Effects of Black Wattle (Acacia mearnsii) Condensed Tannins on Intake, Protozoa Population, Ruminal Fermentation, and Nutrient Digestibility in Jersey Steers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
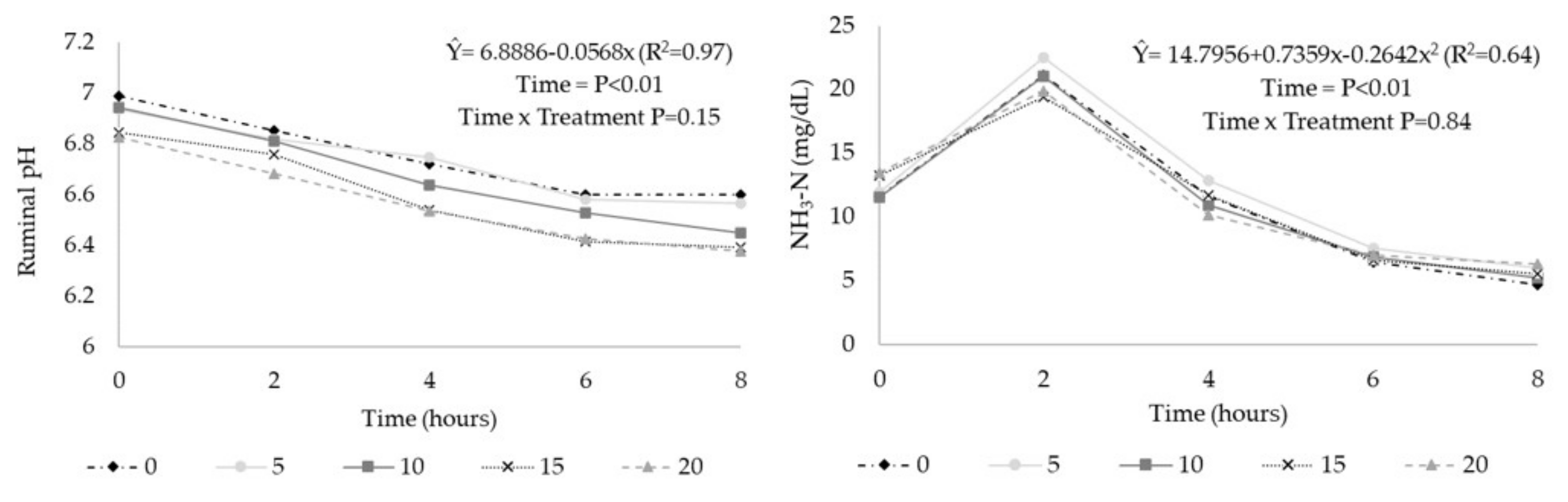
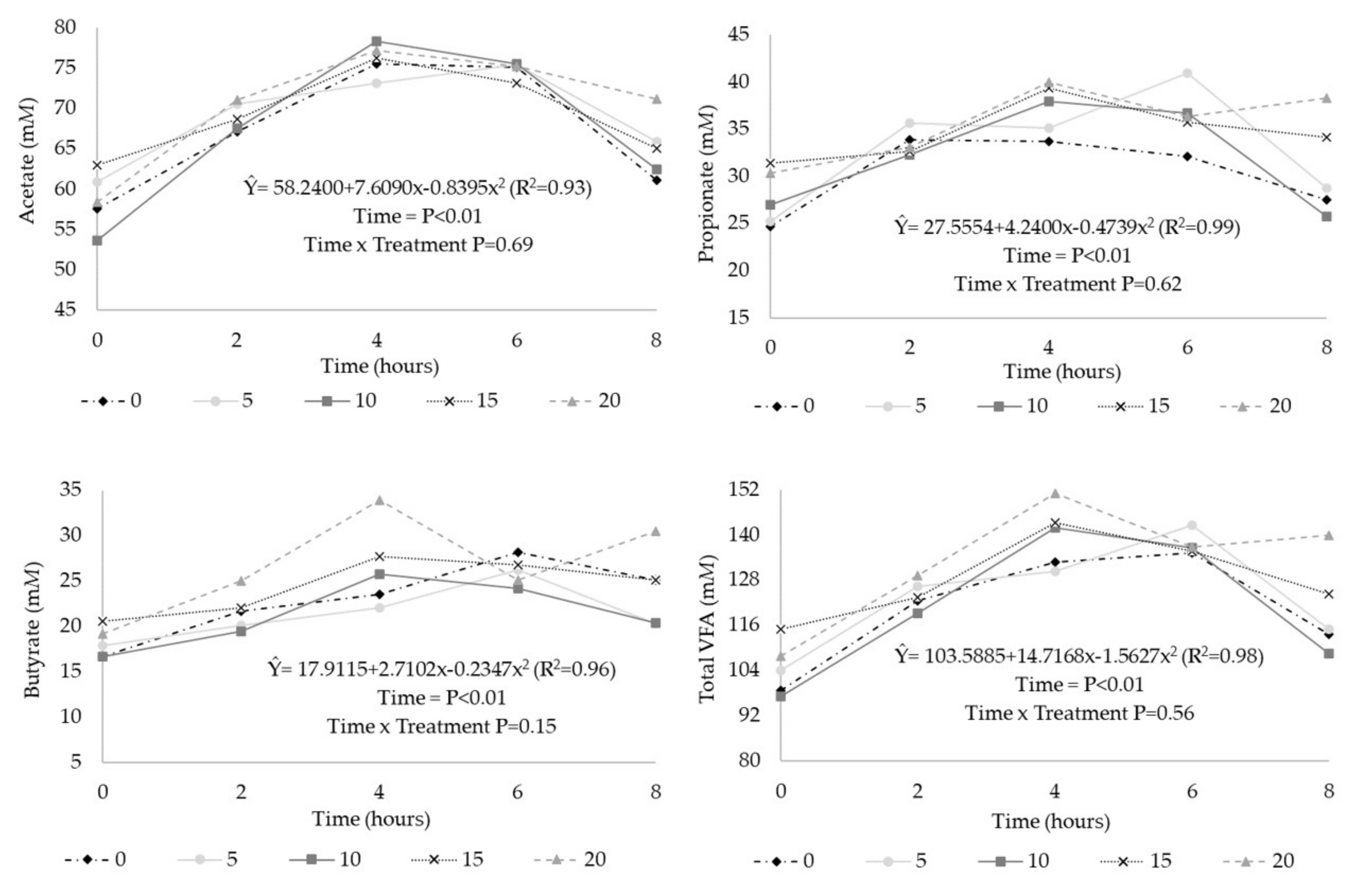
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2004, 2, 191. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Ahnert, S.; Dickhoefer, U.; Schulz, F.; Susenbeth, A. Influence of ruminal Quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance, and urinary purine derivatives excretion in heifers. Livest. Sci. 2015, 177, 63–70. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef]
- Dickhoefer, U.; Ahnert, S.; Susenbeth, A. Effects of quebracho tannin extract on rumen fermentation and yield and composition of microbial mass in heifers. J. Anim. Sci. 2016, 94, 1561–1575. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Capozzolo, M.C.; Lencioni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef]
- Oliveira, S.G.; Berchielli, T.T. Potencialidades da utilização de taninos na conservação de forragens e nutrição de ruminantes-revisão. Arch. Vet. Sci. 2007, 12, 1–9. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Giráldez, F.J.; Mantecón, A.R.; Del Pino, M.C.A. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2005, 109, 65–78. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Dietary phytochemicals as rumen modifiers: A review of the effects on microbial populations. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2009, 96, 363–375. [Google Scholar] [CrossRef]
- Faciola, A.P.; Broderick, G.A.; Hristov, A.; Leão, M.I. Effects of lauric acid on ruminal protozoal numbers and fermentation pattern and milk production in lactating dairy cows. J. Anim. Sci. 2013, 91, 363–373. [Google Scholar] [CrossRef][Green Version]
- Faciola, A.P.; Broderick, G.A. Effects of feeding lauric acid or coconut oil on ruminal protozoa numbers, fermentation pattern, digestion, omasal nutrient flow, and milk production in dairy cows. J. Dairy Sci. 2014, 97, 5088–5100. [Google Scholar] [CrossRef]
