An Assay System to Evaluate Riboflavin/UV-A Corneal Phototherapy Efficacy in a Porcine Corneal Organ Culture Model
Abstract
Simple summary
Abstract
1. Introduction
2. Materials and Methods
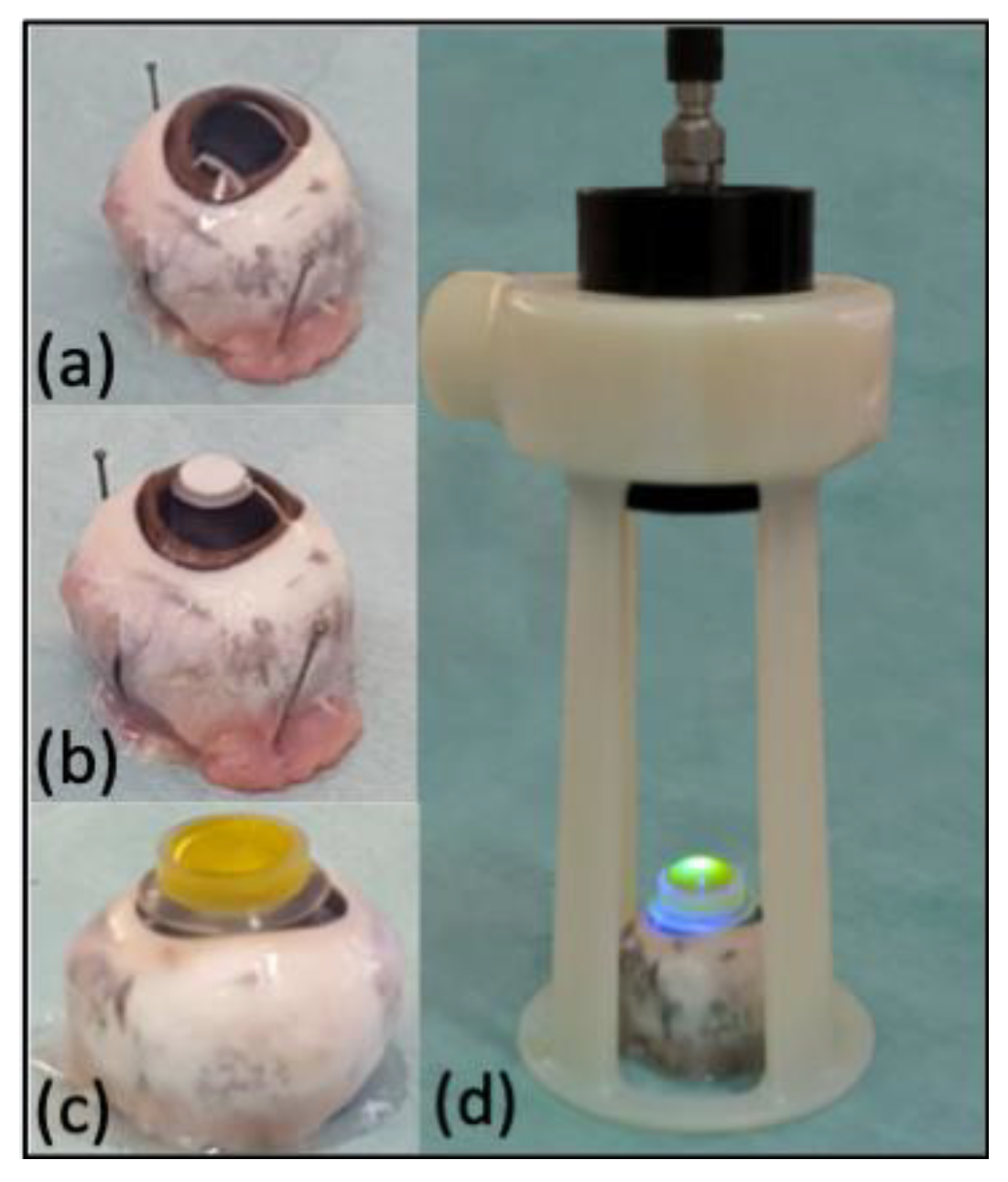
2.1. Eyes Preparation
2.2. Experimental Groups
2.3. Induction of Experimental Lesion and Riboflavin/UV-A Corneal Phototherapy Procedures
2.4. Porcine Organ-Culture Procedure
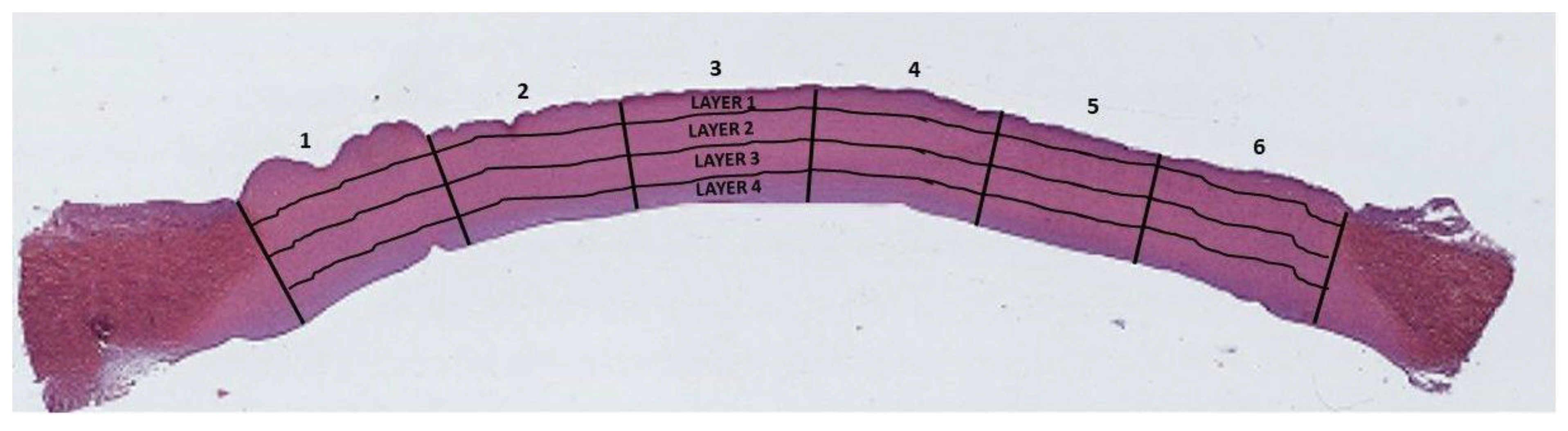
2.5. Histological Staining
2.6. Image Processing and Data Collecting
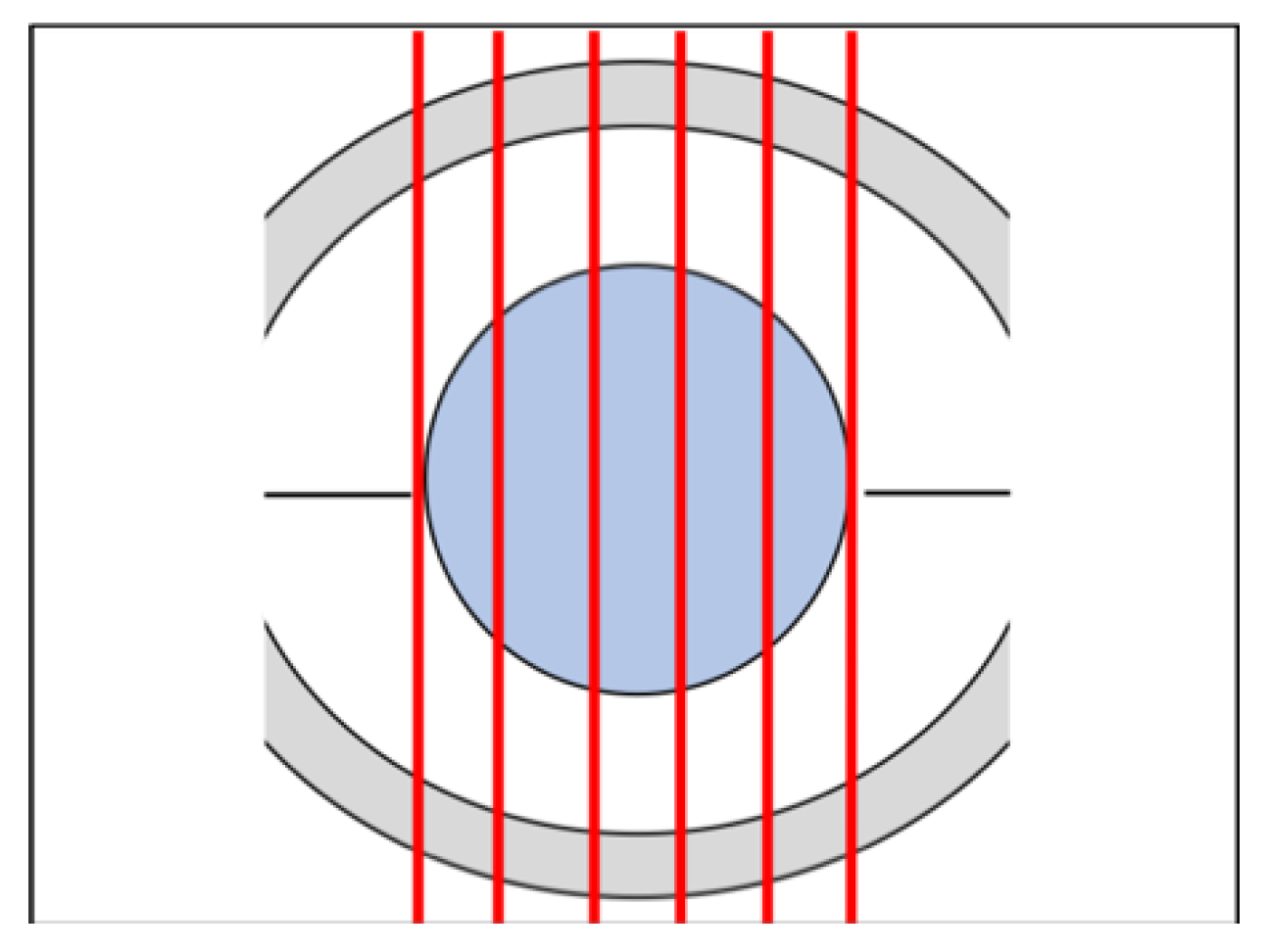
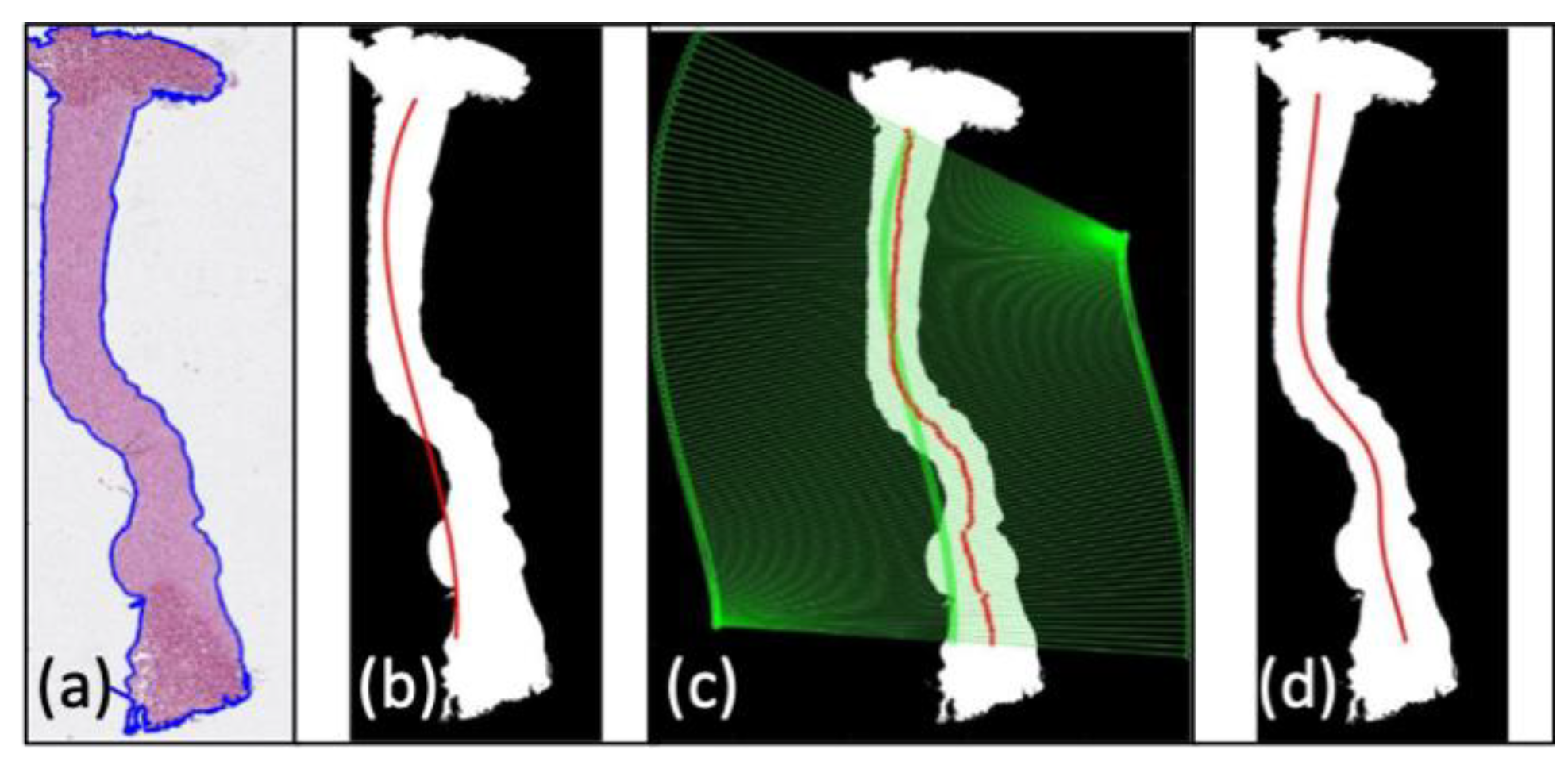
2.6.1. ROI Detection
2.6.2. Collagen Damage Quantification
2.7. Statistical Design and Data Analytics
3. Results
3.1. Corneal Organ Culture Results
3.2. Histological Evaluation of Hematoxylin and Eosin-Stained Sections
3.3. Statistical Analysis
3.4. Statistical Analysis of Brightness Values among Corneal Populations
3.5. Statistical Analysis of Brightness Values Distribution among Layers and Radial Frames
3.6. Statistical Analysis of Brightness Values Distribution among the 24 ROIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanore, M.; Chahory, S.; Payen, G.; Clerc, B. Surgical repair of deep melting ulcers with porcine small intestinal submucosa (SIS) graft in dogs and cats. Veter Ophthalmol. 2007, 10, 93–99. [Google Scholar] [CrossRef]
- Spiess, B.M.; Pot, S.A.; Florin, M.; Hafezi, F. Corneal collagen cross-linking (CXL) for the treatment of melting keratitis in cats and dogs: A pilot study. Veter Ophthalmol. 2013, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Famose, F. Evaluation of accelerated collagen cross?linking for the treatment of melting keratitis in eight dogs. Veter Ophthalmol. 