Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities
Simple Summary
Abstract
1. Introduction
2. Heat Stress Assessment and Principals of Mitigation
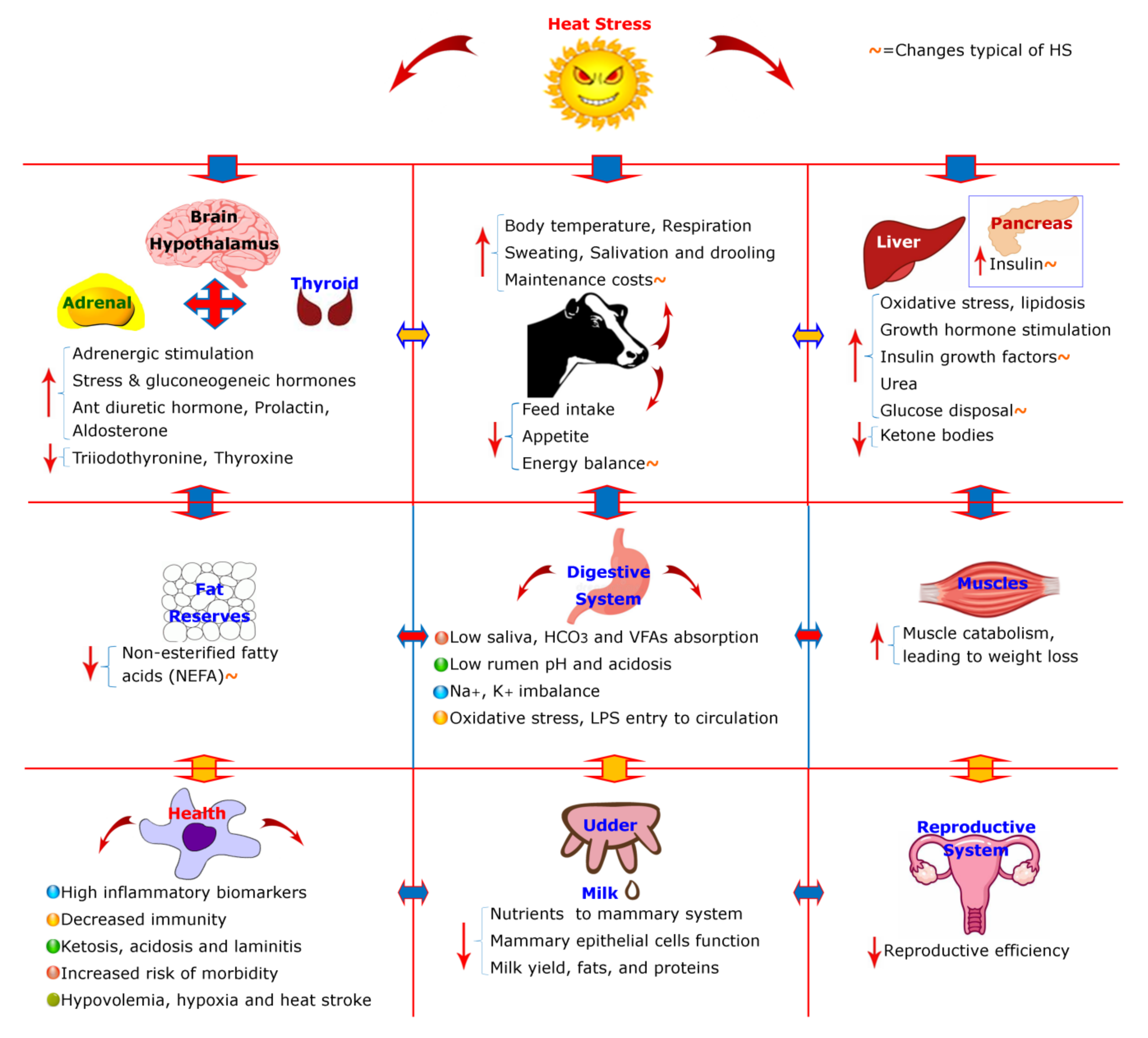
3. Physiological and Behavioral Modifications of the Cattle
4. Hypothalamus–Pituitary–Adrenal Axis and Cellular Level Changes
5. Negative Energy Balance (NEBAL) in Lactating Cows
6. Negative Energy Balance (NEBAL) Typical to Heat Stress
6.1. Insulin and Glucose Axis of Heat Stress
6.2. Insulin and Lipids Metabolism Axis of Heat Stress
6.3. Protein Metabolism in Heat Stress
7. Effects on Milk Production
8. Mechanisms Underlying Lactation Changes
9. Rumen and Gut-Associated Changes
10. Health Consequences of Heat Stress
11. Consequences for Reproductive System
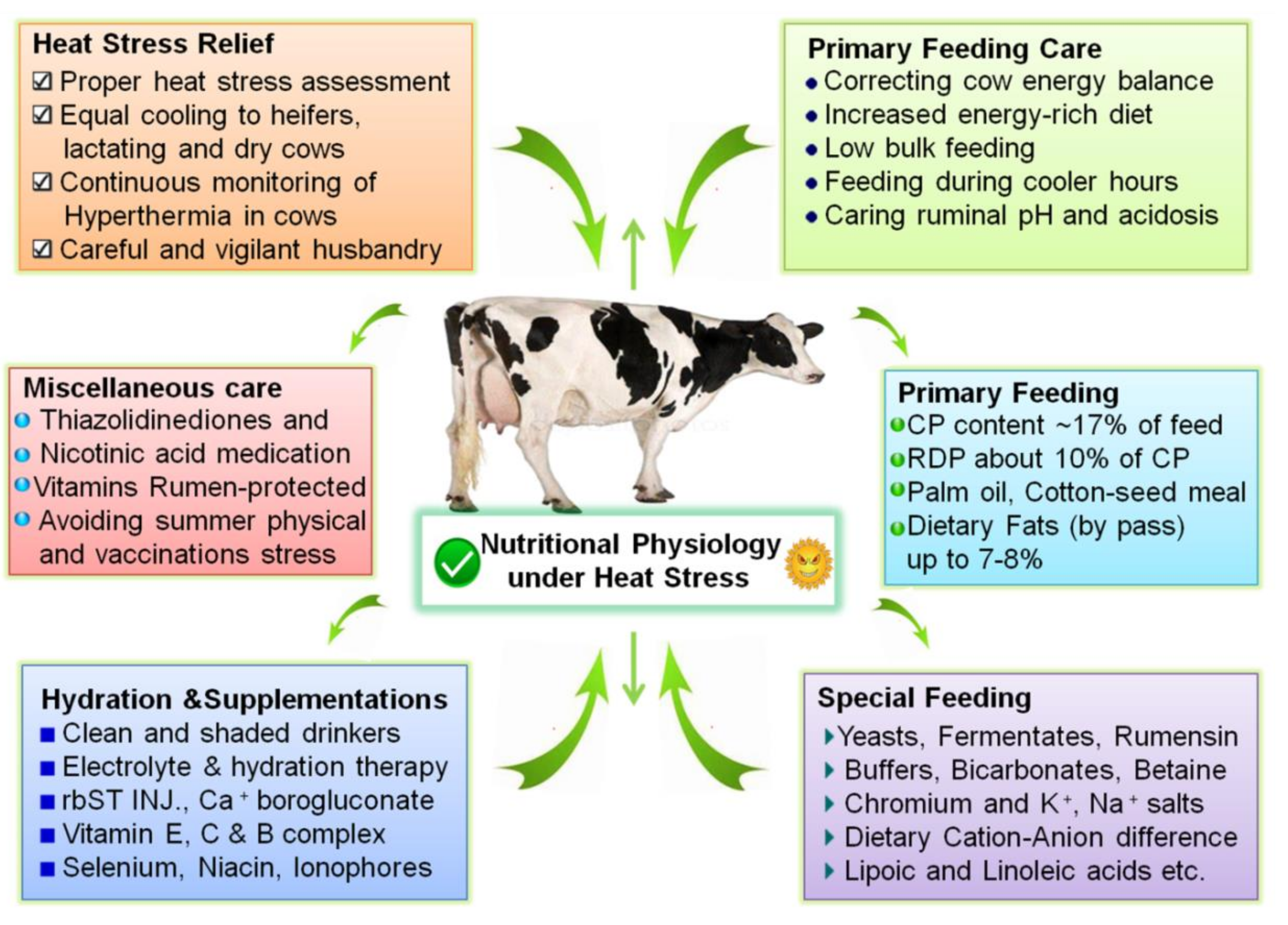
12. Mitigation Strategies
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Bouraoui, R.; Lahmar, M.; Majdoub, A.; Djemali, M.; Belyea, R. The relationship of temperature-humidity index with milk production of dairy cows in a Mediterranean climate. Anim. Res. 2002, 51, 479–491. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.V. Heat Stress Interaction with Shade and Cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Berman, A. An overview of heat stress relief with global warming in perspective. Int. J. Biometeorol. 2019, 63, 493–498. [Google Scholar] [CrossRef]
- Ray, D.; Correa-Calderon, A.; Armstrong, D.; Enns, M.; DeNise, S.; Howison, C. Thermoregulatory responses of Holstein and Brown Swiss Heat-Stressed dairy cows to two different cooling systems. Int. J. Biometeorol. 2004, 48, 142–148. [Google Scholar] [CrossRef]
- Bohmanova, J.; Misztal, I.; Cole, J.B. Temperature-Humidity Indices as Indicators of Milk Production Losses due to Heat Stress. J. Dairy Sci. 2007, 90, 1947–1956. [Google Scholar] [CrossRef]
- Wildridge, A.M.; Thomson, P.C.; Garcia, S.C.; John, A.J.; Jongman, E.C.; Clark, C.E.F.; Kerrisk, K.L. Short communication: The effect of temperature-humidity index on milk yield and milking frequency of dairy cows in pasture-based automatic milking systems. J. Dairy Sci. 2018, 101, 4479–4482. [Google Scholar] [CrossRef]
- Ammer, S.; Lambertz, C.; Von Soosten, D.; Zimmer, K.; Meyer, U.; Dänicke, S.; Gauly, M. Impact of diet composition and temperature-humidity index on water and dry matter intake of high-yielding dairy cows. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018, 102, 103–113. [Google Scholar] [CrossRef]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Legrand, A.; Schütz, K.E.; Tucker, C.B. Using water to cool cattle: Behavioral and physiological changes associated with voluntary use of cow showers. J. Dairy Sci. 2011, 94, 3376–3386. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.L.; Wall, E. Dairy cattle in a temperate climate: The effects of weather on milk yield and composition depend on management. Animal 2015, 9, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Carabaño, M.J.; Logar, B.; Bormann, J.; Minet, J.; Vanrobays, M.-L.; Díaz, C.; Tychon, B.; Gengler, N.; Hammami, H. Modeling heat stress under different environmental conditions. J. Dairy Sci. 2016, 99, 3798–3814. [Google Scholar] [CrossRef]
- Herbut, P.; Angrecka, S. Relationship between THI level and dairy cows’ behaviour during summer period. Ital. J. Anim. Sci. 2018, 17, 226–233. [Google Scholar] [CrossRef]
- Heinicke, J.; Hoffmann, G.; Ammon, C.; Amon, B.; Amon, T. Effects of the daily heat load duration exceeding determined heat load thresholds on activity traits of lactating dairy cows. J. Therm. Biol. 2018, 77, 67–74. [Google Scholar] [CrossRef]
- Pinto, S.; Hoffmann, G.; Ammon, C.; Amon, T. Critical THI thresholds based on the physiological parameters of lactating dairy cows. J. Therm. Biol. 2020, 88, 102523. [Google Scholar] [CrossRef]
- West, J.W.; Mullinix, B.G.; Bernard, J.K. Effects of Hot, Humid Weather on Milk Temperature, Dry Matter Intake, and Milk Yield of Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 232–242. [Google Scholar] [CrossRef]
- Herbut, P.; Angrecka, S.; Godyń, D. Effect of the duration of high air temperature on cow’s milking performance in moderate climate conditions. Ann. Anim. Sci. 2018, 18, 195–207. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Chen, X.; Lu, Y.; Wang, D. Effects of heat stress on body temperature, milk production, and reproduction in dairy cows: A novel idea for monitoring and evaluation of heat stress—A review. Asian-Australas. J. Anim. Sci. 2019, 32, 1332–1339. [Google Scholar] [CrossRef]