- Baah, J.; Ivan, M.; Hristov, A.N.; Koenig, K.M.; Rode, L.M.; McAllister, T.A. Effects of potential dietary antiprotozoal supplements on rumen fermentation and digestibility in heifers. Anim. Feed Sci. Technol. 2007, 137, 126–137. [Google Scholar] [CrossRef]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust. J. Agric. Res. 2005, 56, 961–970. [Google Scholar] [CrossRef]
- Perna Junior, F.; Cassiano, E.C.O.; Martins, M.F.; Romero, L.A.; Zapata, D.C.V.; Pinedo, L.A.; Marino, C.T.; Rodrigues, P.H.M. Effect of tannins-rich extract from Acacia mearnsii or monensin as feed additives on ruminal fermentation efficiency in cattle. Livest. Sci. 2017, 203, 21–29. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef]
- Kozloski, G.V.; Härter, C.J.; Hentz, F.; de Ávila, S.C.; Orlandi, T.; Stefanello, C.M. Intake, digestibility and nutrients supply to wethers fed ryegrass and intraruminally infused with levels of Acacia mearnsii tannin extract. Small Rumin. Res. 2012, 106, 125–130. [Google Scholar] [CrossRef]
- Weiss, W.P. Energy prediction equations for ruminant feeds. In Cornell Nutrition Conference for Feed Manufacturers; Cornell University: Ithaca, NY, USA, 1999; Volume 61, pp. 176–185. [Google Scholar]
- National Research Council—NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academic Press: Washington, DC, USA, 2001; 381p. [Google Scholar]
- Association of Official Analytical Chemists—AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; 125p. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Casali, A.O.; Detmann, E.; Valadares Filho, S.D.C.; Pereira, J.C.; Henriques, L.T.; De Freitas, S.G.; Paulino, M.F. Influência do tempo de incubação e do tamanho de partículas sobre os teores de compostos indigestíveis em alimentos e fezes bovinas obtidos por procedimentos in situ. Rev. Bras. Zootec. 2008, 37, 335–342. [Google Scholar] [CrossRef]
- Vieira, P.F. Efeito do Formaldeído na Proteção de Proteínas e Lipídios em Rações Para Ruminantes. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 1980. [Google Scholar]
- Lazaro, C.Z. Obtenção e Caracterização Filogenética de Consórcio de Bactérias Fototrópicas Púrpuras Não-Sulfurosas Consumidoras de Ácidos Orgânicos Visando a Produção de Hidrogênio em Reator Anaeróbio de Batelada. Master’s Thesis, Universidade de São Paulo, São Carlos, Brazil, 2009. [Google Scholar]