2013, 17, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Gallhoefer, N.S.; Spiess, B.M.; Guscetti, F.; Hilbe, M.; Hartnack, S.; Hafezi, F.; Pot, S.A. Penetration depth of corneal cross-linking with riboflavin and UV -A (CXL) in horses and rabbits. Veter Ophthalmol. 2015, 19, 275–284. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Bazan, H.E. Alkali-Induced Corneal Stromal Melting Prevention by a Novel Platelet-Activating Factor Receptor Antagonist. Arch. Ophthalmol. 2006, 124, 70–78. [Google Scholar] [CrossRef][Green Version]
- Ion, L.; Ionascu, I.; Birtoiu, A. Melting Keratitis in Dogs and Cats. Agric. Agric. Sci. Procedia 2015, 6, 342–349. [Google Scholar] [CrossRef]
- Pot, S.A.; Gallhöfer, N.S.; Matheis, F.L.; Voelter-Ratson, K.; Hafezi, F.; Spiess, B.M. Corneal collagen cross-linking as treatment for infectious and noninfectious corneal melting in cats and dogs: Results of a prospective, nonrandomized, controlled trial. Veter Ophthalmol. 2013, 17, 250–260. [Google Scholar] [CrossRef]
- Edman, A.H.; Makdoumi, K.; Mortensen, J.; Ekesten, B. Corneal cross-linking in 9 horses with ulcerative keratitis. BMC Veter Res. 2013, 9, 128. [Google Scholar]
- Tabibian, D.; Mazzotta, C.; Hafezi, F. PACK-CXL: Corneal cross-linking in infectious keratitis. Eye Vis. 2016, 3, 11. [Google Scholar] [CrossRef]
- Papaioannou, L.; Miligkos, M.; Papathanassiou, M. Corneal Collagen Cross-Linking for Infectious Keratitis. Cornea 2016, 35, 62–67. [Google Scholar] [CrossRef]
- Greenstein, S.A.; Shah, V.P.; Fry, K.L.; Hersh, P.S. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J. Cataract. Refract. Surg. 2011, 37, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, X.; Schwend, T.; Littlechild, S.; Conrad, G.W. Resistance of Corneal RFUVA–Cross-Linked Collagens and Small Leucine-Rich Proteoglycans to Degradation by Matrix Metalloproteinases. Investig. Opthalmol. Vis. Sci. 2013, 54, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Sorkhabi, R.; Sedgipoor, M.; Mahdavifard, A. Collagen cross-linking for resistant corneal ulcer. Int. Ophthalmol. 2012, 33, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Pucci, G.; Barberi, R.C.; Lombardo, M. Interaction of ultraviolet light with the cornea: Clinical implications for corneal crosslinking. J. Cataract. Refract. Surg. 2015, 41, 446–459. [Google Scholar] [CrossRef]
- Escarião, A.C.; Ribeiro, E.; De Almeida Jorge, P.; Soares Leite, E.C.; Brandt, C.T. Therapeutic effect of corneal crosslinking on infectious keratitis. Rev. Bras. Oftalmol. 2013, 72, 366–372. [Google Scholar]
- Said, D.G.; Elalfy, M.S.; Gatzioufas, Z.; El-Zakzouk, E.S.; Hassan, M.A.; Saif, M.Y.S.; Zaki, A.A.; Dua, H.S.; Hafezi, F. Collagen Cross-Linking with Photoactivated Riboflavin (PACK-CXL) for the Treatment of Advanced Infectious Keratitis with Corneal Melting. Ophthalmology 2014, 121, 1377–1382. [Google Scholar] [CrossRef]
- Zamani, M.; Panahi-Bazaz, M.; Asadi, M. Corneal Collagen Cross-linking for Treatment of Non-healing Corneal Ulcers. J. Ophthalmic Vis. Res. 2015, 10, 16–20. [Google Scholar] [CrossRef]
- Chan, T.C.Y.; Lau, T.W.S.; Lee, J.W.; Wong, I.Y.H.; Jhanji, V.; Wong, R.L.M. Corneal collagen cross-linking for infectious keratitis: An update of clinical studies. Acta Ophthalmol. 2015, 93, 689–696. [Google Scholar] [CrossRef]
- Anwar, H.M.; El-Danasoury, A.M.; Hashem, A.N. Corneal collagen crosslinking in the treatment of infectious keratitis. Clin. Ophthalmol. 2011, 5, 1277–1280. [Google Scholar] [CrossRef]
