The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium
Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Animals and Tissue Collection
2.2. In Vitro Endometrial Explant Culture
2.3. Viability of Endometrial Explants
2.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.5. Western Blot Analysis
2.6. Zymography
2.7. Statistical Analysis
3. Results
3.1. Validation of the Viability of Long-Term Endometrial Explant Culture
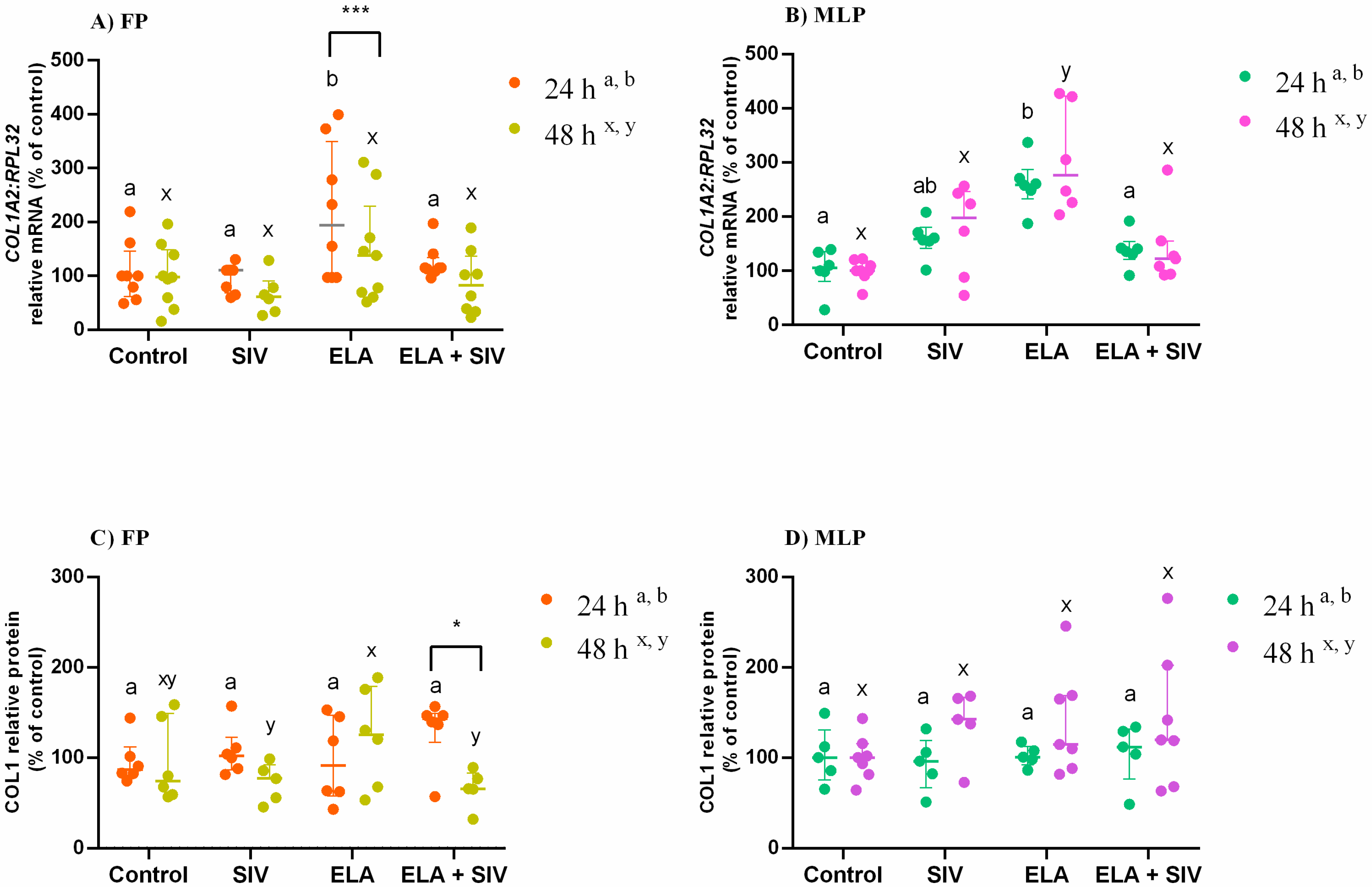
3.2. Inhibitory Effect of Sivelestat on ELA-Induced COL1
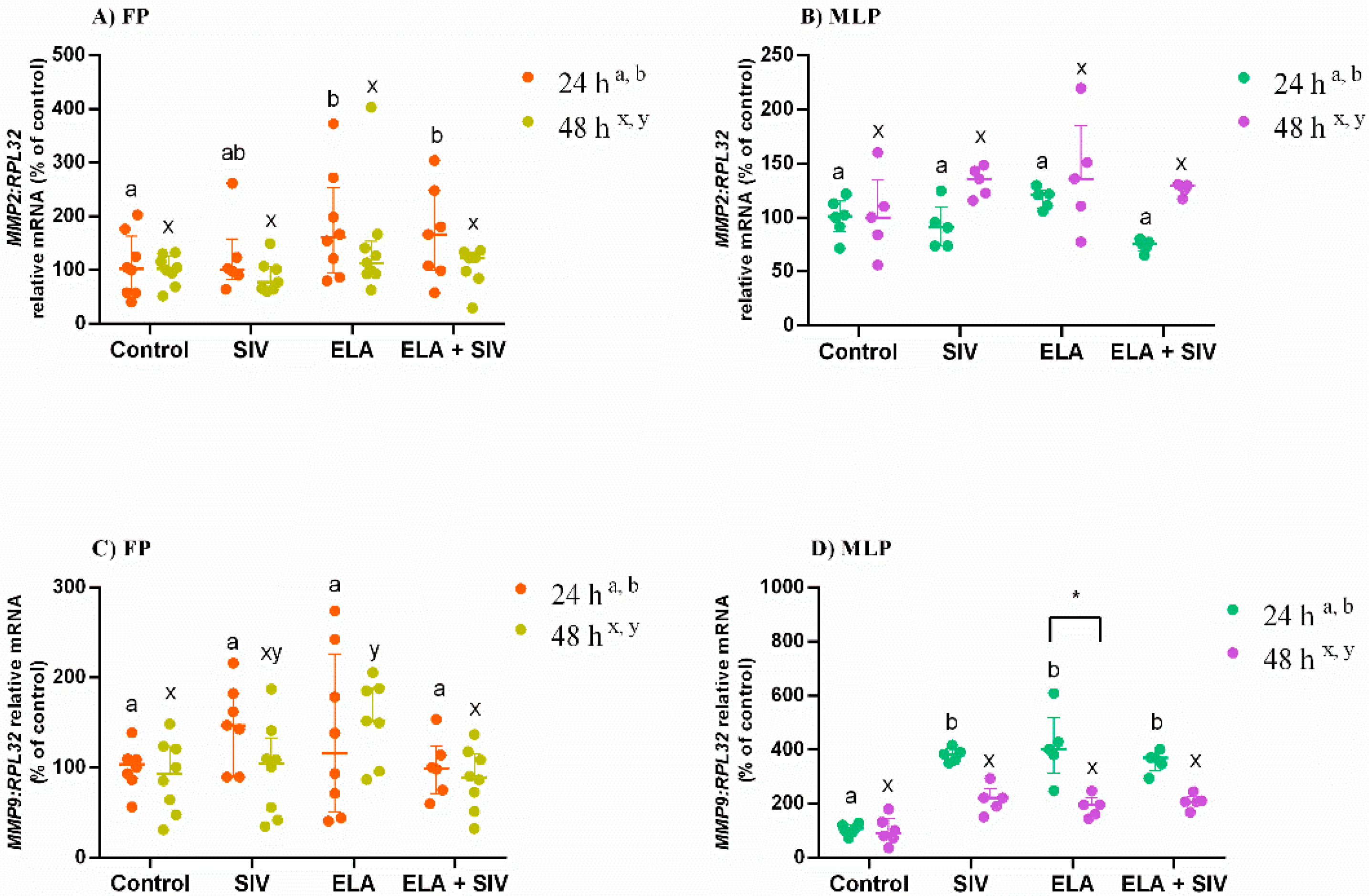
3.3. The Effect of ELA and SIV on MMP Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kotilainen, T.; Huhtinen, M.; Katila, T. Sperm-induced leukocytosis in the equine uterus. Theriogenology 1994, 41, 629–636. [Google Scholar] [CrossRef]
- Katila, T. Onset and Duration of Uterine Inflammatory Response of Mares after Insemination with Fresh Semen. Boil. Reprod. 1995, 52, 515–517. [Google Scholar] [CrossRef]
- Troedsson, M.H. Function of uterine and blood-derived polymorphonuclear neutrophils in mares susceptible and resistant to chronic uterine infection: Phagocytosis and chemotaxis. Boil. Reprod. 1993, 49, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H. Breeding-Induced Endometritis in Mares. Veter Clin. N. Am. Equine Pract. 2006, 22, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Rebordão, M.; Carneiro, C.; Alexandre-Pires, G.; Brito, P.; Pereira, C.; Nunes, T.; Galvão, A.; Leitao, A.; Vilela, C.; Dias, G.M.L.F. Neutrophil extracellular traps formation by bacteria causing endometritis in the mare. J. Reprod. Immunol. 2014, 106, 41–49. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Foster, D.N. Seminal DNase Frees Spermatozoa Entangled in Neutrophil Extracellular Traps. Boil. Reprod. 2005, 73, 1174–1181. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Lovaas, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, U.N. Species-specific interaction of seminal plasma on sperm–neutrophil binding. Anim. Reprod. Sci. 2009, 114, 331–344. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Łukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Constituents of neutrophil extracellular traps induce in vitro collagen formation in mare endometrium. Theriogenology 2018, 113, 8–18. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Łukasik, K.; Szóstek-Mioduchowska, A.; Baclawska, A.; Rebordão, M.R.; Aguiar-Silva, J.; Pinto-Bravo, P.; Skarzynski, D.J.; Ferreira-Dias, G. Elastase inhibition affects collagen transcription and prostaglandin secretion in mare endometrium during the estrous cycle. Reprod. Domest. Anim. 2018, 53, 66–69. [Google Scholar] [CrossRef] [PubMed]
