Comparison of Selenium Source in Preventing Oxidative Stress in Bovine Mammary Epithelial Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Determination of Optimal Culture Time
2.3. Determination of Time and Concentration of H2O2 Treatment
2.4. Cellular Antioxidant Response and Against Oxidative Stress with Different Se Sources
2.5. Statistical Analysis
3. Results
3.1. Determination of Optimal Culture Time
3.2. Determination of Time and Concentration of H2O2 Treatment
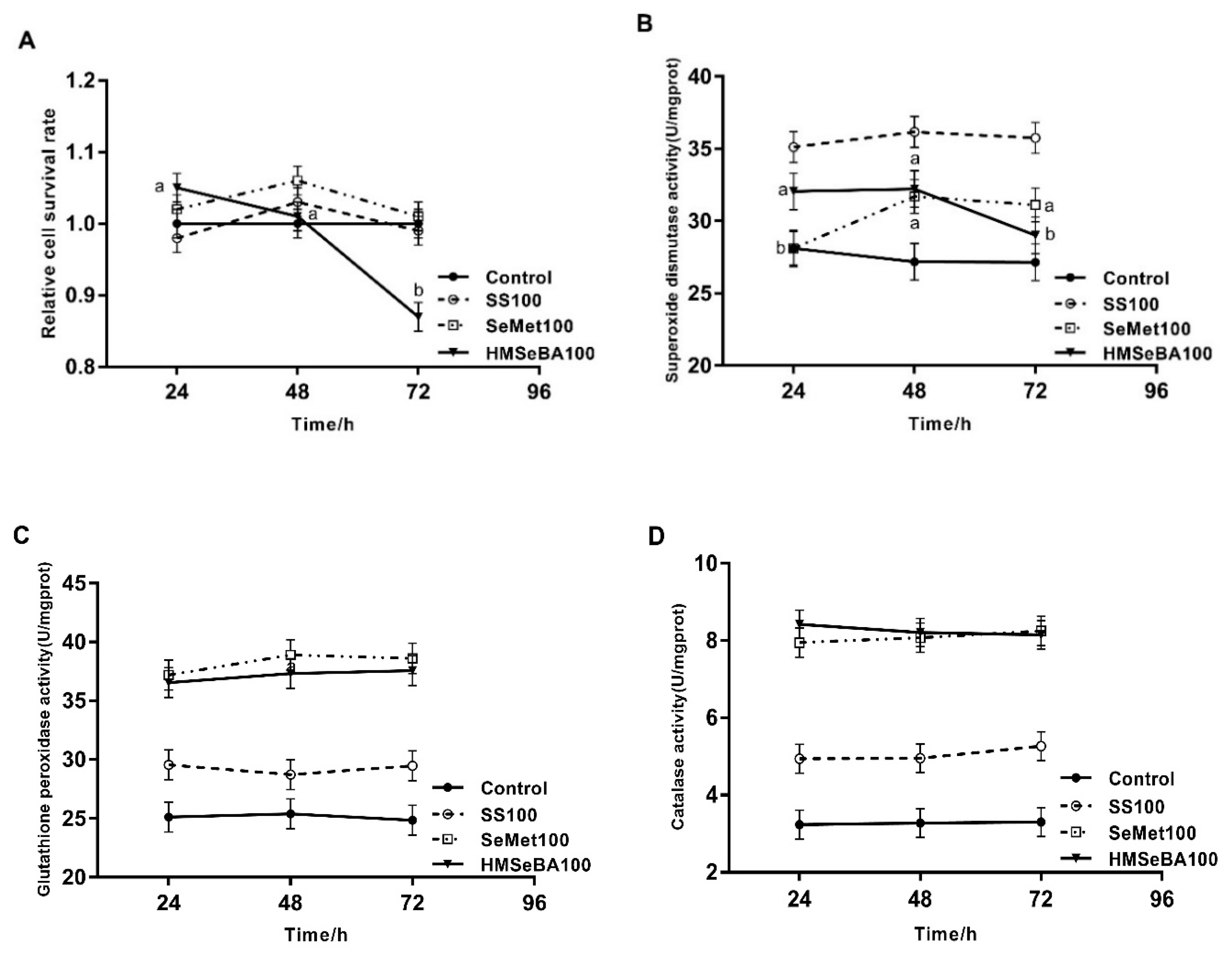
3.3. Cellular Antioxidant Response with Different Se Sources
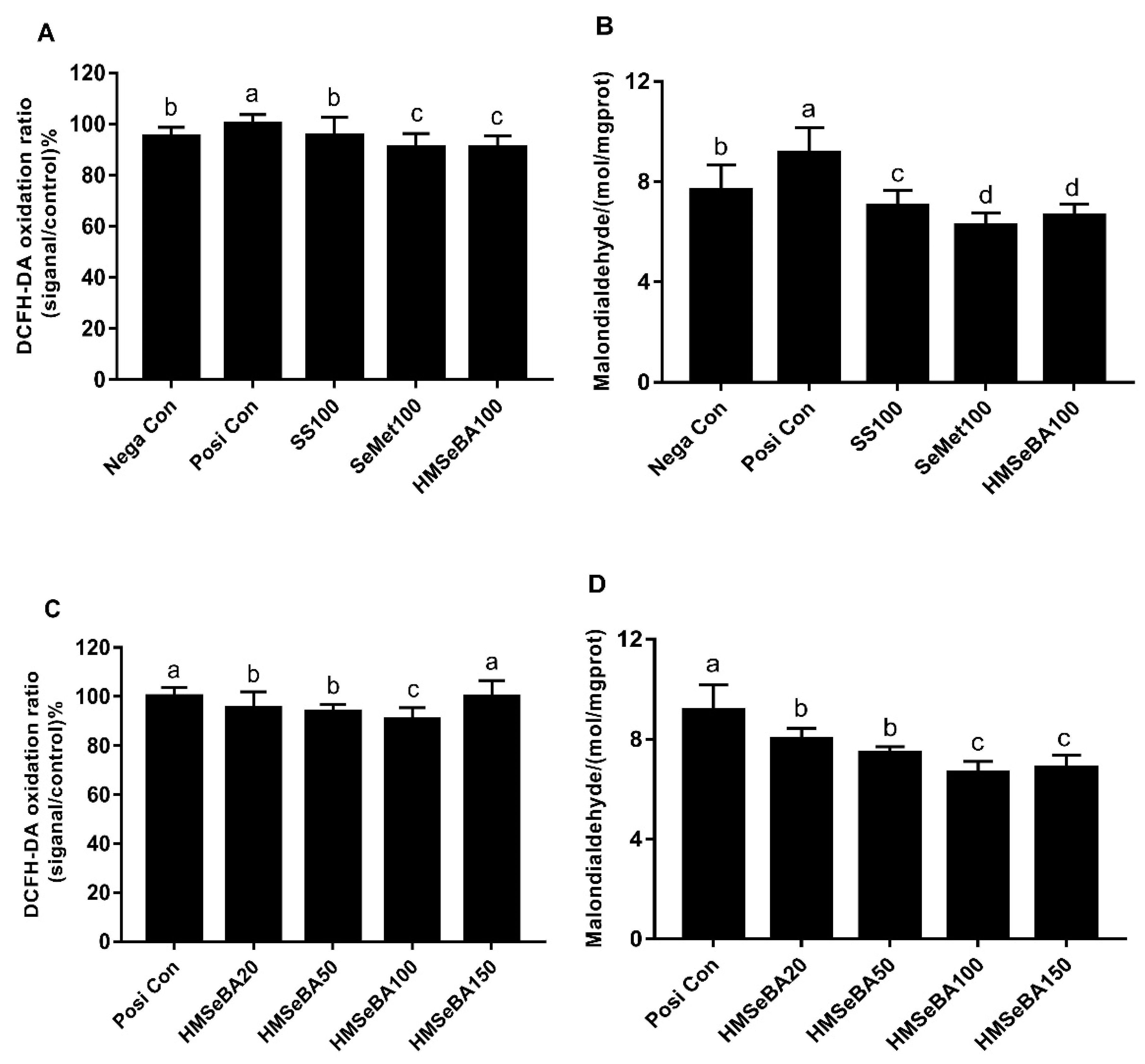
3.4. Effects of Different Se Sources on Protection of BMEC Against Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sordillo, L.M. Factors affecting mammary gland immunity and mastitis susceptibility. Livest. Prod. Sci. 2005, 97, 89–99. [Google Scholar] [CrossRef]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor-3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Ron Kincaid, P. The biological basis for selenium requirements of animals. Prof. Anim. Scien. 1995, 11, 26–29. [Google Scholar] [CrossRef]
- Malbe, M.; Klaassen, M.; Fang, W.; Myllys, V.; Vikerpuur, M.; Nyholm, K.; Sankari, S.; Suoranta, K.; Sandholm, M. Comparisons of selenite and selenium yeast feed supplements on Se-incorporation, mastitis and leucocyte function in Se-deficient dairy cows. Transbound. Emerg. Dis. 2010, 42, 111–121. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on safety and efficacy of hydroxy-analogue of selenomethionine as feed additive for all species. EFSA J. 2013, 11, 3046. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.; Liu, W.; Bu, D.P.; Liu, S.J.; Zhang, K.Z. Hydroxy-selenomethionine: A novel organic selenium source that improves antioxidant status and selenium concentrations in milk and plasma of mid-lactation dairy cows. J. Dairy Sci. 2017, 100, 9602–9610. [Google Scholar] [CrossRef]
