specific patterns in fecal glucocorticoid and androgen levels in zoo-living orangutans (Pongo spp.). Gen. Comp. Endocrinol. 2011, 172, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Cocks, L. Factors affecting mortality, fertility, and well-being in relation to species differences in captive orangutans. Int. J. Primatol. 2007, 28, 421–428. [Google Scholar] [CrossRef]
- Campbell, J.L.; Eisemann, J.H.; Williams, C.V.; Glenn, K.M. Description of the gastrointestinal tract of five lemur species: Propithecus tattersalli, Propithecus verreauxi coquereli, Varecia variegata, Hapalemur griseus, and Lemur catta. Am. J. Primatol. 2000, 52, 133–142. [Google Scholar] [CrossRef]
- Balestri, M.; Barresi, M.; Campera, M.; Serra, V.; Ramanamanjato, J.B.; Heistermann, M.; Donati, G. Habitat degradation and seasonality affect physiological stress levels of Eulemur collaris in littoral forest fragments. PLoS ONE 2014, 9, e107698. [Google Scholar] [CrossRef] [PubMed]
- Ostner, J.; Kappeler, P.; Heistermann, M. Androgen and glucocorticoid levels reflex seasonally occurring social challenges in male red fronted lemurs (Eulemur fulvus rufus). Behav. Ecol. Sociobiol. 2008, 62, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Volfova, M.; Machovcova, Z.; Schwarzenberger, F.; Voslarova, E.; Bedanova, I.; Vecerek, V. Non-invasive assessment of adrenocortical activity as a measure of stress in ring-tailed lemurs (Lemur catta). Berl. Munch. Tierarztl. Wochenschr. 2020, 133, 28–35. [Google Scholar]
- Volfova, M.; Machovcova, Z.; Schwarzenberger, F.; Voslarova, E.; Bedanova, I.; Vecerek, V. The effects of transport stress on the behaviour and adrenocortical activity of the black-and-white ruffed lemur (Varecia variegata). Acta Vet. Brno 2019, 88, 85–92. [Google Scholar] [CrossRef]
