Evolution of In Vitro Antimicrobial Susceptibility of Equine Clinical Isolates in France between 2016 and 2019
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
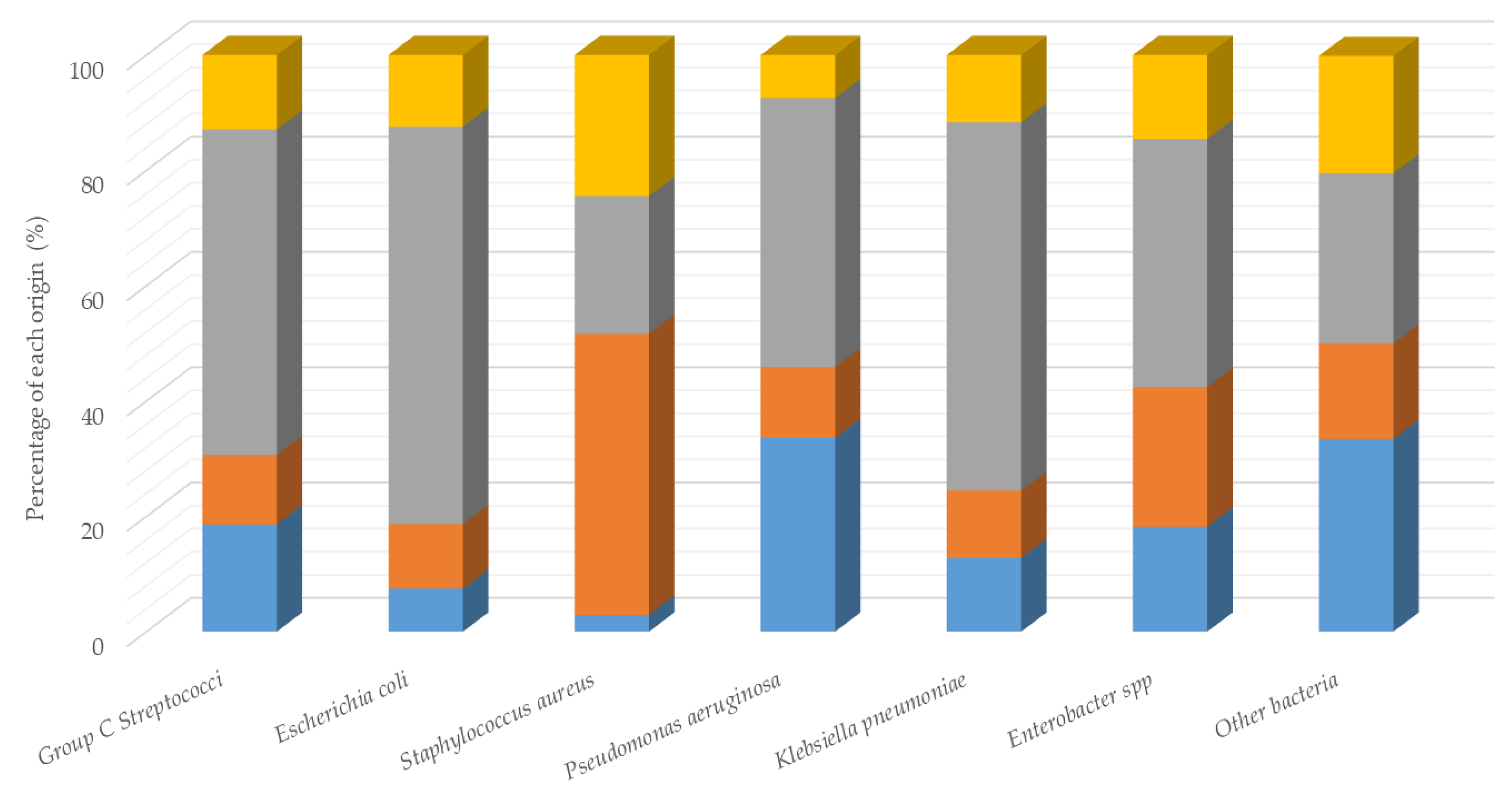
3.1. Identification and Distribution of Bacterial Isolates
3.2. Antimicrobial Susceptibility
3.2.1. GRAM Positive Bacteria
3.2.2. GRAM Negative Bacteria
3.3. Multi-Drug Resistant (MDR) Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization and Antimicrobial Resistance. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 16 March 2020).