- Lombardo, M.; Serrao, S.; Randleman, J.B.; Santhiago, M.R.; Kymionis, G.D.; Hafezi, F. Corneal Cross-linking Standardized Terminology. J. Refract. Surg. 2018, 34, 213. [Google Scholar] [CrossRef]
- Pot, S.A.; Gallhöfer, N.S.; Walser-Reinhardt, L.; Hafezi, F.; Spiess, B.M. Treatment of bullous keratopathy with corneal collagen cross-linking in two dogs. Veter Ophthalmol. 2013, 18, 168–173. [Google Scholar] [CrossRef]
- Famose, F. Evaluation of accelerated collagen cross-linking for the treatment of melting keratitis in ten cats. Veter Ophthalmol. 2013, 18, 95–104. [Google Scholar] [CrossRef]
- Malska, A.; Elks, R.; Gonella, B.; Lin, J.-T. Repair of Severe Melting Ulcers with High Intensity UV-pen (18 to 60 mW/cm2) Corneal Cross-linking (CXL) and Amniotic Membrane Graft. Ophthalmol. Res. Int. J. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Edman, A.H.; Ström, L.; Ekesten, B. Corneal cross-linking (CXL)-A clinical study to evaluate CXL as a treatment in comparison with medical treatment for ulcerative keratitis in horses. Veter Ophthalmol. 2019, 22, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, A.; Gialletti, R.; Cassarani, M.P.; Arcelli, R.; Lombardo, G.; Lombardo, M. Valutazione dell’efficacia della terapia fotochimica corneale con UV-A e riboflavina per il trattamento delle ulcere corneali profonde settiche del cane. In Proceedings of the Internatinal Congress SCIVAC 2019, Rimini, Italy, 24–26 May 2019; pp. 358–359. [Google Scholar]
- Bottós, K.M.; Dreyfuss, J.L.; Regatieri, C.V.; Lima-Filho, A.A.; Schor, P.; Nader, H.B.; Chamon, W. Immunofl uorescence Confocal Microscopy of Porcine Corneas Following Collagen Cross-linking Treatment With Ribofl avin and Ultraviolet A. J. Refract. Surg. 2008, 24, S715–S719. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Wilsch, M.; Spoerl, E.; Seiler, T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea 2004, 23, 503–507. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.-C.; Lee, H.-J.; Cheong, Y.; Jung, G.-B.; Jin, K.-H.; Park, H.-K. Structural response of human corneal and scleral tissues to collagen cross-linking treatment with riboflavin and ultraviolet A light. Lasers Med Sci. 2012, 28, 1289–1296. [Google Scholar] [CrossRef]
- Rieck, P.W.; Gigon, M.; Jaroszewski, J.; Pleyer, U.; Hartmann, C. Increased endothelial survival of organ-cultured corneas stored in FGF-2-supplemented serum-free medium. Investig. Opthalmol. Vis. Sci. 2003, 44, 3826–3832. [Google Scholar] [CrossRef]
- Castro, N.; Gillespie, S.R.; Bernstein, A.M. Ex Vivo Corneal Organ Culture Model for Wound Healing Studies. J. Vis. Exp. 2019, 15, e58562. [Google Scholar] [CrossRef]
- Colombo-Barboza, M.; Colombo-Barboza, G.; Felberg, S.; Dantas, P.E.C.; Sato, E.H. Induction of corneal collagen cross-linking in experimental corneal alkali burns in rabbits. Arq. Bras. Oftalmol. 2014, 77. [Google Scholar] [CrossRef]
- Wollensak, G.J.; Spörl, E.; Herbst, H. Biomechanical efficacy of contact lens-assisted collagen cross-linking in porcine eyes. Acta Ophthalmol. 2018, 97, e84–e90. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Espandar, L.; Mamalis, N.; Prestwich, G.D. A cross-linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Veter Ophthalmol. 2010, 13, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-W.; Zhao, X.-D.; Li, W.-J.; Zhou, X.; Liu, Y. Experimental study on the treatment of rabbit corneal melting after alkali burn with Collagen cross-linking. Int. J. Ophthalmol. 2012, 5, 147–150. [Google Scholar] [PubMed]