- Cockrem, J.F. Individual Variation in Glucocorticoid Stress Responses in Animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Heistermann, M.; Palme, R.; Ganswindt, A. Comparison of different enzyme immunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am. J. Primatol. 2006, 68, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Clark, F.E.; Fitzpatrick, M.; Hartley, A.; King, A.J.; Lee, T.; Routh, A.; Walker, S.L.; George, K. Relationship between behavior, adrenal activity, and environment in zoo-housed Western lowland gorillas (Gorilla gorilla gorilla). Zoo Biol. 2011, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pirovino, M.; Heistermann, M.; Zimmermann, N.; Zingg, R.; Clauss, M.; Codron, D.; Kauphanspeter, F.J.; Steinmetz, W. Fecal glucocorticoid measurements and their relation to rearing, behavior, and environmental factors in the population of pileated gibbons (Hylobates pileatus) held in European zoos. Int. J. Primatol. 2011, 32, 1161–1178. [Google Scholar] [CrossRef]
- Hämäläinen, A.; Heistermann, M.; Fenosoa, Z.S.E.; Raus, C. Evaluating capture stress in wild gray mouse lemurs via repeated fecal sampling: Method validation and the influence of prior experience and handling protocols on stress responses. Gen. Comp. Endocrinol. 2014, 195, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien. Tierarztl. Monatsschr. 2013, 100, 238–246. [Google Scholar]
- Palme, R.; Möstl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Mamm. Biol. 1997, 62, 192–197. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-hall, INC: New Jersey, NJ, USA, 1999. [Google Scholar]
- Grandin, T. Assessment of stress during handling and transport. Sci. J. Anim. Sci. 1997, 75, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Margulis, S.W.; Hoyos, C.; Anderson, M. Effect of felid activity on zoo visitor interest. Zoo Biol. 2003, 22, 587–599. [Google Scholar] [CrossRef]