- A Wynn, T. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Steen, P.E.V.D.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Boil. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Semo, A.P.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 23. [Google Scholar] [CrossRef]
- Harvey, A.P.; Montezano, A.C.; Alves-Lopes, R.; Rios, F.J.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Boil. 2015, 44, 147–156. [Google Scholar] [CrossRef]
- Wang, B.-L.; Tu, Y.; Fu, J.-F.; Zhong, Y.-X.; Fu, G.; Tian, X.-X.; Wang, L.-H.; Gong, L.; Ren, Q. Unbalanced MMP/TIMP-1 expression during the development of experimental pulmonary fibrosis with acute paraquat poisoning. Mol. Med. Rep. 2011, 4, 243–248. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Baclawska, A.; Okuda, K.; Skarzynski, D.J. Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Słowińska, M.; Pacewicz, J.; Skarzynski, D.J.; Okuda, K. Matrix metallopeptidase expression and modulation by transforming growth factor-β1 in equine endometrosis. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Dittrich, A.S.; Kühbandner, I.; Gehrig, S.; Rickert-Zacharias, V.; Twigg, M.; Wege, S.; Taggart, C.C.; Herth, F.; Schultz, C.; Mall, M.A. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur. Respir. J. 2018, 51, 1701910. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.D.; Kliment, C.R.; Metz, H.E.; Kim, K.-H.; Kargl, J.; Agostini, B.A.; Crum, L.T.; Oczypok, E.A.; Oury, T.A.; Houghton, A.M. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J. Leukoc. Boil. 2015, 98, 143–152. [Google Scholar] [CrossRef]
- Takemasa, A.; Ishii, Y.; Fukuda, T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur. Respir. J. 2012, 40, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, N.; Ishizaka, A.; Hirasawa, H.; Shimazaki, S.; Yamamoto, Y.; Sugimoto, H.; Shinozaki, M.; Taenaka, N.; Endo, S.; Ikeda, T.; et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm. Pharmacol. Ther. 2011, 24, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Muramatsu, K.; Yatera, K.; Asakawa, T.; Otsubo, H.; Kubo, T.; Fujino, Y.; Matsuda, S.; Mayumi, T.; Mukae, H. Efficacy of early sivelestat administration on acute lung injury and acute respiratory distress syndrome. Respirology 2016, 22, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.P.R.; Serrão, P.M.; Monteiro, S.; Pessa, P.; Silva, J.R.; Dias, G.M.L.F. Caspase-3-mediated apoptosis and cell proliferation in the equine endometrium during the oestrous cycle. Reprod. Fertil. Dev. 2007, 19, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Rebordão, M.R.; Amaral, A.; Łukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Impairment of the antifibrotic prostaglandin E2 pathway may influence neutrophil extracellular traps–induced fibrosis in the mare endometrium. Domest. Anim. Endocrinol. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Kenney, R.M.; Doig, P.A. Equine endometrial biopsy. Current Therapy in Theriogenology 2: Diagnosis, Treatment, and Prevention of Reproductive Diseases in Small and Large Animals, 2nd ed.; Morrow, D.A., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1986; pp. 723–729. [Google Scholar]
- Szóstek-Mioduchowska, A.; Łukasik, K.; Skarzynski, D.J.; Okuda, K. Effect of transforming growth factor -β1 on α-smooth muscle actin and collagen expression in equine endometrial fibroblasts. Theriogenology 2019, 124, 9–17. [Google Scholar] [CrossRef]
- Nash, D.M.; Lane, E.; Herath, S.; Sheldon, I.M. ORIGINAL ARTICLE: Endometrial Explant Culture for Characterizing Equine Endometritis. Am. J. Reprod. Immunol. 2008, 59, 105–117. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Łukasik, K.; Galvão, A.; Ferreira-Dias, G.M.; Skarzynski, D.J. Impairment of the Interleukin System in Equine Endometrium During the Course of Endometrosis. Boil. Reprod. 2013, 89, 79. [Google Scholar] [CrossRef]
- Voynow, J.A.; Fischer, B.M.; Zheng, S. Proteases and cystic fibrosis. Int. J. Biochem. Cell Boil. 2008, 40, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Misumi, T.; Tanaka, T.; Mikawa, K.; Nishina, K.; Morikawa, O.; Obara, H. Effects of sivelestat, a new elastase inhibitor, on IL-8 and MCP-1 production from stimulated human alveolar epithelial type II cells. J. Anesth. 2006, 20, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, W.R.; Fischer, L.; Roth, K.; Jüllig, A.K.; Stuckenschneider, J.E.; Schwartz, P.; Weimer, M.; Orlowska-Volk, M.; Hanjalic-Beck, A.; Kranz, I.; et al. Critical evaluation of human endometrial explants as an ex vivo model system: A molecular approach. Mol. Hum. Reprod. 2010, 17, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Boil. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Ladner-Keay, C.; Yang, J.; Turner, R.J.; Edwards, R.A. Visible fluorescent detection of proteins in polyacrylamide gels without staining. Anal. Biochem. 2004, 326, 13–20. [Google Scholar] [CrossRef]