- Bonucci, E. Manuale di Istochimica; Lombardo: Roma, Italy, 1981. [Google Scholar]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Grisan, E.; Poletti, A. Ruggeri: Automatic segmentation and disentangling of chromosomes in q-band prometaphase images. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 575–581. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation inference for the general linear model. Neuro Image 2014, 92, 381–397. [Google Scholar] [CrossRef]
- Winkler, A.M.; Webster, M.A.; Brooks, J.C.; Tracey, I.; Smith, S.M.; Nichols, T.E. Non-parametric combination and related permutation tests for neuroimaging. Hum. Brain Mapp. 2016, 37, 1486–1511. [Google Scholar] [CrossRef]
- Grisan, E.; Graïc, J.-M.; Corain, L.; Peruffo, A. Resolving single cells in heavily clustered Nissl-stained images for the analysis of brain cytoarchitecture. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; pp. 427–430. [Google Scholar]
- Graïc, J.-M.; Corain, L.; Peruffo, A.; Cozzi, B.; Swaab, D.F. The bovine anterior hypothalamus: Characterization of the vasopressin-oxytocin containing nucleus and changes in relation to sexual differentiation. J. Comp. Neurol. 2018, 526, 2898–2917. [Google Scholar] [CrossRef]
- Pesarin, F.; Salmaso, L. Permutation Tests for Complex Data: Theory, Applications and Software; Wiley Series in Probability and Statistics: Chichester, UK, 2010. [Google Scholar]
- Bonnini, S.; Corain, L.; Marozzi, M.; Salmaso, L. Nonparametric Hypothesis Testing: Rank and Permutation Methods with Applications in R; Wiley: Chichester, UK, 2014. [Google Scholar]
- Corain, L.; Salmaso, L. Improving power of multivariate combination-based permutation tests. Stat. Comput. 2013, 25, 203–214. [Google Scholar] [CrossRef]