- Schüller, L.K.; Heuwieser, W. Measurement of heat stress conditions at cow level and comparison to climate conditions at stationary locations inside a dairy barn. J. Dairy Res. 2016, 83, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, M.; Sadraoui, R.; Najar, T.; M’rad, M.B.; Rejeb, M.; Sadraoui, R.; Najar, T.; M’rad, M. Ben A Complex Interrelationship between Rectal Temperature and Dairy Cows’ Performance under Heat Stress Conditions. Open J. Anim. Sci. 2016, 06, 24–30. [Google Scholar] [CrossRef]
- Godyń, D.; Herbut, P.; Angrecka, S. Measurements of peripheral and deep body temperature in cattle—A review. J. Therm. Biol. 2019, 79, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, M.; Balboula, A.Z.; Yamanaka, K.; Takahashi, M. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black cow. Anim. Sci. J. 2012, 83, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Bok, J.D.; Lee, H.J.; Lee, H.G.; Kim, D.; Lee, I.; Kang, S.K.; Choi, Y.J. Body Temperature Monitoring Using Subcutaneously Implanted Thermo-loggers from Holstein Steers. Asian-Australas. J. Anim. Sci. 2016, 29, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ipema, A.H.; Goense, D.; Hogewerf, P.H.; Houwers, H.W.J.; Van Roest, H. Pilot study to monitor body temperature of dairy cows with a rumen bolus. Comput. Electron. Agric. 2008, 64, 49–52. [Google Scholar] [CrossRef]
- Peng, D.; Chen, S.; Li, G.; Chen, J.; Wang, J.; Gu, X. Infrared thermography measured body surface temperature and its relationship with rectal temperature in dairy cows under different temperature-humidity indexes. Int. J. Biometeorol. 2019, 63, 327–336. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef]
- Yano, M.; Shimadzu, H.; Endo, T. Modelling temperature effects on milk production: A study on Holstein cows at a Japanese farm. Springerplus 2014, 3, 129. [Google Scholar] [CrossRef]
- Li, G.; Chen, S.; Chen, J.; Peng, D.; Gu, X. Predicting rectal temperature and respiration rate responses in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2020. [Google Scholar] [CrossRef]
- Ji, B.; Banhazi, T.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. Modelling of heat stress in a robotic dairy farm. Part 2: Identifying the specific thresholds with production factors. Biosyst. Eng. 2019. [Google Scholar] [CrossRef]
- Amamou, H.; Beckers, Y.; Mahouachi, M.; Hammami, H. Thermotolerance indicators related to production and physiological responses to heat stress of holstein cows. J. Therm. Biol. 2019, 82, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Carabaño, M.J.; Ramón, M.; Menéndez-Buxadera, A.; Molina, A.; Díaz, C. Selecting for heat tolerance. Anim. Front. 2019, 9, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.D. Bioclimates and Livestock. In Bioclimatology and the Adaptation of Livestock, Chap. 1; Johnson, H.D., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; pp. 3–16. [Google Scholar]
- Horowitz, M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 475–483. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef]
- Gaughan, J.; Lacetera, N.; Valtorta, S.E.; Khalifa, H.H.; Hahn, L.; Mader, T. Response of Domestic Animals to Climate Challenges. In Biometeorology for Adaptation to Climate Variability and Change; Springer: Dordrecht, The Netherlands, 2009; pp. 131–170. [Google Scholar] [CrossRef]
- Ferrell, C.L.; Jenkins, T.G. Cow type and the nutritional environment: Nutritional aspects. J. Anim. Sci. 1985, 61, 725–741. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019. [Google Scholar] [CrossRef]
- Robinson, J.B.; Ames, D.R.; Milliken, G.A. Heat Production of Cattle Acclimated to Cold, Thermoneutrality and Heat When Exposed to Thermoneutrality and Heat Stress. J. Anim. Sci. 1986, 62, 1434–1440. [Google Scholar] [CrossRef]
- Fuquay, J.W. Heat stress as it affects animal production. J. Anim. Sci. 1981, 52, 164–174. [Google Scholar] [CrossRef]
- Fox, D.G.; Tylutki, T.P. Accounting for the Effects of Environment on the Nutrient Requirements of Dairy Cattle. J. Dairy Sci. 1998, 81, 3085–3095. [Google Scholar] [CrossRef]