- Gilda, J.E.; Gomes, A.V. Stain-Free total protein staining is a superior loading control to β-actin for Western blots. Anal. Biochem. 2013, 440, 186–188. [Google Scholar] [CrossRef]
- Posch, A.; Kohn, J.; Oh, K.; Hammond, M.; Liu, N. V3 Stain-free Workflow for a Practical, Convenient, and Reliable Total Protein Loading Control in Western Blotting. J. Vis. Exp. 2013, 50948. [Google Scholar] [CrossRef]
- Comajoan, P.; Gubern, C.; Huguet, G.; Serena, J.; Kádár, E.; Castellanos, M. Evaluation of common housekeeping proteins under ischemic conditions and/or rt-PA treatment in bEnd.3 cells. J. Proteom. 2018, 184, 10–15. [Google Scholar] [CrossRef]
- Manuel, J.A.; Gawronska-Kozak, B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Boil. 2006, 25, 505–514. [Google Scholar] [CrossRef]
- Raykin, J.; Snider, E.; Bheri, S.; Mulvihill, J.J.; Ethier, C.R. A modified gelatin zymography technique incorporating total protein normalization. Anal. Biochem. 2017, 521, 8–10. [Google Scholar] [CrossRef]
- Shimoda, M.; Iwasaki, Y.; Suzuki, S. A Protective Effect of Sivelestat From Ischemia/Reperfusion Injury in a Porcine Hepatectomy Model. Int. Surg. 2019, 103, 191–198. [Google Scholar] [CrossRef]
- Yuan, Q.; Jiang, Y.-W.; Fang, Q.-H. Improving effect of Sivelestat on lipopolysaccharide-induced lung injury in rats. APMIS 2014, 122, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Hilscher, M.B.; Sehrawat, T.; Arab, J.P.; Zeng, Z.; Gao, J.; Liu, M.; Kostallari, E.; Gao, Y.; Simonetto, U.A.; Yaqoob, U.; et al. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology 2019, 157, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Kang, C.M.; Rhee, C.K.; Yoon, H.K.; Kim, Y.K.; Moon, H.S.; Park, S.H. Effects of elastase inhibitor on the epithelial cell apoptosis in bleomycin-induced pulmonary fibrosis. Exp. Lung Res. 2009, 35, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Rosales-Mayor, E.; Dale, G.E.; Dembowsky, K.; Torres, A. The Role of Neutrophil Elastase Inhibitors in Lung Diseases. Chest 2017, 152, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Schwarz, R.I. Collagen I and the fibroblast: High protein expression requires a new paradigm of post-transcriptional, feedback regulation. Biochem. Biophys. Rep. 2015, 3, 38–44. [Google Scholar] [CrossRef]