- Sun, L.L.; Gao, S.T.; Wang, K.; Xu, J.C.; Sanz-Fernandez, M.V.; Baumgard, L.H.; Bu, D.P. Effects of source on bioavailability of selenium, antioxidant status, and performance in lactating dairy cows during oxidative stress-inducing conditions. J. Dairy Sci. 2019, 102, 311–319. [Google Scholar] [CrossRef]
- Jlali, M.; Briens, M.; Rouffineau, F.; Geraert, P.A.; Mercier, Y. Evaluation of the efficacy of 2-hydroxy-4-methylselenobutanoic acid on growth performance and tissue selenium retention in growing pigs. J. Anim. Sci. 2014, 92, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Briens, M.; Yves, M.; Friedrich, R.; Veronique, V.; Pierre-André, G. Comparative study of a new organic selenium source v. seleno-yeast and mineral selenium sources on muscle selenium enrichment and selenium digestibility in broiler chickens. Br. J. Nutr. 2013, 110, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Briens, M.; Yves, M.; Friedrich, R.; Frédéric, M.; Pierre-André, G. 2-Hydroxy-4-methylselenobutanoic acid induces additional tissue selenium enrichment in broiler chickens compared with other selenium sources. Poult. Sci. 2014, 93, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Jlali, M.; Briens, M.; Rouffineau, F.; Mercerand, F.; Geraert, P.A.; Mercier, Y. Effect of 2-hydroxy-4-methylselenobutanoic acid as a dietary selenium supplement to improve the selenium concentration of table eggs. J. Anim. Sci. 2013, 91, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Campo-Sabariz, J.; Moral-Anter, D.; Brufau, M.T.; Briens, M.; Pinloche, E.; Ferrer, R.; Martin-Venegas, R. 2-Hydroxy-(4-methylseleno)butanoic acid is used by intestinal Caco-2 cells as a source of selenium and protects against oxidative stress. J. Nutr. 2019, 149, 2191–2198. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.Q.; Bu, D.P.; Wei, H.Y.; Zhou, L.Y.; Li, F.D.; Juan, J.L. In vitro culture and characterization of a mammary epithelial cell line from Chinese holstein dairy cow. PLoS ONE 2009, 4, e7636. [Google Scholar] [CrossRef]