- Von Borell, E.H. The biology of stress and its application to livestock housing and transportation assessment. J. Anim. Sci. 2001, 79, E260. [Google Scholar] [CrossRef]
- Elvinger, F.; Natzke, R.P.; Hansen, P.J. Interactions of Heat Stress and Bovine Somatotropin Affecting Physiology and Immunology of Lactating Cows. J. Dairy Sci. 1992, 75, 449–462. [Google Scholar] [CrossRef]
- Starkie, R.L.; Hargreaves, M.; Rolland, J.; Febbraio, M.A. Heat stress, cytokines, and the immune response to exercise. Brain. Behav. Immun. 2005, 19, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, J.B. Basic Principles Involved in Adaption of Livestock to Climate Change. In Environmental Stress and Amelioration in Livestock Production; Springer: Berlin/Heidelberg, Germany, 2012; pp. 245–261. [Google Scholar]
- McGuire, M.A.; Beede, D.K.; Collier, R.J.; Buonomo, F.C.; DeLorenzo, M.A.; Wilcox, C.J.; Huntington, G.B.; Reynolds, C.K. Effects of acute thermal stress and amount of feed intake on concentrations of somatotropin, insulin-like growth factor (IGF)-I and IGF-II, and thyroid hormones in plasma of lactating Holstein cows. J. Anim. Sci. 1991, 69, 2050–2056. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Kim, J.W.; Van Amburgh, M.E.; Ehrhardt, R.A.; Frank, S.J.; Boisclair, Y.R. Effect of nutrition on the GH responsiveness of liver and adipose tissue in dairy cows. J. Endocrinol. 2007, 195, 49–58. [Google Scholar] [CrossRef]
- Qu, M.; Wei, S.; Chen, Z.; Wang, G.; Zheng, Y.; Yan, P. Differences of hormones involved in adipose metabolism and lactation between high and low producing Holstein cows during heat stress. Anim. Nutr. 2015, 1, 339–343. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Kim, J.W.; Collier, R.J.; Crooker, B.A.; Boisclair, Y.R.; Baumgard, L.H.; Rhoads, R.P. Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J. Dairy Sci. 2010, 93, 170–179. [Google Scholar] [CrossRef]
- Horowitz, M. Heat acclimation: Phenotypic plasticity and cues to the underlying molecular mechanisms. J. Therm. Biol. 2001, 26, 357–363. [Google Scholar] [CrossRef]
- Yousef, M.K.; Johnson, H.D. Calorigenesis of Cattle as Influenced by Growth Hormone and Environmental Temperature. J. Anim. Sci. 1966, 25, 1076–1082. [Google Scholar] [CrossRef]
- El-Nouty, F.D.; Elbanna, I.M.; Davis, T.P.; Johnson, H.D. Aldosterone and ADH response to heat and dehydration in cattle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 249–255. [Google Scholar] [CrossRef]
- Collier, R.J.; Beede, D.K.; Thatcher, W.W.; Israel, L.A.; Wilcox, C.J. Influences of Environment and Its Modification on Dairy Animal Health and Production. J. Dairy Sci. 1982, 65, 2213–2227. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Baile, C.A.; Forbes, J.M. Control of feed intake and regulation of energy balance in ruminants. Physiol. Rev. 1974, 54, 160–214. [Google Scholar] [CrossRef]
- Volloch, V.; Rits, S. A natural extracellular factor that induces Hsp72, inhibits apoptosis, and restores stress resistance in aged human cells. Exp. Cell Res. 1999, 253, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Mizzen, L.A.; Welch, W.J. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J. Cell Biol. 1988, 106, 1105–1116. [Google Scholar] [CrossRef]
- Kavanagh, K.; Flynn, D.M.; Jenkins, K.A.; Zhang, L.; Wagner, J.D. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E894–E901. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.E.; Kay, J.K.; Collier, R.J.; VanBaale, M.J.; Baumgard, L.H. Effect of supplemental conjugated linoleic acids on heat-stressed brown Swiss and Holstein cows. J. Dairy Sci. 2005, 88, 1732–1740. [Google Scholar] [CrossRef]
- Garverick, H.A.; Harris, M.N.; Vogel-Bluel, R.; Sampson, J.D.; Bader, J.; Lamberson, W.R.; Spain, J.N.; Lucy, M.C.; Youngquist, R.S. Concentrations of nonesterified fatty acids and glucose in blood of periparturient dairy cows are indicative of pregnancy success at first insemination. J. Dairy Sci. 2013, 96, 181–188. [Google Scholar] [CrossRef]
- Bauman, D.E.; Vernon, R.G. Effects of Exogenous Bovine Somatotropin on Lactation. Annu. Rev. Nutr. 1993, 13, 437–461. [Google Scholar] [CrossRef]
- Galster, A.D.; Clutter, W.E.; Cryer, P.E.; Collins, J.A.; Bier, D.M. Epinephrine plasma thresholds for lipolytic effects in man: Measurements of fatty acid transport with [l-13C]palmitic acid. J. Clin. Invest. 1981, 67, 1729–1738. [Google Scholar] [CrossRef]