- Romano, M.C.; Rodas, A.Z.; Valdez, R.A.; Hernández, S.E.; Galindo, F.; Canale, D.; Brousset, D.M. Stress in wildlife species: Noninvasive monitoring of glucocorticoids. Neuroimmunomodulation 2010, 17, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.A.; Swaisgood, R.R.; Czekala, N.M.; Steinman, K.; Lindburg, D.G. Monitoring stress in captive giant pandas (Ailuropoda melanoleuca): Behavioral and hormonal responses to ambient noise. Zoo Biol. 2004, 23, 147–164. [Google Scholar] [CrossRef]
- Cavigelli, S.A. Behavioural patterns associated with faecal cortisol levels in free rating female Ring-tailed lemurs, Lemur catta. Anim. Behav. 1999, 57, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Cavigelli, S.A.; Dubovick, T.; Levash, W.; Jolly, A.; Pitts, A. Female dominance status and fecal corticoids in a kooperative breeder with low reproductive skew: Ring-Tailed lemurs (Lemur catta). Horm. Behav. 2003, 43, 166–179. [Google Scholar] [CrossRef]
- Gould, L.; Ziegler, T.E.; Wittwer, D.J. Effects of reproductive and social variables on fecal glucocorticoid levels in a sample of adult male ring-tailed lemurs (Lemur catta) at the Beza Nahafaly Reserve, Madagascar. Am. J. Primatol. 2005, 67, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.A.; Moss, S.E.W.; Pomeroy, P.; Speakman, J.R.; Fedak, M.A. Effects of handling regime and sex on changes in cortisol, thyroid hormones and body mass in fasting grey seal pups. Comp. Biochem. Phys. A 2012, 161, 69–76. [Google Scholar] [CrossRef] [PubMed]
- McGillivray, C.L. Husbandry Manual for Black and Ehite Ruffed Lemur—Varecia variegata variegata (Mammalia: Lemuridae); Western Sydney Institute of Tafe: Richmond, Australia, 2007. [Google Scholar]
- Quine, H. Husbandry Guidelines for White Fronted Brown Lemur (Eulemur fulvus albifrons); Western Sydney Institute of Tafe: Richmond, Australia, 2009. [Google Scholar]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. J. Am. Assoc. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar]