- Food and Agriculture Organisation of the United Nations and Antimicrobial Resistance. Available online: http://www.fao.org/antimicrobial-resistance/fr/ (accessed on 16 March 2020).
- World Organisation for Animal Health-OIE and Antimicrobial Resistance. Available online: https://www.oie.int/en/for-the-media/amr/ (accessed on 16 March 2020).
- The ECOANTIBIO Plan 2012–2017. Available online: https://agriculture.gouv.fr/plan-ecoantibio-2012-2017-lutte-contre-lantibioresistance (accessed on 25 March 2020).
- ECOANTIBIO2. Available online: https://agriculture.gouv.fr/le-plan-ecoantibio-2-2017-2021 (accessed on 25 March 2020).
- Chipangura, J.K.; Chetty, T.; Kgoete, M.; Naidoo, V. Prevalence of antimicrobial resistance from bacterial culture and susceptibility record from horse samples in South Africa. Prev. Vet. Med. 2017, 148, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Greenwood, S.; Boison, J.O.; Chirino-Trejo, M.; Dowling, P.M. Bacterial isolates from equine infections in western Canada (1998–2003). Can. Vet. J. 2008, 49, 153–160. [Google Scholar] [PubMed]
- Malo, A.; Cluzel, C.; Labrecque, O.; Beauchamp, G.; Lavoie, J.P.; Leclere, M. Evolution of in vitro antimicrobial resistance in an equine hospital over 3 decades. Can. Vet. J. 2016, 57, 747–751. [Google Scholar]
- van Spijk, J.N.; Schmitt, S.; Fürst, A.E.; Schoster, A. A retrospective analysis of antimicrobial resistance in bacterial pathogens in an equine hospital (2012–2015). Schweiz. Arch. Tierheilkd. 2016, 158, 433–442. [Google Scholar] [CrossRef]
- Johns, I.C.; Adams, E.L. Trends in antimicrobial resistance in equine bacterial isolates: 1999–2012. Vet. Rec. 2015, 176, 334. [Google Scholar] [CrossRef]
- Duchesne, R.; Castagnet, S.; Maillard, K.; Petry, S.; Cattoir, V.; Giard, J.C.; Leon, A. In vitro antimicrobial susceptibility of equine clinical isolates from France, 2006–2016. J. Glob. Antimicrob. Resist. 2019, 19, 144–153. [Google Scholar] [CrossRef]
- Bourély, C.; Cazeau, G.; Jarrige, N.; Haenni, M.; Gay, E.; Leblond, A. Antimicrobial resistance in bacteria isolated from diseased horses in France. Equine Vet. J. 2020, 52, 112–119. [Google Scholar] [CrossRef]
- CA-SFM/EUCAST. Available online: https://www.sfm-microbiologie.org/2019/05/06/casfm-eucast-2019-v2/ (accessed on 3 April 2020).
- Schmiedel, J.; Falgenhauer, L.; Domann, E.; Bauerfeind, R.; Prenger-Berninghoff, E.; Imirzalioglu, C.; Chakraborty, T. Multiresistant extended-spectrum β-lactamase-producing Enterobacteriaceae from humans, companion animals and horses in central Hesse, Germany. BMC Microbiol. 2014, 12, 14–187. [Google Scholar] [CrossRef]
- EMA/CVMP/AWP/401740/2013. Reflection Paper on the Risk of Antimicrobial Resistance Transfer from Companion Animals. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-risk-antimicrobial-resistance-transfer-companion-animals_en.pdf (accessed on 25 March 2020).
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 26 March 2020).