- Randle, P.J. Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes. Metab. Rev. 1998, 14, 263–283. [Google Scholar] [CrossRef]
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Kim, J.W.; Leury, B.J.; Baumgard, L.H.; Segoale, N.; Frank, S.J.; Bauman, D.E.; Boisclair, Y.R. Insulin Increases the Abundance of the Growth Hormone Receptor in Liver and Adipose Tissue of Periparturient Dairy Cows. J. Nutr. 2004, 134, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Melendez, P.; Marin, M.P.; Robles, J.; Rios, C.; Duchens, M.; Archbald, L. Relationship between serum nonesterified fatty acids at calving and the incidence of periparturient diseases in Holstein dairy cows. Theriogenology 2009, 72, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Sundrum, A. Metabolic Disorders in the Transition Period Indicate that the Dairy Cows’ Ability to Adapt is Over stressed. Animals 2015, 5, 978–1020. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- O’Brien, M.D.; Rhoads, R.P.; Sanders, S.R.; Duff, G.C.; Baumgard, L.H. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 2010, 38, 86–94. [Google Scholar] [CrossRef]
- Ahmed, N.; Berridge, M.V. Transforming oncogenes regulate glucose transport by increasing transporter affinity for glucose: Contrasting effects of oncogenes and heat stress in a murine marrow-derived cell line. Life Sci. 1998, 63, 1887–1903. [Google Scholar] [CrossRef]
- Tao, S.; Thompson, I.M.; Monteiro, A.P.A.; Hayen, M.J.; Young, L.J.; Dahl, G.E. Effect of cooling heat-stressed dairy cows during the dry period on insulin response. J. Dairy Sci. 2012, 95, 5035–5046. [Google Scholar] [CrossRef]
- Ferrannini, E.; Camastra, S.; Coppack, S.W.; Fliser, D.; Golay, A.; Mitrakou, A. Insulin action and non-esterified fatty acids. Proc. Nutr. Soc. 1997, 56, 753–761. [Google Scholar] [CrossRef]
- Dunshea, F.R.; Bell, A.W.; Trigg, T.E. Non-esterified fatty acid and glycerol kinetics and fatty acid re-esterification in goats during early lactation. Br. J. Nutr. 1990, 64, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K. ADSA foundation scholar award: Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Streffer, C. Aspects of metabolic change after hyperthermia. Recent Results Cancer Res. 1988, 107, 7–16. [Google Scholar] [PubMed]
- Zhou, Z.; Loor, J.J.; Piccioli-Cappelli, F.; Librandi, F.; Lobley, G.E.; Trevisi, E. Circulating amino acids in blood plasma during the peripartal period in dairy cows with different liver functionality index. J. Dairy Sci. 2016, 99, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian-Australas. J. Anim. Sci. 2018, 31, 47–53. [Google Scholar] [CrossRef]
- Bell, A.W.; Burhans, W.S.; Overton, T.R. Protein nutrition in late pregnancy, maternal protein reserves and lactation performance in dairy cows. Proc. Nutr. Soc. 2000, 59, 119–126. [Google Scholar] [CrossRef]
- Sejian, V.; Indu, S.; Naqvi, S.M.K. Impact of short term exposure to different environmental temperature on the blood biochemical and endocrine responses of Malpura ewes under semi-arid tropical environment. Indian J. Anim. Sci. 2013, 83, 1155–1159. [Google Scholar]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Shwartz, G.; Rhoads, M.L.; Vanbaale, M.J.; Rhoads, R.P.; Baumgard, L.H. Effects of a supplemental yeast culture on heat-stressed lactating Holstein cows. J. Dairy Sci. 2009, 92, 935–942. [Google Scholar] [CrossRef]
- Schneider, P.L.; Beede, D.K.; Wilcox, C.J. Nycterohemeral Patterns of Acid-Base Status, Mineral Concentrations and Digestive Function of Lactating Cows in Natural or Chamber Heat Stress Environments2. J. Anim. Sci. 1988, 66, 112–125. [Google Scholar] [CrossRef]
- Cowley, F.C.; Barber, D.G.; Houlihan, A.V.; Poppi, D.P. Immediate and residual effects of heat stress and restricted intake on milk protein and casein composition and energy metabolism. J. Dairy Sci. 2015, 98, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Designing Foods: Animal Product Options in the Marketplace; The National Academies Press: Washington, DC, USA, 1988; ISBN 978-0-309-03795-2. [Google Scholar] [CrossRef]