- D’Agosto, M.; Carneiro, M.E. Evaluation of lugol solution used for counting rumen ciliates. Rev. Bras. Zool. 1999, 16, 725–729. [Google Scholar] [CrossRef]
- Dehority, B.A. Evaluation of subsampling and fixation procedures used for counting rumen protozoa. Appl. Environ. Microbiol. 1984, 48, 182–185. [Google Scholar] [CrossRef]
- Ogimoto, K.; Imai, S. Atlas of Rumen Microbiology; Japan Scientific Societies Press: Tokyo, Japan, 1981; 231p. [Google Scholar]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Review: Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Krueger, W.K.; Gutierrez-Bañuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Getachew, G.; Pittroff, W.; Putnam, D.H.; Dandekar, A.; Goyal, S.; DePeters, E.J. The influence of addition of gallic acid, tannic acid, or quebracho tannins to alfalfa hay on in vitro rumen fermentation and microbial protein synthesis. Anim. Feed Sci. Technol. 2008, 140, 444–461. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- McNabb, W.C.; Peters, J.S.; Foo, L.Y.; Waghorn, G.C.; Jackson, F.S. Effect of condensed tannins prepared from several forages on the in vitro precipitation of ribulose-1,5-bisphosphate carboxylase (Rubisco) protein and its digestion by trypsin (EC 2.4.21.4) and chymotrypsin (EC 2.4.21.1). J. Sci. Food Agric. 1998, 77, 201–212. [Google Scholar] [CrossRef]
- Ríspoli, T.B.; Rodrigues, I.L.; Martins Neto, R.G.; Kazama, R.; Prado, O.P.P.; Zeoula, L.M.; Arcuri, P.B. Protozoários ciliados do rúmen de bovinos e bubalinos alimentados com dietas suplementadas com monensina ou própolis. Pesqui. Agropecu. Bras. 2009, 44, 92–97. [Google Scholar] [CrossRef]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; McAllister, T.A.; Chouinard, P.Y. Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extracts. J. Dairy Sci. 2008, 91, 4765–4777. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, K.; Pries, M.; Tholen, E.; Schmithausen, A.J.; Büscher, W.; Südekum, K.H. Effect of condensed tannins in rations of lactating dairy cows on production variables and nitrogen use efficiency. Animal 2018, 12, 1847–1855. [Google Scholar] [CrossRef]
- Koenig, K.M.; Beauchemin, K.A. Effect of feeding condensed tannins in high protein finishing diets containing corn distillers’ grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 2018, 96, 4398–4413. [Google Scholar] [CrossRef]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.S.; Young, A.J.; Min, B.R. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef]