- Arboretti, R.; Bonnini, S.; Corain, L.; Salmaso, L. A permutation approach for ranking of multivariate populations. J. Multivar. Anal. 2014, 132, 39–57. [Google Scholar] [CrossRef]
- Corain, L.; Arboretti, R.; Bonnini, S. Ranking of Multivariate Populations-A Permutation Approach with Applications; Chapman & Hall/CRC: Boca Raton, FL, USA, 2016. [Google Scholar]
- Peruffo, A.; Corain, L.; Bombardi, C.; Centelleghe, C.; Grisan, E.; Graïc, J.-M.; Bontempi, P.; Grandis, A.; Cozzi, B. The motor cortex of the sheep: Laminar organization, projections and diffusion tensor imaging of the intracranial pyramidal and extrapyramidal tracts. Brain Struct. Funct. 2019, 224, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Arboretti, R.; Bolzan, M.; Bonnini, S.; Corain, L.; Solmi, F. Advantages of Closed Testing Method for Multiple Comparison Procedures. Commun. Stat. Simul. Comput. 2012, 41, 746–763. [Google Scholar]
- Leonard, B.; Cosert, K.; Winkler, M.; Marangakis, A.; Thomasy, S.M.; Murphy, C.J.; Jester, J.V.; Raghunathan, V. Stromal Collagen Arrangement Correlates with Stiffness of the Canine Cornea. Bioengergy 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Z.; Dean, T. The mechanics of multi-point sandwich forming. Int. J. Mach. Tools Manuf. 2008, 48, 1495–1503. [Google Scholar] [CrossRef]
- Poldrack, R.A. Region of interest analysis for fMRI. Soc. Cogn. Affect. Neurosci. 2007, 2, 67–70. [Google Scholar] [CrossRef] [PubMed]

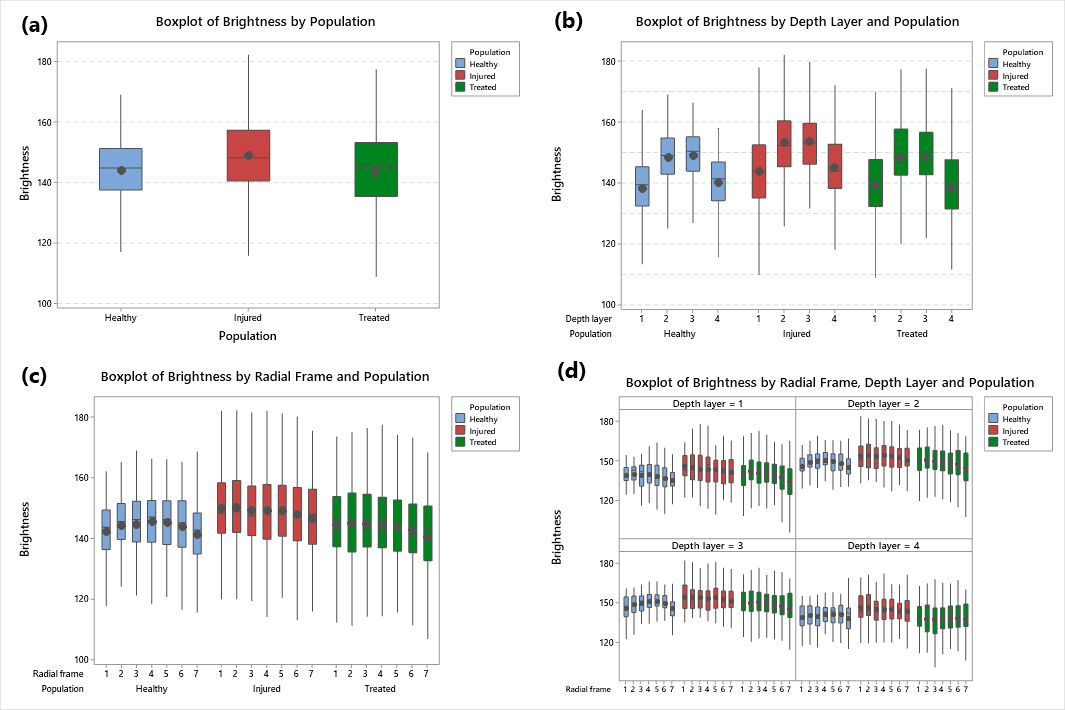
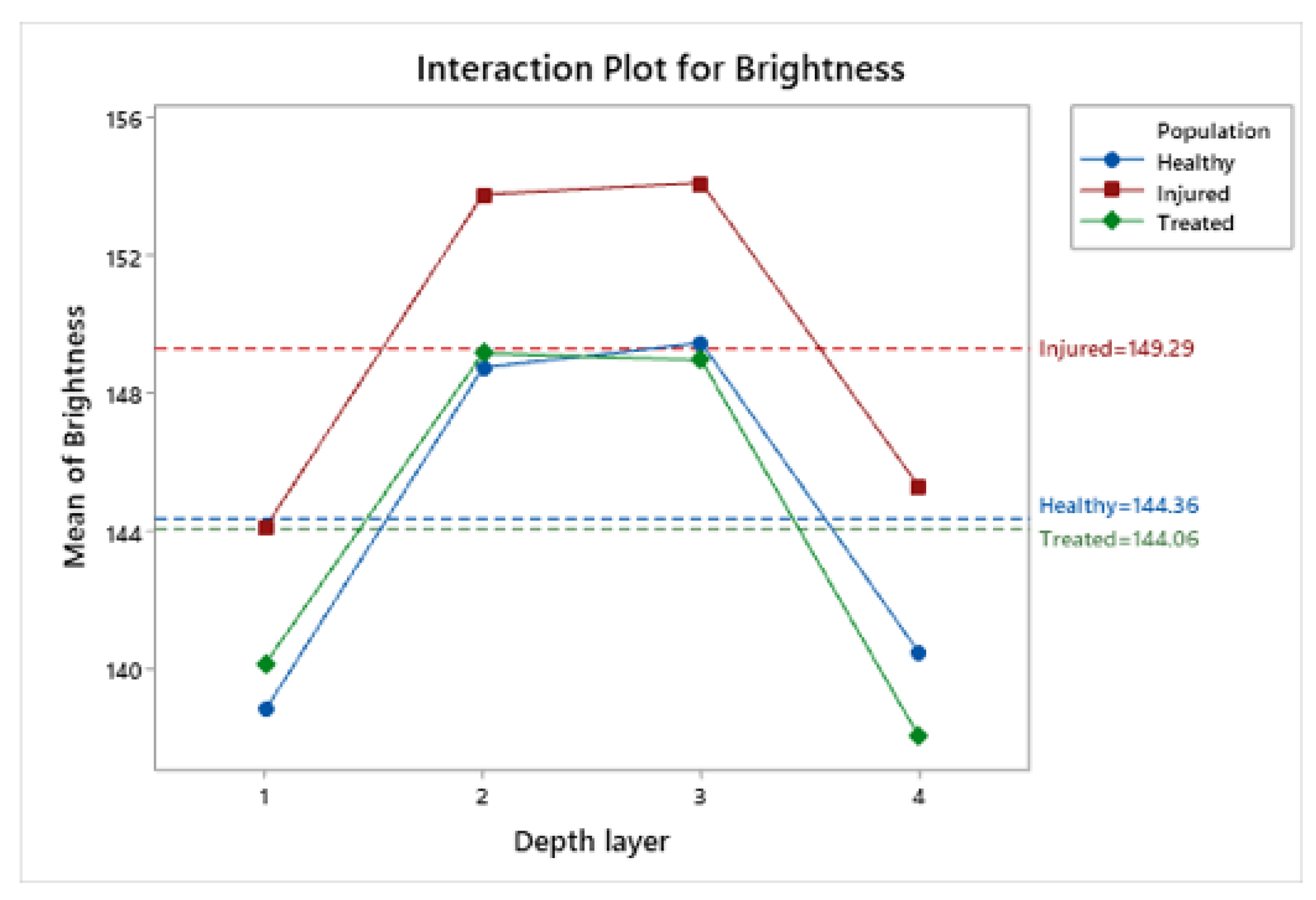
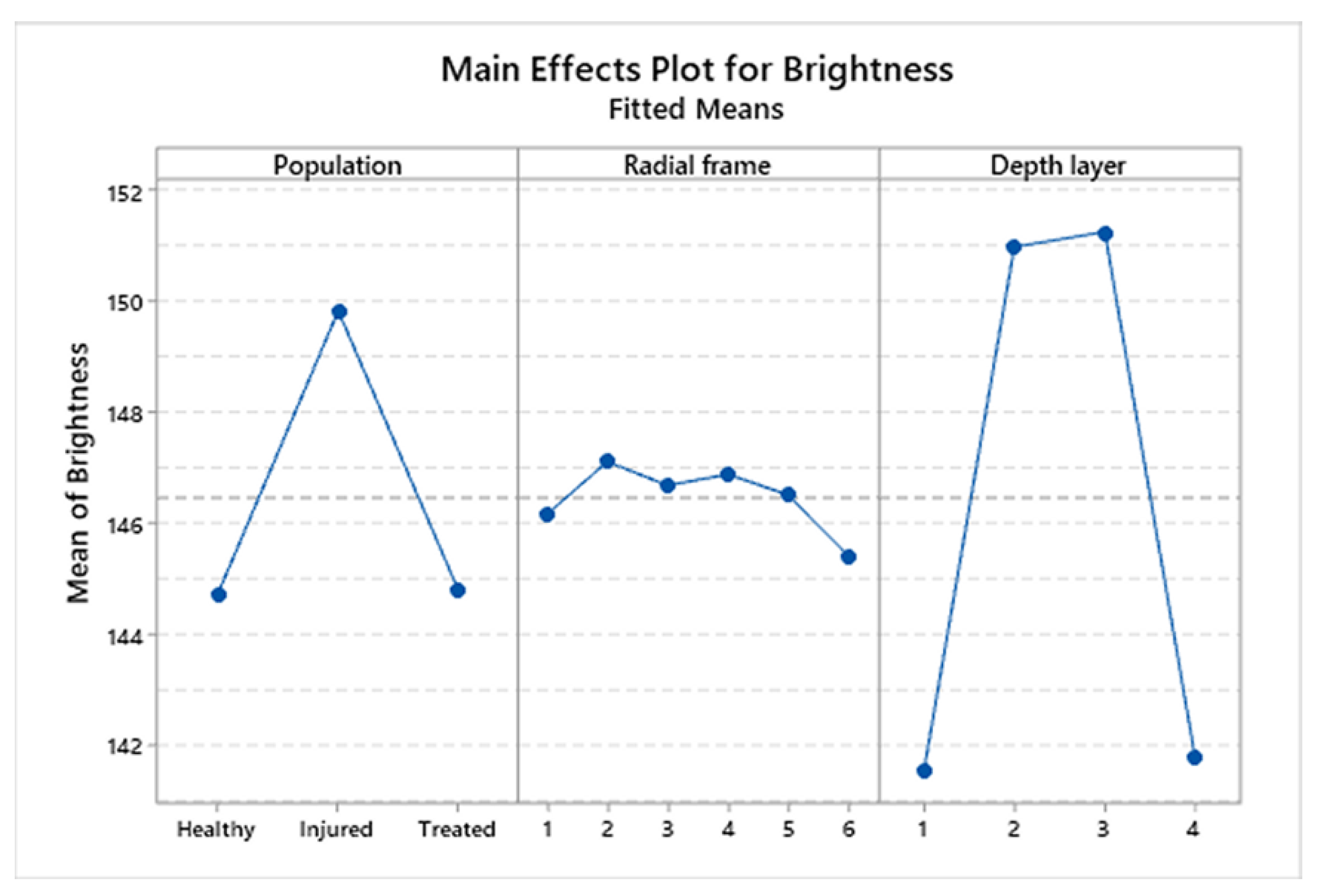
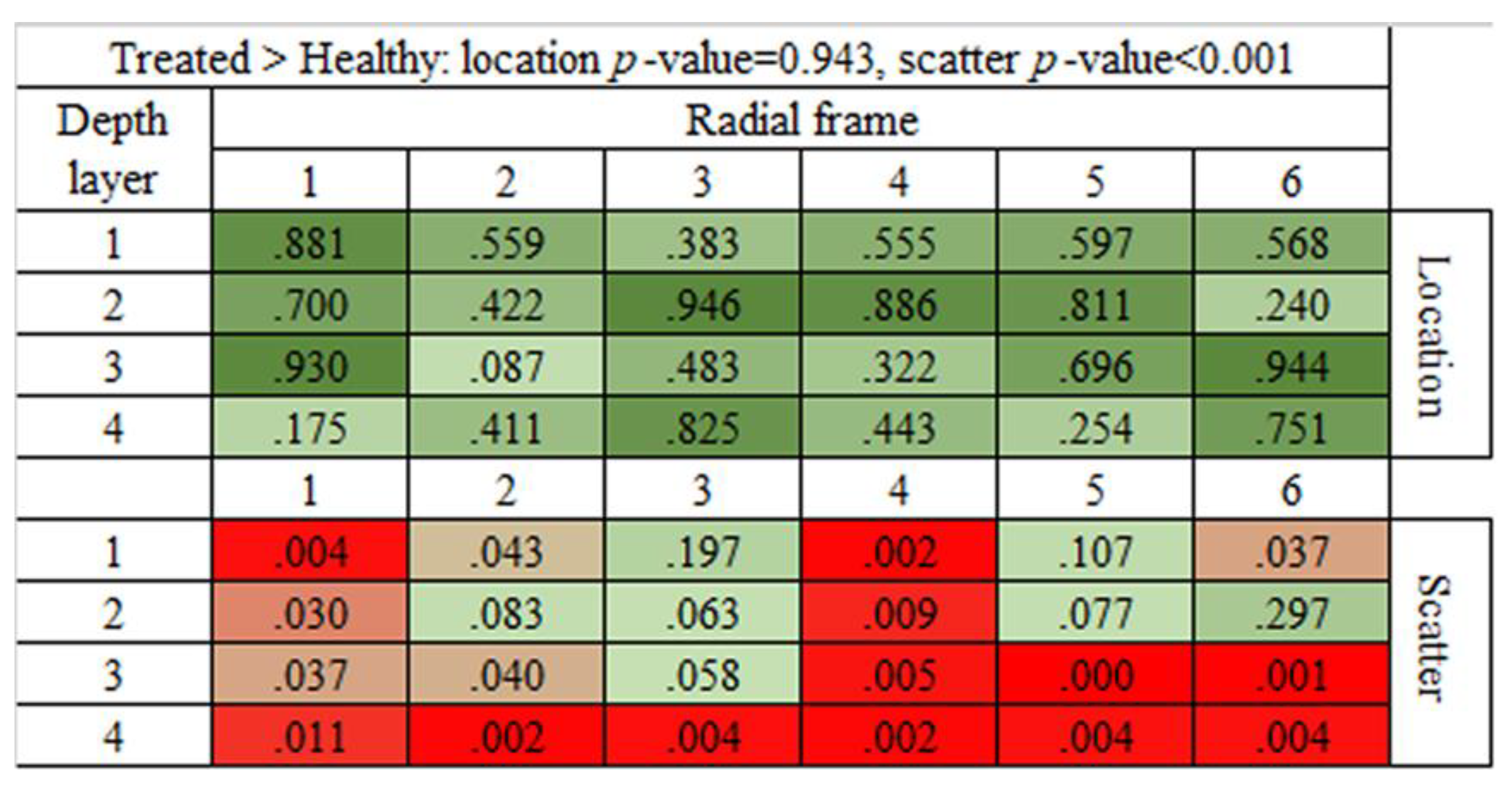
| Population | Mean | Median | St.Dev | CV | IQR |
|---|---|---|---|---|---|
| Healthy | 144.36 | 145.39 | 10.24 | 7.09 | 13.72 |
| Injured | 149.29 | 148.33 | 12.66 | 8.48 | 16.63 |
| Treated | 144.06 | 145.7 | 14.24 | 9.88 | 17.55 |