- Roti Roti, J.L. Cellular responses to hyperthermia (40–46 °C): Cell killing and molecular events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, R.P.; La Noce, A.J.; Wheelock, J.B.; Baumgard, L.H. Short communication: Alterations in expression of gluconeogenic genes during heat stress and exogenous bovine somatotropin administration. J. Dairy Sci. 2011, 94, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.T.; Guo, J.; Quan, S.Y.; Nan, X.M.; Fernandez, M.V.S.; Baumgard, L.H.; Bu, D.P. The effects of heat stress on protein metabolism in lactating Holstein cows. J. Dairy Sci. 2017, 100, 5040–5049. [Google Scholar] [CrossRef]
- Pragna, P.; Archana, P.R.; Aleena, J.; Sejian, V.; Krishnan, G.; Bagath, M.; Manimaran, A.; Beena, V.; Kurien, E.K.; Varma, G.; et al. Heat stress and dairy cow: Impact on both milk yield and composition. Int. J. Dairy Sci. 2017, 12, 1–11. [Google Scholar]
- Archer, S.C.; Mc Coy, F.; Wapenaar, W.; Green, M.J. Association of season and herd size with somatic cell count for cows in Irish, English, and Welsh dairy herds. Vet. J. 2013, 196, 515–521. [Google Scholar] [CrossRef]
- Bernabucci, U.; Basiricò, L.; Morera, P.; Dipasquale, D.; Vitali, A.; Piccioli Cappelli, F.; Calamari, L. Effect of summer season on milk protein fractions in Holstein cows. J. Dairy Sci. 2015, 98, 1815–1827. [Google Scholar] [CrossRef]
- Bickerstaffe, R.; Annison, E.F.; Linzell, J.L. The metabolism of glucose, acetate, lipids and amino acids in lactating dairy cows. J. Agric. Sci. 1974, 82, 71–85. [Google Scholar] [CrossRef]
- Joksimović-Todorović, M.; Davidović, V.; Hristov, S.; Stanković, B. Effect of heat stress on milk production in dairy cows. Biotechnol. Anim. Husb. 2011, 27, 1017–1023. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Bernabucci, U. Climatic effects on productive traits in livestock. Vet. Res. Commun. 2006, 30, 75–81. [Google Scholar] [CrossRef]
- Liu, Z.; Ezernieks, V.; Wang, J.; Arachchillage, N.W.; Garner, J.B.; Wales, W.J.; Cocks, B.G.; Rochfort, S. Heat Stress in Dairy Cattle Alters Lipid Composition of Milk. Sci. Rep. 2017, 7, 961. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, K.; Hayasaka, K.; Yamazaki, T.; Shirai, T.; Osawa, T.; Terawaki, Y.; Nagamine, Y.; Masuda, Y.; Suzuki, M. Effects of heat stress on production, somatic cell score and conception rate in Holsteins. Anim. Sci. J. 2017, 88, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.A.K.; Contreras-Jodar, A.; Love, S.; Mehaba, N.; Such, X.; Caja, G. Milk yield, milk composition, and milk metabolomics of dairy goats intramammary-challenged with lipopolysaccharide under heat stress conditions. Sci. Rep. 2020, 10, 5055. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Horst, E.A.; Abuajamieh, M.; Mayorga, E.J.; Fernandez, M.V.S.; Baumgard, L.H. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef]
- Silanikove, N.; Shapiro, F.; Shinder, D. Acute heat stress brings down milk secretion in dairy cows by up-regulating the activity of the milk-borne negative feedback regulatory system. BMC Physiol. 2009, 9, 13. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Wang, J.P.; Bu, D.P.; Wang, J.Q.; Huo, X.K.; Guo, T.J.; Wei, H.Y.; Zhou, L.Y.; Rastani, R.R.; Baumgard, L.H.; Li, F.D. Effect of saturated fatty acid supplementation on production and metabolism indices in heat-stressed mid-lactation dairy cows. J. Dairy Sci. 2010, 93, 4121–4127. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 2002, 85, 2173–2179. [Google Scholar] [CrossRef]
- Dechow, C.D.; Goodling, R.C. Mortality, culling by sixty days in milk, and production profiles in high- and low-survival Pennsylvania herds. J. Dairy Sci. 2008, 91, 4630–4639. [Google Scholar] [CrossRef]
- Vitali, A.; Segnalini, M.; Bertocchi, L.; Bernabucci, U.; Nardone, A.; Lacetera, N. Seasonal pattern of mortality and relationships between mortality and temperature-humidity index in dairy cows. J. Dairy Sci. 2009, 92, 3781–3790. [Google Scholar] [CrossRef]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The Impact of Heat Load on Cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Gantner, V.; Kuterovac, K.; Potočnik, K. 11. Effect of Heat Stress on Metabolic Disorders Prevalence Risk and Milk Production in Holstein Cows in Croatia. Ann. Anim. Sci. 2016, 16, 451–461. [Google Scholar] [CrossRef]
- Basiricò, L.; Bernabucci, U.; Morera, P.; Lacetera, N.; Nardone, A. Gene expression and protein secretion of apolipoprotein B100 (ApoB100) in transition dairy cows under hot or thermoneutral environments. Ital. J. Anim. Sci. 2009, 8, 592–594. [Google Scholar] [CrossRef]