- Miranda, S.G.; Wang, Y.J.; Purdie, N.G.; Osborne, V.R.; Coomber, B.L.; Cant, J.P. Selenomethionine stimulates expression of glutathione peroxidase 1 and 3 and growth of bovine mammary epithelial cells in primary culture. J Dairy Sci. 2009, 92, 2670–2683. [Google Scholar] [CrossRef]
- Miranda, S.G.; Purdie, N.G.; Osborne, V.R.; Coomber, B.L.; Cant, J.P. Selenomethionine increases proliferation and reduces apoptosis in bovine mammary epithelial cells under oxidative stress. J. Dairy Sci. 2011, 94, 165–173. [Google Scholar] [CrossRef]
- Wilde, C.J.; Addey, C.V.P.; Li, P.; Fernig, D.G. Programmed cell death in bovine mammary tissue during lactation and involution. Exp. Physiol. 1997, 82, 943–953. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Korhola, M.; Vainio, A.; Edelmann, K. Selenium yeast. Ann. Clin. Res. 1986, 18, 65. [Google Scholar] [PubMed]
- Gong, J.; Ni, L.; Wang, D.; Shi, B.; Yan, S. Effect of dietary organic selenium on milk selenium concentration and antioxidant and immune status in midlactation dairy cows. Livest. Sci. 2014, 70, 84–90. [Google Scholar] [CrossRef]
- Bansal, M.P.; Tranum, K. Growth characteristics and selenium status changes of yeast cells with inorganic and organic selenium supplementation: Selenium, a chemopreventive agent. J. Med. Food 2002, 5, 85–90. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Velichko, O.A. Selenium in poultry nutrition: From sodium selenite to organic selenium sources. J.Poult. Sci. 2018, 55, 79–93. [Google Scholar] [CrossRef]
- Chu, F.F.; Esworthy, R.S.; Doroshow, J.H.; Doan, K.; Liu, X.F. Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood 1992, 79, 3233–3238. [Google Scholar] [CrossRef]