- Abdela, N. Sub-acute ruminal acidosis (SARA) and its consequence in dairy cattle: A review of past and recent research at global prospective. Achiev. Life Sci. 2016, 10, 187–196. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Castro-Montoya, J.M.; Makkar, H.P.S.; Becker, K. Chemical composition of rumen microbial fraction and fermentation parameters as affected by tannins and saponins using an in vitro rumen fermentation system. Can. J. Anim. Sci. 2011, 91, 433–448. [Google Scholar] [CrossRef]
- Cieslak, A.; Zmora, P.; Pers-Kamczyc, E.; Szumacher-Strabel, M. Effects of tannins source (Vaccinium vitis idaea L.) on rumen microbial fermentation in vivo. Anim. Feed Sci. Technol. 2012, 176, 102–106. [Google Scholar] [CrossRef]
- Orlandi, T.; Kozloski, G.V.; Alves, T.P.; Mesquita, F.R.; Ávila, S.C. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Anim. Feed Sci. Technol. 2015, 210, 37–45. [Google Scholar] [CrossRef]
| Item | Tifton 85 Hay | Ground Corn | Soybean Meal |
|---|---|---|---|
| Dry matter (g/kg of fresh matter) | 850 | 907 | 889 |
| Organic matter | 920 | 985 | 925 |
| Ether extract(EE) | 16.7 | 20.0 | 12.0 |
| Crude protein(CP) | 113 | 82.7 | 479 |
| Neutral detergent fiber | 739 | 113 | 195 |
| Non fiber carbohydrates (NFC) 1 | 110 | 782 | 302 |
| Ingredients | Levels of Condensed Tannins Inclusion (g/kg of DM) | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Tifton 85 hay | 600 | 600 | 600 | 600 | 600 |
| Ground corn | 337 | 328 | 322 | 314 | 307 |
| Soybean meal | 52.1 | 55.3 | 55.1 | 57.2 | 58.4 |
| Mineral mix 1 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Condensed tannin extract 2 | - | 6.20 | 12.4 | 18.7 | 24.9 |
| Chemical Composition 3 | |||||
| DM (g/kg of fresh matter) | 868 | 866 | 868 | 868 | 873 |
| OM | 936 | 936 | 935 | 935 | 935 |
| EE | 19.3 | 17.9 | 19.9 | 20.9 | 20.4 |
| CP | 122 | 117 | 121 | 119 | 121 |
| Estimated RDP 4 | 88.8 | 85.5 | 88.8 | 87.6 | 88.8 |
| Estimated RUP 4 | 33.0 | 31.6 | 32.8 | 32.1 | 32.5 |
| NDF | 490 | 486 | 488 | 487 | 487 |
| NDFap | 447 | 444 | 444 | 443 | 444 |