- Ferrandière, M.; Cattier, B.; Dequin, P.F. Septicemia and meningitis due to Streptococcus zooepidemicus. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Friederichs, J.; Hungerer, S.; Werle, R.; Militz, M.; Bühren, W. Human bacterial arthritis caused by Streptococcus zooepidemicus: Report of a case. Int. J. Inf. Dis. 2010, e233–e235. [Google Scholar] [CrossRef] [PubMed]
- Björnsdóttir, S.; Harris, S.R.; Svansson, V.; Gunnarsson, E.; Gammeljord, K.; Steward, K.F.; Newton, J.R.; Robinson, C.; Charbonneau, A.R.; Parkhill, J.; et al. Genomic dissection of an Icelandic epidemic of respiratory disease in horses and associated zoonotic cases. mBio 2017, 8, e00826-17. [Google Scholar] [CrossRef]
- Waller, A.S. Strangles: A pathogenic legacy of the war horse. Vet. Rec. 2016, 178, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.S. Science-in-brief: Streptococcus zooepidemicus: A versatile opportunistic pathogen that hedges its bets in horses. Equine Vet. J. 2017, 49, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.G.; Timoney, J.F.; Newton, J.R.; Hines, M.T.; Waller, A.S.; Buchanan, B.R. Streptococcus equi Infections in Horses: Guidelines for Treatment, Control, and Prevention of Strangles-Revised Consensus Statement. J. Vet. Intern Med. 2018, 32, 633–647. [Google Scholar] [CrossRef]
- Petersen, M.R.; Skive, B.; Christoffersen, M.; Lu, K.; Nielsen, J.M.; Troedsson, M.H.; Bojesen, A.M. Activation of persistent Streptococcus equi subspecies zooepidemicus in mares with subclinical endometritis. Vet. Microbiol. 2015, 179, 119–125. [Google Scholar] [CrossRef]
- Muranaka, M.; Yamanaka, T.; Katayama, Y.; Niwa, H.; Oku, K.; Matsumura, T.; Oyamada, T. Time-related Pathological Changes in Horses Experimentally Inoculated with Equine Influenza A Virus. J. Eq. Sci. 2012, 23, 17–26. [Google Scholar] [CrossRef]
- Paillot, R.; Prowse, L.; Montesso, F.; Huang, C.M.; Barnes, H.; Escala, J. Whole inactivated equine influenza vaccine: Efficacy against a representative clade 2 equine influenza virus, IFNgamma synthesis and duration of humoral immunity. Vet. Microbiol. 2013, 162, 396–407. [Google Scholar] [CrossRef]
- Guérin, F.; Fines-Guyon, M.; Meignen, P.; Delente, G.; Fondrinier, C.; Bourdon, N.; Cattoir, V.; Léon, A. Nationwide molecular epidemiology of methicillin-resistant Staphylococcus aureus responsible for horse infections in France. BMC Microbiol. 2017, 17, 104. [Google Scholar] [CrossRef]
- Theelen, M.J.; Wilson, W.D.; Edman, J.M.; Magdesian, K.G.; Kass, P.H. Temporal trends in in vitro antimicrobial susceptibility patterns of bacteria isolated from foals with sepsis: 1979-2010. Equine Vet. J. 2014, 46, 161–168. [Google Scholar] [CrossRef]
- Davis, H.A.; Stanton, M.B.; Thungrat, K.; Boothe, D.M. Uterine bacterial isolates from mares and their resistance to antimicrobials: 8296 cases (2003–2008). J. Am. Vet. Med. Assoc. 2013, 242, 977–983. [Google Scholar] [CrossRef] [PubMed]
| Antibiotic Category | Year | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|
| (Number of Strains) | (692) | (598) | (454) | (374) | ||
| Penicillins | PEN | 0.1 | 0.3 | 0 | 0 | |
| AMX ** (p = 0.016) | 0.7 | 0.2 | 0 | 0 | ||