- Rangel-Negrín, A.; Flores-Escobar, E.; Chavira, R.; Canales-Espinosa, D.; Dias, P.A.D. Physiological and analytical validations of fecal steroid hormone measures in black howler monkeys. Primates 2014, 55, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J.; Perdue, B.M.; Powell, D.M.; Forthman, D.L.; Bloomsmith, M.A.; Maple, T.L. Behavioral and hormonal consequences of transporting giant pandas from China to the United states. J. Appl. Anim. Welf. Sci. 2012, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Shutt, K.; Setchell, J.M.; Heistermann, M. Non-invasive monitoring of physiological stress in the estern lowland gorila (Gorilla gorilla gorilla): Validation of fecal glucocorticoid assay and methods for practical application in the field. Gen. Comp. Endocrinol. 2012, 179, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Boccia, M.L.; Laudenslager, M.L.; Reite, M.L. Individual differences in Macaques’ responses to stressors based on social and physiological factors: Implications for primate welfare and research outcomes. Lab. Anim. 1995, 29, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.K.; Hunt, K.E.; Brown, J.L.; Cooper, K.; Crockett, C.M.; Bechert, U.; Millspaugh, J.J.; Larson, S.; Monfort, S.L. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 2000, 120, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Cavigelli, S.A.; Caruso, M.J. Sex, social status and physiological stress in primates: The importace of social and glucocorticoid dynamics. Philos. Trans. R. Soc. 2015, 370, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Starling, A.P.; Charpentier, M.J.E.; Fitzpatrick, C.; Scordato, E.S.; Drea, C.H.M. Seasonality, sociality, and reproduction: Long-Term stressors of ring-tailed lemurs (Lemur catta). Horm. Behav. 2010, 57, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Requena, M.; Palme, R. Measuring faecal glucocorticoid metabolites as a non-invasive tool for monitoring adrenocortical activity in South american camelids. Anim. Welf. 2013, 22, 25–31. [Google Scholar] [CrossRef]