| NFC 5 | 348 | 358 | 349 | 351 | 350 |
| Estimated TDN 4 | 648 | 642 | 637 | 631 | 626 |
| Variables | Condensed Tannins Inclusion (g/kg of DM) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | CT | L | Q | ||
| DMI (kg/d) | 8.90 | 8.25 | 9.00 | 8.42 | 8.75 | 0.324 | 0.17 | 0.87 | 0.47 |
| DMI (g/kg BW) | 11.8 | 10.9 | 12.0 | 11.0 | 11.5 | 0.447 | 0.13 | 0.67 | 0.59 |
| OMI (kg/d) | 8.34 | 7.73 | 8.42 | 7.88 | 8.19 | 0.301 | 0.17 | 0.83 | 0.46 |
| CPI (kg/d) | 1.07 | 1.01 | 1.10 | 1.04 | 1.07 | 0.040 | 0.25 | 0.81 | 0.76 |
| EEI (kg/d) | 0.17 | 0.15 | 0.18 | 0.18 | 0.18 | 0.011 | 0.10 | 0.08 | 0.70 |
| NDFI (kg/d) | 4.36 | 3.96 | 4.38 | 4.06 | 4.29 | 0.184 | 0.15 | 0.91 | 0.33 |
| NDFI (g/kg BW) | 5.79 | 5.25 | 5.86 | 5.34 | 5.65 | 0.249 | 0.11 | 0.75 | 0.40 |
| NFCI (kg/d) | 3.10 | 2.97 | 3.15 | 2.94 | 3.04 | 0.141 | 0.57 | 0.65 | 0.85 |
| TDNI (kg/d) | 6.92 | 6.42 | 6.93 | 6.59 | 6.86 | 0.293 | 0.33 | 0.95 | 0.39 |
| Variables | Condensed Tannins Inclusion (g/kg of DM) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | CT | L | Q | ||
| DMD | 67.4 | 68.2 | 65.6 | 69.0 | 68.1 | 1.30 | 0.16 | 0.44 | 0.46 |
| OMD | 73.1 | 73.9 | 70.6 | 74.2 | 72.7 | 1.18 | 0.07 | 0.84 | 0.49 |
| CPD 1 | 70.4 | 68.9 | 66.1 | 68.0 | 64.0 | 1.70 | 0.03 | 0.01 | 0.98 |
| EED | 69.5 | 67.3 | 72.3 | 76.0 | 72.9 | 3.00 | 0.11 | 0.04 | 0.71 |
| NDFD | 58.6 | 57.9 | 55.9 | 59.1 | 57.7 | 1.77 | 0.48 | 0.89 | 0.43 |
| NFCD | 85.8 | 86.1 | 84.2 | 85.5 | 87.0 | 0.964 | 0.14 | 0.40 | 0.05 |
| TDN | 79.4 | 78.3 | 78.0 | 80.0 | 79.3 | 0.979 | 0.35 | 0.30 | 0.49 |
| Item | Condensed Tannins Inclusion (g/kg of DM) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | CT | L | Q | ||
| Entodinium | 1.21 | 1.03 | 1.10 | 1.08 | 0.86 | 0.120 | 0.13 | 0.03 | 0.51 |
| Dasytricha | 0.04 | 0.02 | 0.05 | 0.04 | 0.02 | 0.012 | 0.15 | 0.68 | 0.34 |
| Isotricha | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.006 | 0.10 | 0.05 | 0.25 |
| Charonina | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.007 | 0.43 | 0.19 | 0.45 |
| Eremoplast | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.005 | 0.78 | 0.30 | 0.59 |
| Metadinium | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.004 | 0.84 | 0.81 | 0.95 |
| Total | 1.31 | 1.11 | 1.20 | 1.18 | 0.93 | 0.005 | 0.12 | 0.03 | 0.45 |