| OXA | 0.1 | 0.3 | 0 | 0 | ||
| AMC ** (p = 0.011) | 0.7 | 0.0 * | 0 | 0 | ||
| (p = 0.037) | ||||||
| Cephalosporins | 3rd | CEF ** (p = 0.049) | 0.4 | 0 | 0 | 0 |
| 4th | CEQ | 0.1 | 0 | 0 | 0 | |
| Aminoglycosides | STR HC ** (p < 0.0001) | 5.5 | 3.8 | 0.0 * | 0.5 | |
| KAN HC ** (p < 0.0001) | 5.3 | 4.5 | 0.2 * | 0 | ||
| (p < 0.0001) | ||||||
| GENHC | 0.6 | 1.2 | 0.2 | 0 | ||
| Tetracycline | TET ** (p < 0.0001) | 82.1 | 87.0 * | 71.6 * | 58.6 * | |
| (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | ||||
| Macrolides | ERY ** (p < 0.0001) | 11.1 | 22.1* | 10.3 * | 2.1 * | |
| (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | ||||
| Rifampicin | RIF | 15.5 | 47.8 * | 22.0 * | 16.6 * | |
| (p < 0.0001) | (p < 0.0001) | (p = 0.049) | ||||
| Sulphonamides | SXT ** (p < 0.0001) | 4.8 | 15.6 * | 0.7 * | 0 | |
| (p < 0.0001) | (p < 0.0001) | |||||
| Antibiotic Category | Year | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|
| (Number of Strains) | (139) | (118) | (118) | (107) | ||
| Penicillins | PEN | 43.9 | 47.5 | 47.5 | 56.1 | |
| AMX | 43.2 | 46.6 | 47.5 | 55.1 | ||
| OXA ** (p = 0.045) | 15.8 | 22.9 | 22.0 | 27.1 | ||
| AMC ** (p = 0.021) | 17.3 | 22.9 | 22.6 | 28.0 | ||
| Cephalosporins | 2nd | FOX | 17.3 | 22.9 | 22.6 | 28.0 |
| 3rd | CEF ** (p = 0.045) | 17.3 | 22.9 | 22.6 | 28.0 | |
| 4th | CEQ ** (p = 0.031) | 17.3 | 22.9 | 22.6 | 28.0 | |
| Aminoglycosides | STR | 20.9 | 11.0 * | 11.0 | 17.8 | |
| (p = 0.033) | ||||||
| KAN ** (p = 0.003) | 23.0 | 31.4 | 32.2 | 41.1 | ||
| GEN ** (p = 0.001) | 21.6 | 30.5 | 32.2 | 41.1 | ||
| Tetracycline | TET ** (p = 0.01) | 27.3 | 35.6 | 35.6 | 43.9 | |
| Macrolides | ERY | 5.8 | 5.1 | 4.2 | 8.4 | |
| Rifampicin | RIF ** (p < 0.001) | 2.9 | 11.0 * | 15.3 | 16.8 | |
| (p = 0.009) | ||||||
| Sulphonamides | SXT ** (p = 0.008) | 6.5 | 3.4 | 12.7 * | 14.0 | |
| (p = 0.008) | ||||||
| Fluoroquinolones | ENO ** (p = 0.002) | 1.4 | 2.5 | 5.9 | 9.3 | |
| MAR ** (p = 0.002) | 1.4 | 1.7 | 5.9 | 9.3 | ||
| Antibiotic Category | Year | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|
| (Number of Strains) | (344) | (325) | (372) | (341) | ||
| Penicillins | AMX ** (p = 0.02) | 39.5 | 33.2 | 27.4 | 32.8 | |
| AMC ** (p < 0.0001) | 31.4 | 21.2 * | 18.3 | 19.4 | ||
| (p = 0.003) | ||||||
| Cephalosporins | 3rd | CEF | 6.1 | 5.8 | 6.2 | 3.5 |
| 4th | CEQ | 5.8 | 5.8 | 6.2 | 3.8 | |
| Aminoglycosides | STR ** (p = 0.005) | 33.1 | 26.2* | 28.2 | 43.4 * | |
| (p = 0.048) | (p < 0.0001) | |||||
| KAN | 9.0 | 8.9 | 9.1 | 11.1 | ||
| GEN | 6.1 | 7.1 | 8.9 | 6.7 | ||
| Tetracycline | TET | 20.6 | 21.2 | 23.1 | 22.6 | |
| Sulphonamides | SXT | 31.4 | 28.3 | 28.8 | 32.6 | |
| Quinolones/Fluoroquinolones | NAL | 4.9 | 3.4 | 5.4 | 3.2 | |
| FLU | 4.9 | 3.4 | 5.4 | 3.2 | ||
| ENO | 3.2 | 3.4 | 2.4 | 3.2 | ||
| MAR | 2.9 | 3.4 | 2.4 | 2.9 | ||
| Antibiotic Category | Year | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|
| (Number of Strains) | (59) | (70) | (75) | (64) | ||
| Cephalosporin | 4th | CEQ | 11.9 | 14.3 | 14.7 | 12.5 |
| Aminoglycosides | GEN | 10.2 | 8.6 | 14.7 | 10.9 | |
| Fluoroquinolones | MAR | 1.7 | 0.0 | 0.0 | 4.7 | |