- Dantzer, B.; McAdam, A.G.; Palme, R.; Fletcher, Q.E.; Boutin, S.; Humphries, M.M.; Boonstra, R. Fecal cortisol metabolite levels in free-ranging North American red squirrels: Assay validation and the effects of reproductive condition. Gen. Comp. Endocrinol. 2010, 167, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Carrera, S.C.; Sen, S.; Heistermann, M.; Lu, A.; Beehner, J.C. Low rank and primiparity increase fecal glucocorticoid metabolites across gestation in wild geladas. Gen. Comp. Endocrinol. 2020, 293, 113494. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.L.; Sandel, A.A.; Bray, J.; Oldenkamp, R.E.; Reddy, R.B.; Hare, B.A. Group Size Predicts Social but Not Nonsocial Cognition in Lemurs. PLoS ONE 2013, 8, e66359. [Google Scholar] [CrossRef] [PubMed]
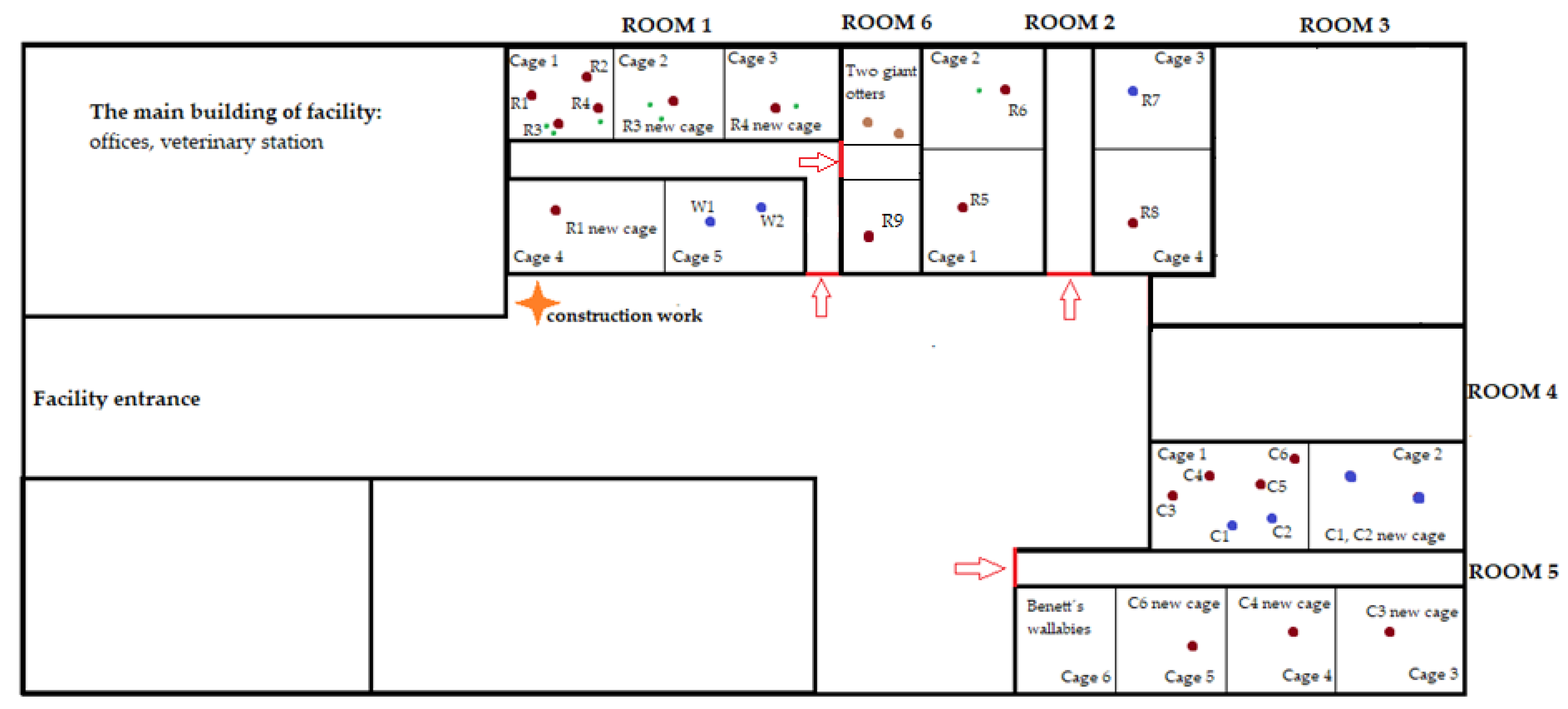
| Species | Animal | Sex | Number of Offspring | Birth | Original Zoo | Arrival to the Facility |
|---|---|---|---|---|---|---|
| Ring-tailed lemur | R1 | F | unknown | Zoo Wroclaw | 1 month before observation | |
| R2 | F | unknown | Zoo Wroclaw | |||
| R3 | F | 2 | March 2002 | Zoo Wroclaw | ||
| R4 | F | 1 | June 2009 | Zoo Wroclaw | ||
| R5 | F | March 2014 | zoo Duisburg | 3 weeks before observation | ||
| R6 | F | 1 | March 2012 | zoo Duisburg | ||
| R7 | F | March 2014 | zoo Bernburg | |||
| R8 | M | unknown | Zoo Wroclaw | |||
| R9 | F | March 2016 | Hamerton zoo park | first day of observation | ||
| White-headed lemur | W1 | M | April 2011 | Olmense zoo | 2 weeks before observation | |
| W2 | M | April 2012 | Olmense zoo | |||