| Variables | Condensed Tannins Inclusion (g/kg DM) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | CT | L | Q | ||
| pH 1 | 6.75 | 6.73 | 6.67 | 6.59 | 6.57 | 0.040 | <0.01 | <0.01 | 0.87 |
| N-NH3 (mg/dL) | 11.0 | 12.1 | 11.1 | 11.3 | 11.3 | 1.02 | 0.86 | 0.90 | 0.78 |
| Total VFA (mM) 2 | 121 | 124 | 122 | 128 | 133 | 5.11 | 0.09 | 0.01 | 0.36 |
| Acetate (%) 3 | 56.0 | 56.3 | 56.3 | 54.0 | 53.5 | 1.08 | 0.02 | <0.01 | 0.22 |
| Propionate (%) | 24.9 | 26.5 | 26.1 | 26.8 | 26.7 | 0.91 | 0.17 | 0.23 | 0.61 |
| Butyrate (%) 4 | 19.0 | 17.1 | 17.4 | 19.1 | 19.6 | 1.28 | 0.01 | 0.07 | <0.01 |
| Acetate:propionate | 2.31 | 2.17 | 2.13 | 2.04 | 2.06 | 0.095 | 0.17 | 0.02 | 0.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, A.S.; Zambom, M.A.; Faccenda, A.; Fischer, M.L.; Anschau, F.A.; Venturini, T.; Tinini, R.C.R.; Dessbesell, J.G.; Faciola, A.P. Effects of Black Wattle (Acacia mearnsii) Condensed Tannins on Intake, Protozoa Population, Ruminal Fermentation, and Nutrient Digestibility in Jersey Steers. Animals 2020, 10, 1011. https://doi.org/10.3390/ani10061011
Avila AS, Zambom MA, Faccenda A, Fischer ML, Anschau FA, Venturini T, Tinini RCR, Dessbesell JG, Faciola AP. Effects of Black Wattle (Acacia mearnsii) Condensed Tannins on Intake, Protozoa Population, Ruminal Fermentation, and Nutrient Digestibility in Jersey Steers. Animals. 2020; 10(6):1011. https://doi.org/10.3390/ani10061011
Chicago/Turabian StyleAvila, Andre S., Maximiliane A. Zambom, Andressa Faccenda, Maria L. Fischer, Fernando A. Anschau, Tiago Venturini, Rodrigo C. R. Tinini, Jessica G. Dessbesell, and Antonio P. Faciola. 2020. "Effects of Black Wattle (Acacia mearnsii) Condensed Tannins on Intake, Protozoa Population, Ruminal Fermentation, and Nutrient Digestibility in Jersey Steers" Animals 10, no. 6: 1011. https://doi.org/10.3390/ani10061011
APA StyleAvila, A. S., Zambom, M. A., Faccenda, A., Fischer, M. L., Anschau, F. A., Venturini, T., Tinini, R. C. R., Dessbesell, J. G., & Faciola, A. P. (2020). Effects of Black Wattle (Acacia mearnsii) Condensed Tannins on Intake, Protozoa Population, Ruminal Fermentation, and Nutrient Digestibility in Jersey Steers. Animals, 10(6), 1011. https://doi.org/10.3390/ani10061011