| Antibiotic Category | Year | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|
| (Number of Strains) | (31) | (33) | (56) | (60) | ||
| Penicillins | AMC | 12.9 | 42.4 * | 16.1 * | 10.0 | |
| (p = 0.009) | (p = 0.006) | |||||
| Cephalosporins | 3rd | CEF | 9.7 | 21.2 | 5.4 * | 10.0 |
| (p = 0.022) | ||||||
| 4th | CEQ | 9.7 | 21.2 | 5.4 * | 10.0 | |
| (p = 0.022) | ||||||
| Aminoglycosides | STR ** (p = 0.008) | 29.0 | 48.5 | 25.0 * | 13.3 | |
| (p = 0.024) | ||||||
| KAN | 3.2 | 12.1 | 7.1 | 6.7 | ||
| GEN | 6.5 | 21.2 | 7.1 | 6.7 | ||
| Tetracycline | TET ** (p = 0.017) | 25.8 | 48.5 | 25.0 * | 13.3 | |
| (p = 0.024) | ||||||
| Sulphonamides | SXT ** (p=0.006) | 32.3 | 51.5 | 26.8 * | 15.0 | |
| (p = 0.019) | ||||||
| Quinolones/Fluoroquinolones | NAL ** (p = 0.049) | 19.4 | 21.2 | 8.9 | 8.3 | |
| FLU | 12.9 | 21.2 | 8.9 | 8.3 | ||
| ENO | 9.7 | 18.2 | 3.6 * | 5.0 | ||
| (p = 0.02) | ||||||
| MAR | 3.2 | 9.1 * | 1.8 | 3.3 | ||
| (p = 0.02) | ||||||
| Antibiotic Category | Year | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|
| (Number of Strains) | (38) | (46) | (52) | (29) | ||
| Cephalosporins | 3rd | CEF | 15.8 | 30.4 | 34.6 | 27.6 |
| 4th | CEQ | 13.2 | 21.7 | 21.2 | 10.3 | |
| Aminoglycosides | STR ** (p = 0.022) | 23.7 | 50.0 * | 44.2 | 55.2 | |
| (p = 0.025) | ||||||
| KAN ** (p = 0.044) | 18.4 | 41.3 * | 36.5 | 44. | ||
| (p = 0.024) | 8 | |||||
| GEN | 18.4 | 45.7 * | 42.3 | 41.4 | ||
| (p = 0.008) | ||||||
| Tetracycline | TET | 21.1 | 32.6 | 36.5 | 37.9 | |
| Sulphonamides | SXT | 21.1 | 45.7 | 42.3 | 48.3 | |
| Quinolones/Fluoroquinolones | NAL | 21.1 | 17.4 | 23.1 | 27.6 | |
| FLU | 21.1 | 17.4 | 23.1 | 27.6 | ||
| ENO | 7.9 | 8.7 | 13.5 | 10.3 | ||
| MAR | 2.6 | 4.3 | 7.7 | 6.9 | ||
| Streptococcus (Group C) ** (p < 0.001) | Staphylococcus aureus ** (p = 0.029) | E. coli | Klebsiella pneumoniae ** (p = 0.001) | Enterobacter spp. ** (p = 0.048) | |
|---|---|---|---|---|---|
| 2016 | 10.7 | 24.5 | 22.7 | 38.7 | 26.3 |
| 2017 | 18.9 | 31.4 | 21.2 | 51.5 | 45.6 |
| 2018 | 3.1 | 33.1 | 21.8 | 26.8 | 44.2 |
| 2019 | 0.5 | 37.4 | 22.6 | 11.7 | 51.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Léon, A.; Castagnet, S.; Maillard, K.; Paillot, R.; Giard, J.-C. Evolution of In Vitro Antimicrobial Susceptibility of Equine Clinical Isolates in France between 2016 and 2019. Animals 2020, 10, 812. https://doi.org/10.3390/ani10050812
Léon A, Castagnet S, Maillard K, Paillot R, Giard J-C. Evolution of In Vitro Antimicrobial Susceptibility of Equine Clinical Isolates in France between 2016 and 2019. Animals. 2020; 10(5):812. https://doi.org/10.3390/ani10050812
Chicago/Turabian StyleLéon, Albertine, Sophie Castagnet, Karine Maillard, Romain Paillot, and Jean-Christophe Giard. 2020. "Evolution of In Vitro Antimicrobial Susceptibility of Equine Clinical Isolates in France between 2016 and 2019" Animals 10, no. 5: 812. https://doi.org/10.3390/ani10050812
APA StyleLéon, A., Castagnet, S., Maillard, K., Paillot, R., & Giard, J.-C. (2020). Evolution of In Vitro Antimicrobial Susceptibility of Equine Clinical Isolates in France between 2016 and 2019. Animals, 10(5), 812. https://doi.org/10.3390/ani10050812