| Collared brown lemur | C1 | M | April 2015 | Hamerton zoo park | first day of observation | |
| C2 | M | April 2015 | Hamerton zoo park | |||
| C3 | F | March 2015 | Hamerton zoo park | |||
| C4 | F | unknown | Hamerton zoo park | |||
| C5 | F | April 2016 | Hamerton zoo park | |||
| C6 | F | April 2015 | Hamerton zoo park |
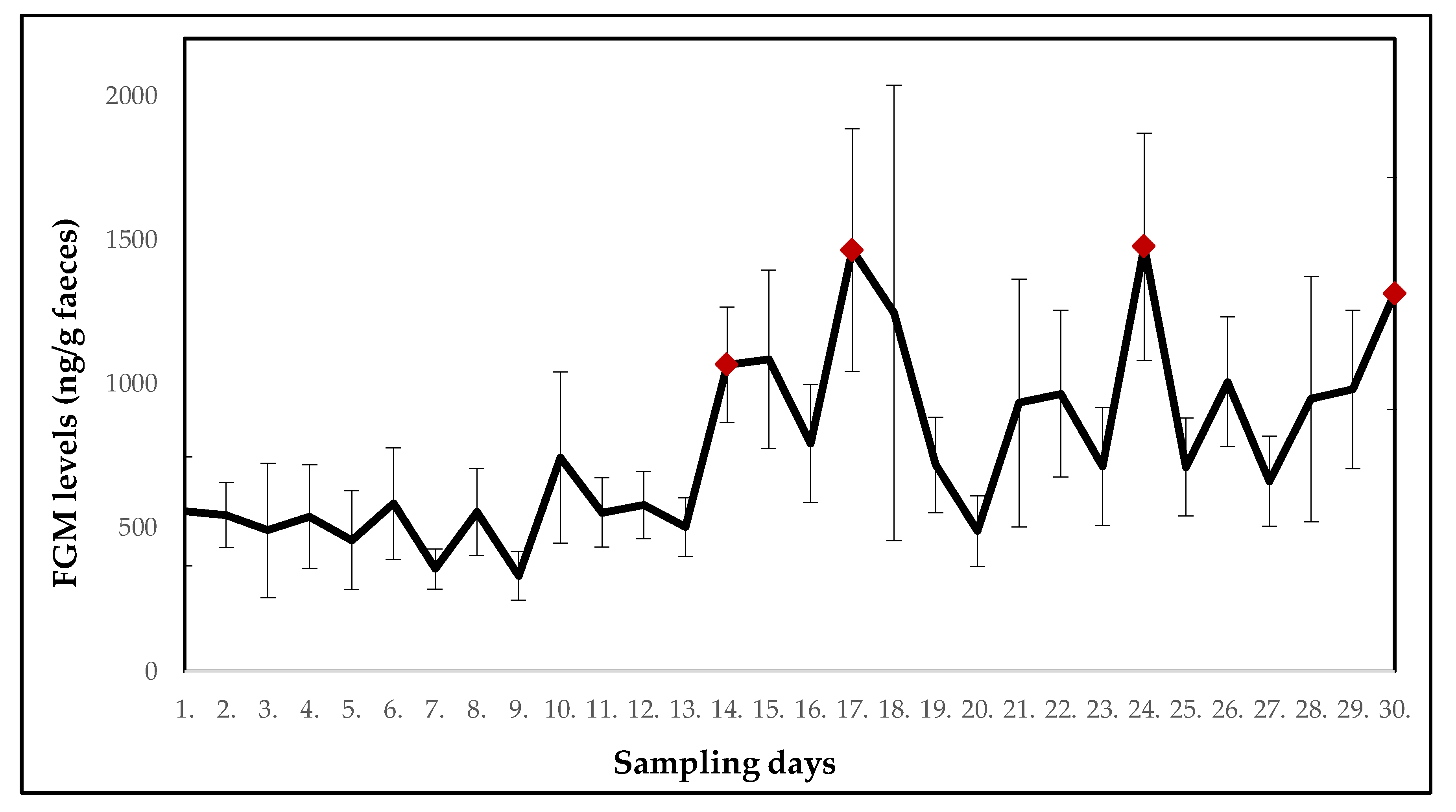
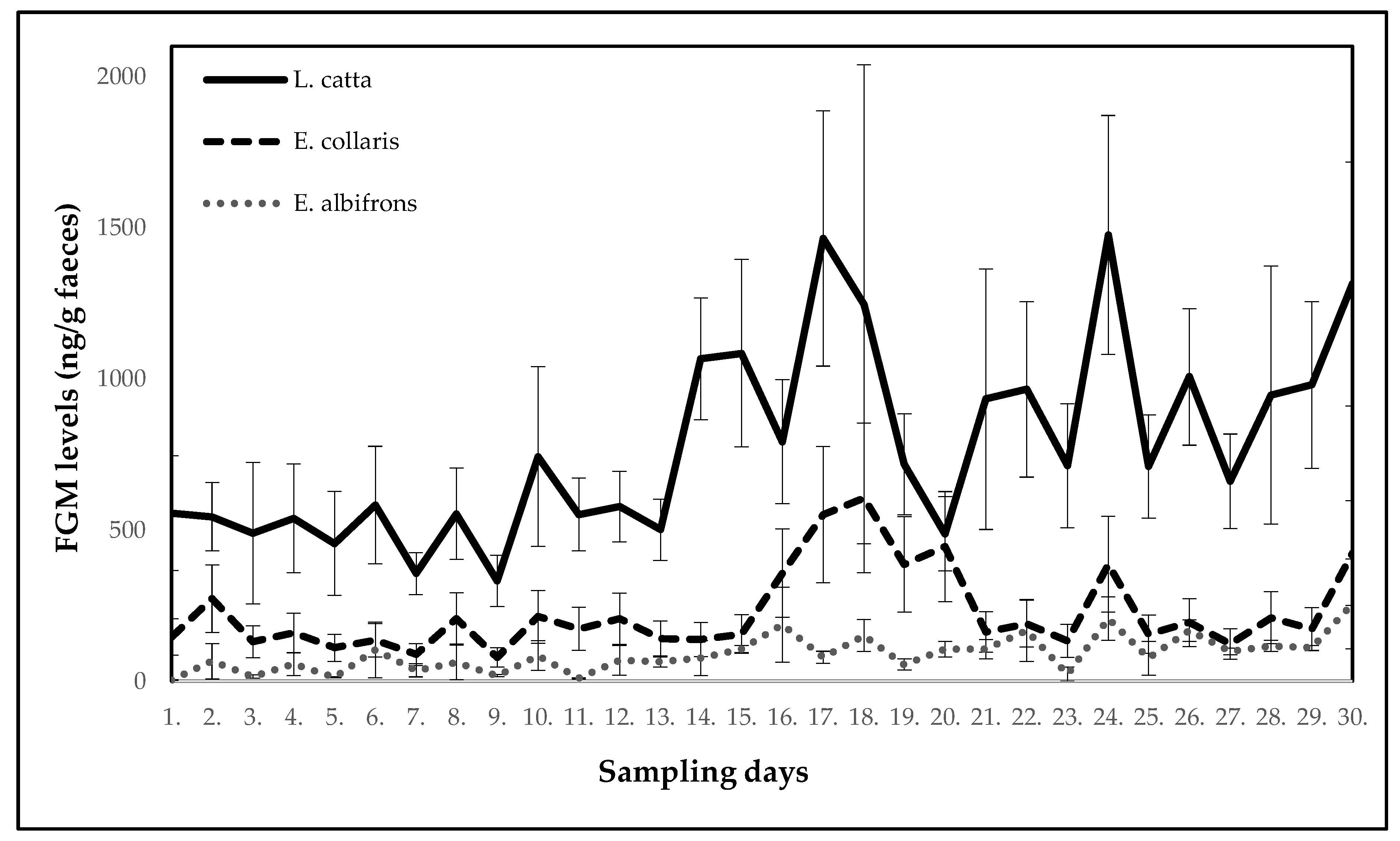
| Ring-Tailed Lemur (Lemur catta) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Mean ± SE (ng/g faeces) | 556.5 ± 189.7 | 544.3 ± 113.0 | 490.1 ± 233.8 | 538.5 ± 180.0 | 456.1 ± 171.7 | 582.9 ± 194.2 | 355.7 ± 69.7 | 554.2 ± 151.6 | 332.0 ± 84.7 | 743.5 ± 297.3 |
| p-value | - | 0.099 | 0.263 | 0.491 | 0.646 | 0.954 | 0.344 | 0.774 | 0.160 | 0.456 |
| Potential stressor | DR | DR | DR | DR | DR | DR | DR | DR | DR | DR |
| Day | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Mean ± SE (ng/g faeces) | 552.2 ± 120.3 | 577.7 ± 116.9 | 501.1 ± 101.6 | 1065.9 ± 201.4 | 1085.2 ± 310.0 | 792.1 ± 205.2 | 1464.3 ± 421.9 | 1246.7 ± 791.8 | 717.4 ± 166.7 | 488.0 ± 122.4 |
| p-value | 1.000 | 0.886 | 0.863 | 0.037 | 0.097 | 0.197 | 0.006 | 0.796 | 0.547 | 0.752 |
| Potential stressor | DR | DR | construction work | construction work | DR | relocation of two females with offspring | DR | DR | DR | DR |
| Day | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Mean ± SE (ng/g faeces) | 933.0 ± 430.9 | 965.5 ± 290.3 | 712.5 ± 204.8 | 1476.2 ± 395.2 | 710.4 ± 170.3 | 1006.6 ± 225.8 | 661.3 ± 156.4 | 946.9 ± 426.6 | 979.8 ± 275.5 | 1314.0 ± 403.2 |
| p-value | 0.863 | 0.178 | 0.863 | 0.018 | 0.841 | 0.136 | 0.605 | 0.752 | 0.109 | 0.010 |
| Potential stressor | DR | DR | relocation of one female | DR | DR | DR | DR | DR | relocation of giant otters | DR |
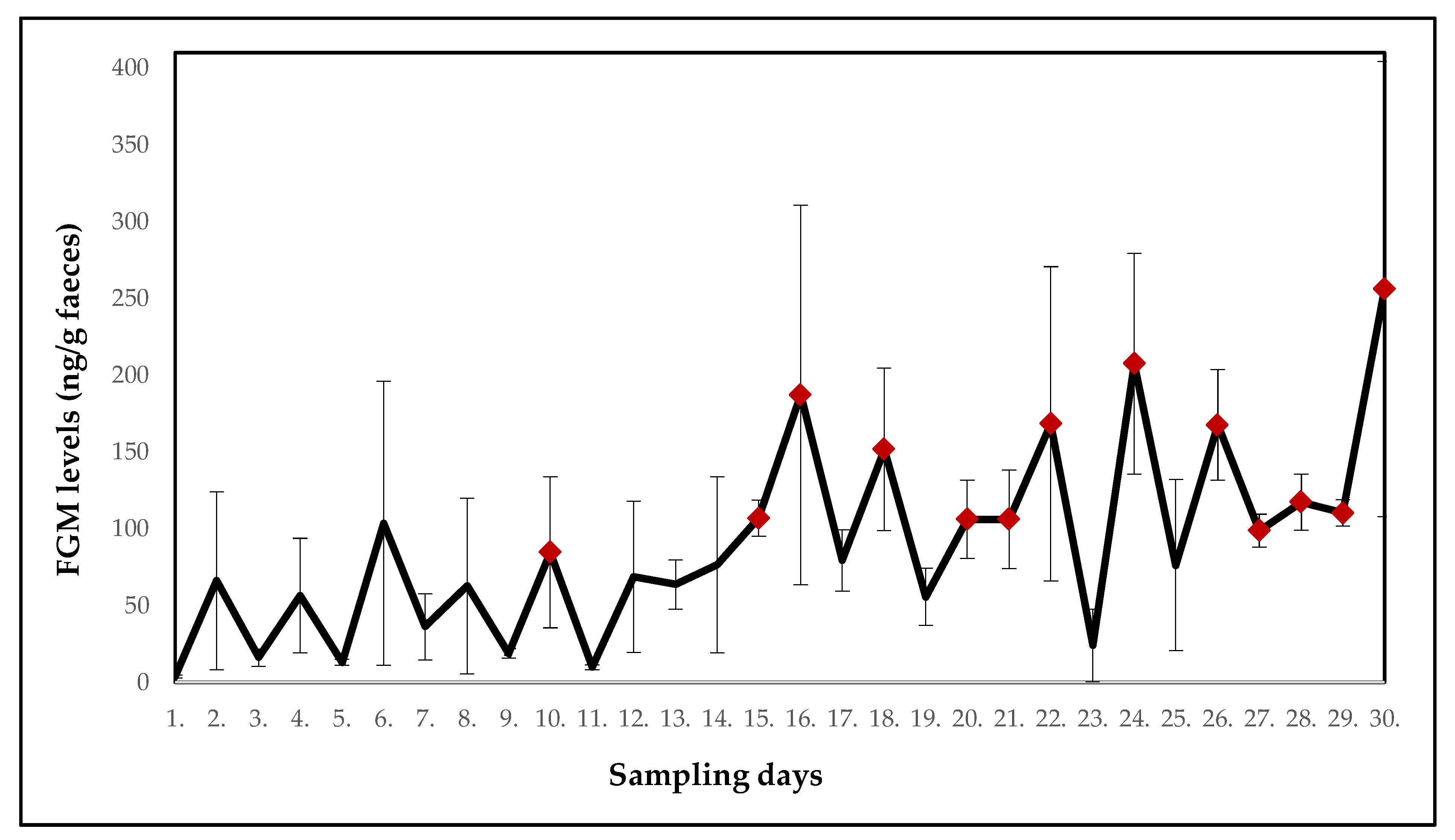