- Nissinen, L.; Kähäri, V. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2571–2580. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Boil. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Nothnick, W.B. Regulation of uterine matrix metalloproteinase-9 and the role of microRNAs. Semin. Reprod. Med. 2008, 26, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Iida, J.; McCarthy, J.B. Expression of collagenase-1 (MMP-1) promotes melanoma growth through the generation of active transforming growth factor-? Melanoma Res. 2007, 17, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [PubMed]
- Kobayashi, T.; Kim, H.; Liu, X.; Sugiura, H.; Kohyama, T.; Fang, Q.; Wen, F.-Q.; Abe, S.; Wang, X.; Atkinson, J.J.; et al. Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L1006–L1015. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, M.; Billings, P.C.; Pacifici, M.; Leboy, P.S.; Kirsch, T. Authentic Matrix Vesicles Contain Active Metalloproteases (MMP). J. Boil. Chem. 2001, 276, 11347–11353. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M. Molecular Determinants of Metalloproteinase Substrate Specificity: Matrix Metalloproteinase Substrate Binding Domains, Modules, and Exosites. Mol. Biotechnol. 2002, 22, 51–86. [Google Scholar] [CrossRef]
- Dayer, C.; Stamenkovic, I. Recruitment of Matrix Metalloproteinase-9 (MMP-9) to the Fibroblast Cell Surface by Lysyl Hydroxylase 3 (LH3) Triggers Transforming Growth Factor-β (TGF-β) Activation and Fibroblast Differentiation. J. Boil. Chem. 2015, 290, 13763–13778. [Google Scholar] [CrossRef]
- Hattori, N.; Mochizuki, S.; Kishi, K.; Nakajima, T.; Takaishi, H.; D’Armiento, J.; Okada, Y. MMP-13 Plays a Role in Keratinocyte Migration, Angiogenesis, and Contraction in Mouse Skin Wound Healing. Am. J. Pathol. 2009, 175, 533–546. [Google Scholar] [CrossRef]
- Bracher, V.; Neuschaefer, A.; Allen, W.R. The Effect of Intra-Uterine Kerosene Infusion on the Endometrium of mares. J. Reprod. Fert. Suppl. 1991, 44, 706–707. [Google Scholar]
- Podico, G.; Canisso, I.F.; Roady, P.J.; Austin, S.M.; Carossino, M.; Balasuriya, U.; Ellerbrock, R.E.; Lima, F.S.; Ferreira-Dias, G.; Douglas, R.H. Uterine responses and equine chorionic gonadotropin concentrations after two intrauterine infusions with kerosene post early fetal loss in mares. Theriogenology 2020, 147, 202–210. [Google Scholar] [CrossRef]

| Gene (Accession Number) | Sequence 5′-3′ | Amplicon |
|---|---|---|
| COL1A2 (XM_001492939.3) | Forward: CAAGGGCATTAGGGGACACA | 196 |
| Reverse: ACCCACACTTCCATCGCTTC | ||
| MMP2 (XM_001493281.2) | Forward: TCCCACTTTGATGACGACGA | 115 |