- Lindmark-Månsson, H.; Chen, J.; Paulsson, M.; Aldéna, G.; Rend, B.; Ladensteind, R.; Åkesson, B. The effect of storage and heat treatment on glutathione peroxidase in bovine milk and whey. Int. Dairy J. 2001, 11, 71–81. [Google Scholar] [CrossRef]
- Avissar, N.; Slemmon, J.R.; Palmer, I.S.; Cohen, H.J. Partial sequence of human plasma glutathione peroxidase and immunologic identification of milk glutathione peroxidase as the plasma enzyme. J. Nutr. 1991, 121, 1243–1249. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Jamwal, A.; Niyogi, S. Dose and chemical species-specific effects of selenium against arsenite toxicity in cultured hepatocytes of rainbow trout (Oncorhynchus mykiss). Metallomics 2017, 9, 744–756. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sanchez-Perez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
| Genes 1 | Accession Number | Primer Sequences |
|---|---|---|
| GPX1 | NM_174076.3 | F: CGCTGGTCCTATCCATCCC R: GCTCACATCTGGCACTTTATTC |
| GPX3 | NM_174077.5 | F: GCTGGCAAATACATCCTCTT R: GGGAAGCCCAGAATGACC |
| SOD1 | NM_174615.2 | F: AAACCAGATGACTTGGGCAGAG R: AGGCCAAACGGCTTCCAG |
| CAT | NM_001035386.2 | F: ACGGCGACTATCCTCTTA R: AAGCCAACTGTTCAACCT |
| RPS9 | NM_001101152.2 | F: ATCCCGTCCTTCATCGTGC R: CCCTTCTTGGCGTTCTTCC |
| UXT | NM_001037471.2 | F: TGGACCATCGTGACAAGGTA R: TGAAGTGTCTGGGACCACTG |
| Concentration of H2O2 (μM) | Culture Time (h) | SEM | |||
|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | ||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 0.02 |
| 10 | 0.99 | 0.99 | 0.99 | 0.98 | 0.02 |
| 30 | 0.99 | 0.99 | 0.98 | 0.98 | 0.03 |
| 50 | 0.98 | 0.97 | 0.96 | 0.96 | 0.02 |
| 100 | 0.96 | 0.95 | 0.95 | 0.94 | 0.02 |
| Items | Treatment 1 | SEM | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | SS100 | SeMet100 | HM20 | HM50 | HM100 | HM150 | Trt 2 | Source 3 | HMSeBA level 4 | |||
| Linear | Quadratic | |||||||||||