- Ronchi, B.; Lacetera, N.; Bernabucci, U.; Nardone, A.; VeriniSupplizi, A. Distinct and common effects of heat stress and restricted feeding on metabolic status of Holstein heifers. Zootec. Nutr. Anim. 1999, 25, 11–20. [Google Scholar]
- Lambert, G.P.; Gisolfi, C.V.; Berg, D.J.; Moseley, P.L.; Oberley, L.W.; Kregel, K.C. Selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. 2002, 92, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Baumgardner, K.R.; Oberley, T.D.; Gisolfi, C. V Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. 1999, 276, G1195–G1203. [Google Scholar] [CrossRef]
- Hall, D.M.; Buettner, G.R.; Oberley, L.W.; Xu, L.; Matthes, R.D.; Gisolfi, C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Hear. Circ. Physiol. 2001, 280, 2. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat Stress Reduces Intestinal Barrier Integrity and Favors Intestinal Glucose Transport in Growing Pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Leon, L.R. Heat stroke and cytokines. Prog. Brain Res. 2007, 162, 481–524. [Google Scholar]
- Waldron, M.R.; Kulick, A.E.; Bell, A.W.; Overton, T.R. Acute experimental mastitis is not causal toward the development of energy-related metabolic disorders in early postpartum dairy cows. J. Dairy Sci. 2006, 89, 596–610. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, R.; Liu, Y.; Zhu, W.; Mao, S. Intravenous lipopolysaccharide challenge alters ruminal bacterial microbiota and disrupts ruminal metabolism in dairy cattle. Br. J. Nutr. 2014, 112, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.-E.; Kang, D.-W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Bernabucci, U.; Scalia, D.; Ronchi, B.; Kuzminsky, G.; Nardone, A. Lymphocyte functions in dairy cows in hot environment. Int. J. Biometeorol. 2005, 50, 105–110. [Google Scholar] [CrossRef]
- Morse, D.; DeLorenzo, M.A.; Wilcox, C.J.; Collier, R.J.; Natzke, R.P.; Bray, D.R. Climatic Effects on Occurrence of Clinical Mastitis. J. Dairy Sci. 1988, 71, 848–853. [Google Scholar] [CrossRef]
- Waage, S.; Sviland, S.; Ødegaard, S.A. Identification of Risk Factors for Clinical Mastitis in Dairy Heifers. J. Dairy Sci. 1998, 81, 1275–1284. [Google Scholar] [CrossRef]
- Chirico, J.; Jonsson, P.; Kjellberg, S.; Thomas, G. Summer mastitis experimentally induced by Hydrotaeairritans exposed to bacteria. Med. Vet. Entomol. 1997, 11, 187–192. [Google Scholar] [CrossRef]
- Giesecke, W.H. The effect of stress on udder health of dairy cows. Onderstepoort J. Vet. Res. 1985, 52, 175–193. [Google Scholar]
- Hein, K.G.; Allrich, R.D. Influence of exogenous adrenocorticotropic hormone on estrous behavior in cattle. J. Anim. Sci. 1992, 70, 243–247. [Google Scholar] [CrossRef]
- Roman-Ponce, H.; Thatcher, W.W.; Buffington, D.E.; Wilcox, C.J.; Van Horn, H.H. Physiological and Production Responses of Dairy Cattle to a Shade Structure in a Subtropical Environment. J. Dairy Sci. 1977, 60, 424–430. [Google Scholar] [CrossRef]
- Gilad, E.; Meidan, R.; Berman, A.; Graber, Y.; Wolfenson, D. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows. J. Reprod. Fertil. 1993, 99, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.S.; Prakash, B.S. Seasonal variation and circadian rhythmicity of the prolactin profile during the summer months in repeat-breeding Murrah buffalo heifers. Reprod. Fertil. Dev. 2007, 19, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Paes, V.M.; Vieira, L.A.; Correia, H.H.V.; Sa, N.A.R.; Moura, A.A.A.; Sales, A.D.; Rodrigues, A.P.R.; Magalhães-Padilha, D.M.; Santos, F.W.; Apgar, G.A.; et al. Effect of heat stress on the survival and development of in vitro cultured bovine preantral follicles and on in vitro maturation of cumulus–oocyte complex. Theriogenology 2016, 86, 994–1003. [Google Scholar] [CrossRef]
- Putney, D.J.; Malayer, J.R.; Gross, T.S.; Thatcher, W.W.; Hansen, P.J.; Drost, M. Heat Stress-Induced Alterations in the Synthesis and Secretion of Proteins and Prostaglandins by Cultured Bovine Conceptuses and Uterine Endometrium1. Biol. Reprod. 1988, 39, 717–728. [Google Scholar] [CrossRef]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvão, K.; Santos, J.E.P. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef]
- Hansen, P.J. Exploitation of genetic and physiological determinants of embryonic resistance to elevated temperature to improve embryonic survival in dairy cattle during heat stress. Theriogenology 2007, 68, S242–S249. [Google Scholar] [CrossRef]