| White-Headed Lemur (Eulemur albifrons) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Mean ± SE (ng/g faeces) | 3.7 ± 1.0 | 66.1 ± 57.9 | 16.1 ± 5.6 | 56.5 ± 37.3 | 13.1 ± 2.1 | 103.5 ± 92.5 | 36.2 ± 21.6 | 62.7 ± 57.2 | 18.8 ± 3.2 | 84.7 ± 49.2 |
| p-value | - | 0.178 | 0.602 | 0.246 | 0.672 | 0.071 | 0.301 | 0.141 | 0.365 | 0.041 |
| Potential stressor | DR | DR | DR | DR | DR | DR | DR | DR | DR | DR |
| Day | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Mean ± SE (ng/g faeces) | 9.8 ± 1.5 | 68.8 ± 49.2 | 63.7 ± 16.1 | 76.6 ± 57.3 | 106.8 ± 11.7 | 187.1 ± 123.6 | 79.3 ± 19.9 | 151.7 ± 52.9 | 55.7 ± 18.6 | 106.2 ± 25.6 |
| p-value | 0.649 | 0.125 | 0.168 | 0.054 | 0.021 | 0.013 | 0.086 | 0.010 | 0.111 | 0.017 |
| Potential stressor | DR | DR | construction work | construction work | DR | relocation of three females | DR | DR | DR | DR |
| Day | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Mean ± SE (ng/g faeces) | 106.2 ± 32.2 | 168.3 ± 102.3 | 23.9 ± 23.7 | 207.5 ± 71.9 | 76.3 ± 55.7 | 167.6 ± 35.9 | 98.7 ± 10.7 | 117.3 ± 18.3 | 110.3 ± 8.5 | 256.0 ± 148.2 |
| p-value | 0.016 | 0.013 | 0.625 | 0.002 | 0.058 | 0.002 | 0.033 | 0.008 | 0.013 | 0.005 |
| Potential stressor | DR | DR | relocation of one female | DR | DR | DR | DR | DR | relocation of giant otters | DR |
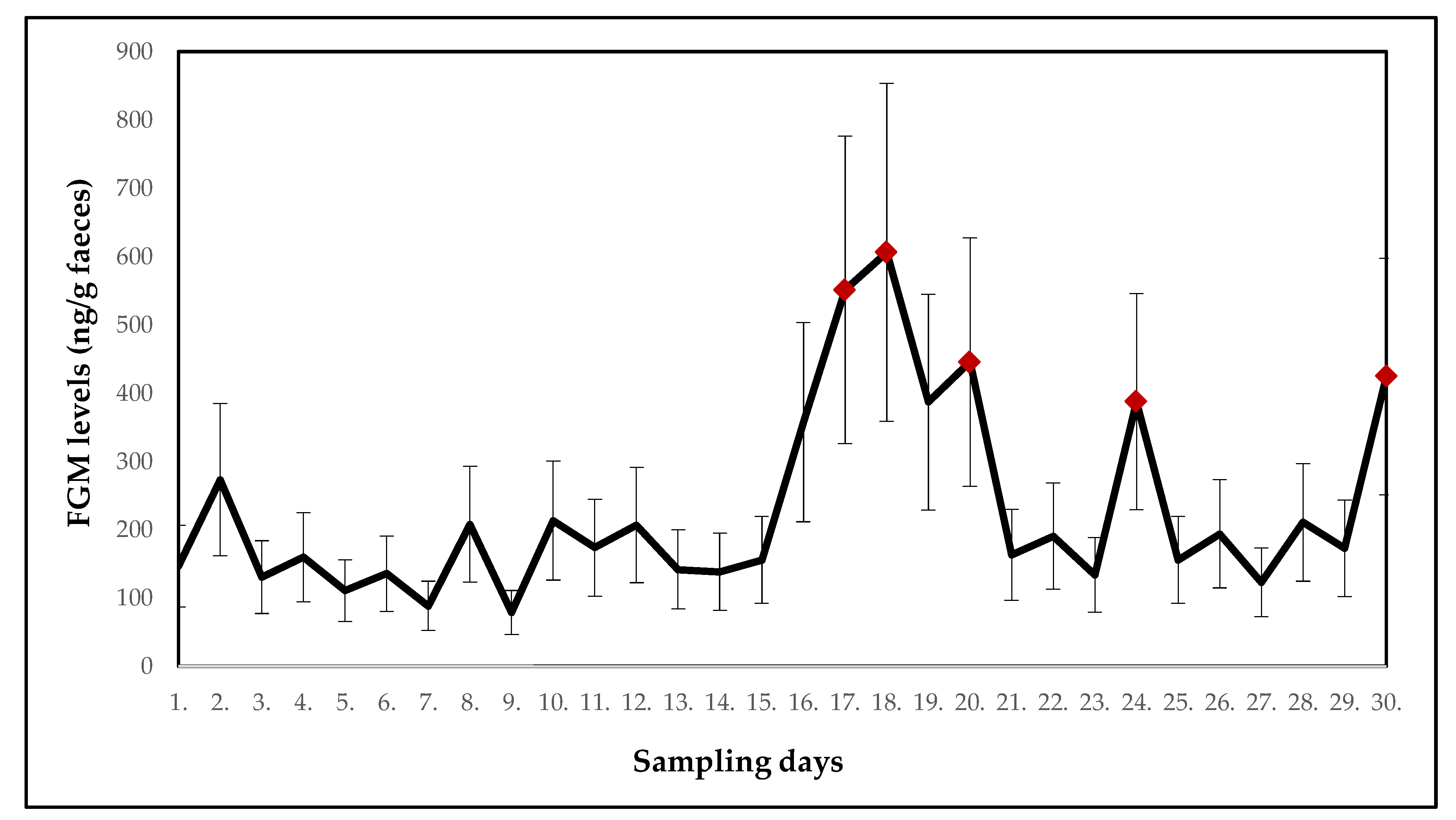
| Collared Brown Lemur (Eulemur collaris) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Mean ± SE (ng/g faeces) | 146.7 ± 59.9 | 273.4 ± 111.6 | 130.6 ± 53.3 | 159.5 ± 65.1 | 110.5 ± 45.1 | 135.4 ± 55.3 | 88.5 ± 36.1 | 207.9 ± 84.9 | 78.8 ± 32.2 | 213.3 ± 87.1 |
| p-value | - | 0.213 | 0.413 | 0.803 | 0.545 | 0.854 | 0.393 | 1.000 | 0.177 | 0.721 |
| Potential stressor | DR | DR | relocation of wallabies | DR | DR | DR | DR | DR | DR | DR |
| Day | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Mean ± SE (ng/g faeces) | 173.6 ± 70.9 | 206.8 ± 84.4 | 141.9 ± 57.9 | 138.5 ± 56.5 | 156.0 ± 63.7 | 357.6 ± 146.0 | 551.1 ± 225.0 | 606.1 ± 247.4 | 386.9 ± 158.0 | 445.5 ± 181.9 |