| Cell survival rate | 1 | 0.99 | 0.97 | 0.97 | 0.97 | 1.01 | 0.99 | 0.02 | 0.11 | 0.31 | 0.18 | 0.42 |
| Antioxidant enzymes activity, U/mgprot | ||||||||||||
| Glutathione peroxidase | 28.0 c | 32.9 b | 37.2 a | 32.8 | 33.4 | 36.5 a | 36.9 | 1.2 | 0.05 | <0.01 | 0.17 | 0.1 |
| Catalase | 3.26 c | 4.66 b | 7.55 a | 3.54 | 6.21 | 6.96 a | 7.31 | 0.36 | <0.01 | 0.01 | 0.42 | 0.05 |
| Superoxide dismutase | 27.9 c | 35.2 a | 30.2 b | 30.7 | 32.1 | 31.6 b | 32.6 | 1.4 | <0.01 | 0.04 | 0.4 | 0.23 |
| Gene expression fold change relative to control | ||||||||||||
| Glutathione peroxidase 1 | 1 | 1.2 | 1.2 | 1.1 | 1.2 | 1.2 | 1.2 | 0.1 | 0.43 | 0.24 | 0.48 | 0.69 |
| Glutathione peroxidase 3 | 1 b | 1.1 b | 1.4 a | 1.2 | 1.2 | 1.5 a | 1.3 | 0.1 | <0.01 | 0.02 | 0.76 | 0.04 |
| Catalase | 1 | 1 | 0.9 | 1.1 | 1.1 | 1 | 1 | 0.1 | 0.36 | 0.54 | 0.41 | 0.35 |
| Superoxide dismutase 1 | 1 | 1.1 | 1.2 | 1.2 | 1 | 1.1 | 1.1 | 0.1 | 0.24 | 0.43 | 0.77 | 0.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Wang, F.; Wu, Z.; Ma, L.; Baumrucker, C.; Bu, D. Comparison of Selenium Source in Preventing Oxidative Stress in Bovine Mammary Epithelial Cells. Animals 2020, 10, 842. https://doi.org/10.3390/ani10050842
Sun L, Wang F, Wu Z, Ma L, Baumrucker C, Bu D. Comparison of Selenium Source in Preventing Oxidative Stress in Bovine Mammary Epithelial Cells. Animals. 2020; 10(5):842. https://doi.org/10.3390/ani10050842
Chicago/Turabian StyleSun, Lingling, Fang Wang, Zhaohai Wu, Lu Ma, Craig Baumrucker, and Dengpan Bu. 2020. "Comparison of Selenium Source in Preventing Oxidative Stress in Bovine Mammary Epithelial Cells" Animals 10, no. 5: 842. https://doi.org/10.3390/ani10050842
APA StyleSun, L., Wang, F., Wu, Z., Ma, L., Baumrucker, C., & Bu, D. (2020). Comparison of Selenium Source in Preventing Oxidative Stress in Bovine Mammary Epithelial Cells. Animals, 10(5), 842. https://doi.org/10.3390/ani10050842