- Collier, R.J.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H. Invited review: Genes involved in the bovine heat stress response. J. Dairy Sci. 2008, 91, 445–454. [Google Scholar] [CrossRef]
- Dikmen, S.; Hansen, P.J. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef]
- Silva, P.R.B.; Machado, K.S.; Da Silva, D.N.L.; Moraes, J.G.N.; Keisler, D.H.; Chebel, R.C. Effects of recombinant bovine somatotropin during the periparturient period on innate and adaptive immune responses, systemic inflammation, and metabolism of dairy cows. J. Dairy Sci. 2015, 98, 4449–4464. [Google Scholar] [CrossRef]
- Tao, S.; Bubolz, J.W.; Do Amaral, B.C.; Thompson, I.M.; Hayen, M.J.; Johnson, S.E.; Dahl, G.E. Effect of heat stress during the dry period on mammary gland development. J. Dairy Sci. 2011, 94, 5976–5986. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K.; Cicela, T.M.; LaCount, D.W. Responses of primiparous and multiparous holstein cows to additional energy from fat or concentrate during summer. J. Dairy Sci. 2003, 86, 1306–1314. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Pohler, K.G.; Mulliniks, J.T.; Ríus, A.G. Lowering rumen-degradable and rumen-undegradable protein improved amino acid metabolism and energy utilization in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2018, 101, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Melendez, P.; Donovan, G.; Risco, C.; Littell, R.; Goff, J. Effect of calcium-energy supplements on calving-related disorders, fertility and milk yield during the transition period in cows fed anionic diets. Theriogenology 2003, 60, 843–854. [Google Scholar] [CrossRef]
- Leng, R.A.; Steel, J.W.; Luick, J.R. Contribution of propionate to glucose synthesis in sheep. Biochem. J. 1967, 103, 785–790. [Google Scholar] [CrossRef]
- Barreras, A.; Castro-Pérez, B.I.; López-Soto, M.A.; Torrentera, N.G.; Montaño, M.F.; Estrada-Angulo, A.; Ríos, F.G.; Dávila-Ramos, H.; Plascencia, A.; Zinn, R.A. Influence of Ionophore Supplementation on Growth Performance, Dietary Energetics and Carcass Characteristics in Finishing Cattle during Period of Heat Stress. Asian-Australas. J. Anim. Sci. 2013, 26, 1553–1561. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Wheelock, J.B.; Sanders, S.R.; Moore, C.E.; Green, H.B.; Waldron, M.R.; Rhoads, R.P. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows1. J. Dairy Sci. 2011, 94, 5620–5633. [Google Scholar] [CrossRef]
- Wildman, C.D.; West, J.W.; Bernard, J.K. Effect of dietary cation-anion difference and dietary crude protein on performance of lactating dairy cows during hot weather. J. Dairy Sci. 2007, 90, 1842–1850. [Google Scholar] [CrossRef]
- Erdman, R.A. Dietary Buffering Requirements of the Lactating Dairy Cow: A Review. J. Dairy Sci. 1988, 71, 3246–3266. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Spain, J.N.; Spiers, D.E. Supplementation of Nicotinic Acid for Lactating Holstein Cows under Heat Stress Conditions. J. Dairy Sci. 1997, 80, 1200–1206. [Google Scholar] [CrossRef]
- Gomez-Alarcon, R.A.; Huber, J.T.; Higginbotham, G.E.; Wiersma, F.; Ammon, D.; Taylor, B. Influence of feeding Aspergillus oryzae fermentation extract on the milk yields, eating patterns, and body temperatures of lactating cows. J. Anim. Sci. 1991, 69, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.G.S.; Rutigliano, H.M.; Cerri, R.L.; Robinson, P.H.; Santos, J.E.P. Effect of feeding Saccharomyces Cerevisiae on performance of dairy cows during summer heat stress. Anim. Feed Sci. Technol. 2009, 150, 175–186. [Google Scholar] [CrossRef]
- Arieli, A.; Adin, G.; Bruckental, I. The effect of protein intake on performance of cows in hot environmental temperatures. J. Dairy Sci. 2004, 87, 620–629. [Google Scholar] [CrossRef]
- Chan, S.C.; Huber, J.T.; Chen, K.H.; Simas, J.M.; Wu, Z. Effects of Ruminally Inert Fat and Evaporative Cooling on Dairy Cows in Hot Environmental Temperatures. J. Dairy Sci. 1997, 80, 1172–1178. [Google Scholar] [CrossRef]
- Melo, R.P.; Castro, L.P.; Cardoso, F.F.; Barbosa, E.F.; Melo, L.Q.; Silva, R.B.; Pereira, R.A.N.; Pereira, M.N. 1328 Supplementation of palm oil to lactating dairy cows fed a high fat diet during summer. J. Anim. Sci. 2016, 94, 640. [Google Scholar] [CrossRef]