| Reverse: TTGCCGTTGAAGAGGAAAGG | ||
| MMP9 (NM_001111302.1) | Forward: GCGGTAAGGTGCTGCTGTTC | 177 |
| Reverse: GAAGCGGTCCTGGGAGAAGT | ||
| RPL32 (XM_001492042.6) | Forward: AGCCATCTACTCGGCGTCA | 144 |
| Reverse: GTCAATGCCTCTGGGTTTCC |
| Estrous Cycle Phase | FP | MLP | ||||||
|---|---|---|---|---|---|---|---|---|
| Time of Treatment | 24 h | 48 h | 24 h | 48 h | ||||
| Treatment | Control | TGFβ1 (10 ng/mL) | Control | TGFβ1 (10 ng/mL) | Control | TGFβ1 (10 ng/mL) | Control | TGFβ1 (10 ng/mL) |
| COL1A2 transcription (fold increase) | 0.66 ± 0.06 a | 0.97 ± 0.04 b | 1.02 ± 0.86 a | 1.82 ± 0.25 b | 1.00 ± 0.24 a | 2.75 ± 0.47 b | 1.00 ± 0.24 a | 3.86 ± 0.48 b |
| COL1 protein (fold increase) | 1.34 ± 0.05 a | 1.93 ± 0.12 b | 1.37 ± 0.05 a | 1.33 ± 0.05 a | 0.71 ± 0.54 a | 1.06 ± 0.01 b | 0.58 ± 0.02 a | 0.87 ± 0.004 b |
| Time of Incubation | LDH Activity (%) |
|---|---|
| 1 h | 94.3 ± 0.9 a |
| 24 h | 92.6 ± 0.5 a |
| 48 h | 89.0 ± 0.6 b |
| Time of Treatment | 24 h | 48 h | ||
|---|---|---|---|---|
| Treatment | Control | OXT (10−7 M) | Control | OXT (10−7 M) |
| PGF2α secretion (ng/mg) | 7.3 ± 0.8 a | 16.0 ± 1.3 b | 7.6 ± 0.9 a | 14.0 ± 3.2 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, A.; Fernandes, C.; Rebordão, M.R.; Szóstek-Mioduchowska, A.; Lukasik, K.; Gawronska-Kozak, B.; Telo da Gama, L.; Skarzynski, D.J.; Ferreira-Dias, G. The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium. Animals 2020, 10, 863. https://doi.org/10.3390/ani10050863
Amaral A, Fernandes C, Rebordão MR, Szóstek-Mioduchowska A, Lukasik K, Gawronska-Kozak B, Telo da Gama L, Skarzynski DJ, Ferreira-Dias G. The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium. Animals. 2020; 10(5):863. https://doi.org/10.3390/ani10050863
Chicago/Turabian StyleAmaral, Ana, Carina Fernandes, Maria Rosa Rebordão, Anna Szóstek-Mioduchowska, Karolina Lukasik, Barbara Gawronska-Kozak, Luís Telo da Gama, Dariusz J. Skarzynski, and Graça Ferreira-Dias. 2020. "The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium" Animals 10, no. 5: 863. https://doi.org/10.3390/ani10050863
APA StyleAmaral, A., Fernandes, C., Rebordão, M. R., Szóstek-Mioduchowska, A., Lukasik, K., Gawronska-Kozak, B., Telo da Gama, L., Skarzynski, D. J., & Ferreira-Dias, G. (2020). The In Vitro Inhibitory Effect of Sivelestat on Elastase Induced Collagen and Metallopeptidase Expression in Equine Endometrium. Animals, 10(5), 863. https://doi.org/10.3390/ani10050863