| p-value | 0.803 | 0.915 | 0.521 | 0.830 | 0.521 | 0.695 | 0.010 | 0.034 | 0.240 | 0.047 |
| Potential stressor | DR | DR | DR | DR | DR | relocation of two males | DR | relocation of one female | DR | DR |
| Day | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Mean ± SE (ng/g faeces) | 163.1 ± 66.6 | 190.8 ± 77.9 | 133.9 ± 54.7 | 387.4 ± 158.2 | 155.7 ± 63.6 | 194.0 ± 79.2 | 122.9 ± 50.2 | 210.5 ± 85.9 | 172.8 ± 70.5 | 424.2 ± 173.2 |
| p-value | 0.775 | 0.498 | 0.721 | 0.028 | 0.873 | 0.748 | 0.775 | 0.618 | 0.901 | 0.015 |
| Potential stressor | DR | DR | relocation of one male | DR | DR | DR | DR | DR | relocation of two males and one female | DR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volfova, M.; Machovcova, Z.; Voslarova, E.; Bedanova, I.; Vecerek, V. Comparison of the Glucocorticoid Concentrations between Three Species of Lemuridae Kept in a Temporary Housing Facility. Animals 2020, 10, 1013. https://doi.org/10.3390/ani10061013
Volfova M, Machovcova Z, Voslarova E, Bedanova I, Vecerek V. Comparison of the Glucocorticoid Concentrations between Three Species of Lemuridae Kept in a Temporary Housing Facility. Animals. 2020; 10(6):1013. https://doi.org/10.3390/ani10061013
Chicago/Turabian StyleVolfova, Martina, Zuzana Machovcova, Eva Voslarova, Iveta Bedanova, and Vladimir Vecerek. 2020. "Comparison of the Glucocorticoid Concentrations between Three Species of Lemuridae Kept in a Temporary Housing Facility" Animals 10, no. 6: 1013. https://doi.org/10.3390/ani10061013
APA StyleVolfova, M., Machovcova, Z., Voslarova, E., Bedanova, I., & Vecerek, V. (2020). Comparison of the Glucocorticoid Concentrations between Three Species of Lemuridae Kept in a Temporary Housing Facility. Animals, 10(6), 1013. https://doi.org/10.3390/ani10061013