- Gregus, Z.; Stein, A.F.; Varga, F.; Klaassen, C.D. Effect of lipoic acid on biliary excretion of glutathione and metals. Toxicol. Appl. Pharmacol. 1992, 114, 88–96. [Google Scholar] [CrossRef]
- Diesel, B.; Kulhanek-Heinze, S.; Höltje, M.; Brandt, B.; Höltje, H.D.; Vollmar, A.M.; Kiemer, A.K. α-lipoic acid as a directly binding activator of the insulin receptor: Protection from hepatocyte apoptosis. Biochemistry 2007, 46, 2146–2155. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Baumgard, L.H.; Suagee, J.K.; Sanders, S.R. Nutritional Interventions to Alleviate the Negative Consequences of Heat Stress. Adv. Nutr. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Jenkins, T.C. Fat in Lactation Rations: Review. J. Dairy Sci. 1980, 63, 1–14. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Lima, F.S.; Ganda, E.K.; Foditsch, C.; Meira, E.B.S.; Machado, V.S.; Teixeira, A.G.V.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Effect of trace mineral supplementation on selected minerals, energy metabolites, oxidative stress, and immune parameters and its association with uterine diseases in dairy cattle. J. Dairy Sci. 2014, 97, 4281–4295. [Google Scholar] [CrossRef]
- Rungruang, S.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H.; De Veth, M.J.; Collier, R.J. A dose-response evaluation of rumen-protected niacin in thermoneutral or heat-stressed lactating Holstein cows. J. Dairy Sci. 2014, 97, 5023–5034. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Ooie, T.; Takahashi, N.; Shinohara, T.; Nakagawa, M.; Yonemochi, H.; Hara, M.; Yoshimatsu, H.; Saikawa, T. Pioglitazone but not glibenclamide improves cardiac expression of heat shock protein 72 and tolerance against ischemia/reperfusion injury in the heredity insulin-resistant rat. Diabetes 2006, 55, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, K.M.; Overton, T.R. Effects of plane of nutrition and 2,4-thiazolidinedione on insulin responses and adipose tissue gene expression in dairy cattle during late gestation. J. Dairy Sci. 2011, 94, 6021–6035. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, G.; Unal, R.; Pokrovskaya, I.; Yao-Borengasser, A.; Phanavanh, B.; Lecka-Czernik, B.; Rasouli, N.; Kern, P.A. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: Effects of obesity, insulin resistance, and TZD treatment. J. Lipid Res. 2006, 47, 2444–2450. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Guan, R.; Shi, K.; Wei, Z.; Liu, J.; Liu, H. Effects of Dietary Rumen-Protected Betaine Supplementation on Performance of Postpartum Dairy Cows and Immunity of Newborn Calves. Animals 2019, 9, 167. [Google Scholar] [CrossRef]
- Mirzaei, M.; Ghorbani, G.R.; Khorvash, M.; Rahmani, H.R.; Nikkhah, A. Chromium improves production and alters metabolism of early lactation cows in summer. J. Anim. Physiol. Anim. Nutr. (Berl.) 2011, 95, 81–89. [Google Scholar] [CrossRef]
- Dos Santos Ribeiro, L.; Zandonadi Brandão, F.; De Rezende Carvalheira, L.; De Freitas Goes, T.J.; De Almeida Torres Filho, R.; Romano Quintão, C.C.; De Fátima Ávila Pires, M.; De Almeida Camargo, L.S.; De Carvalho, B.C. Chromium supplementation improves glucose metabolism and vaginal temperature regulation in Girolando cows under heat stress conditions in a climatic chamber. Trop. Anim. Health Prod. 2019, 1–8. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Abd El-Hack, M.E.; Abdelnour, S.A.; Hendy, Y.A.; Ghanem, H.A.; Alsafy, S.A.; Khafaga, A.F.; Noreldin, A.E.; Shaheen, H.; Samak, D.; et al. Potential use of chromium to combat thermal stress in animals: A review. Sci. Total Environ. 2020, 707, 135996. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. https://doi.org/10.3390/ani10050793
Sammad A, Wang YJ, Umer S, Lirong H, Khan I, Khan A, Ahmad B, Wang Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals. 2020; 10(5):793. https://doi.org/10.3390/ani10050793
Chicago/Turabian StyleSammad, Abdul, Ya Jing Wang, Saqib Umer, Hu Lirong, Imran Khan, Adnan Khan, Baseer Ahmad, and Yachun Wang. 2020. "Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities" Animals 10, no. 5: 793. https://doi.org/10.3390/ani10050793
APA StyleSammad, A., Wang, Y. J., Umer, S., Lirong, H., Khan, I., Khan, A., Ahmad, B., & Wang, Y. (2020). Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals, 10(5), 793. https://doi.org/10.3390/ani10050793