| Population | Depth Layer | Mean | St.Dev | 95% CI |
|---|---|---|---|---|
| Healthy | 138.81 | 9.87 | (137.54; 140.08) | |
| Injured | 1 | 144.08 | 12.71 | (142.45; 145.72) |
| Treated | 140.15 | 13.13 | (138.56; 141.74) | |
| Healthy | 148.72 | 8.98 | (147.57; 149.88) | |
| Injured | 2 | 153.74 | 11.64 | (152.24; 155.23) |
| Treated | 149.14 | 12.80 | (147.59; 150.70) | |
| Healthy | 149.44 | 8.57 | (148.33; 150.54) | |
| Injured | 3 | 154.07 | 11.20 | (152.62; 155.51) |
| Treated | 148.94 | 13.26 | (147.33; 150.55) | |
| Healthy | 140.46 | 8.82 | (139.33; 141.60) | |
| Injured | 4 | 145.26 | 11.61 | (143.77; 146.75) |
| Treated | 138.03 | 14.13 | (136.32; 139.75) |
| Term | F-Value | p-Value |
|---|---|---|
| Population | 63.48 | <0.001 |
| Radial frame | 1.37 | 0.234 |
| Depth layer | 166.28 | <0.001 |
| Population × Radial frame | 0.93 | 0.505 |
| Population × Depth layer | 1.22 | 0.293 |
| Radial frame × Depth layer | 0.28 | 0.997 |
| Difference of Populations | Diff. of Means | 95% CI | Adj. p-Value |
|---|---|---|---|
| Injured—Healthy | 1503 | (1141; 1865) | <0.001 |
| Treated—Healthy | 15 | (−337; 367) | 0.995 |
| Treated—Injured | −1488 | (−1840; −1136) | <0.001 |
| Population | Mean | Variance | ||||
|---|---|---|---|---|---|---|
| Healthy | Injured | Treated | Healthy | Injured | Treated | |
| Healthy | - | 1.000 | 0.070 | - | 1.000 | 1.000 |
| Injured | 0.001 | - | 0.001 | 0.001 | - | 1.000 |
| Treated | 0.931 | 1.000 | - | 0.001 | 1.000 | - |
| ranking | 2 | 1 | 2 | 3 | 2 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perazzi, A.; Gomiero, C.; Corain, L.; Iacopetti, I.; Grisan, E.; Lombardo, M.; Lombardo, G.; Salvalaio, G.; Contin, R.; Patruno, M.; et al. An Assay System to Evaluate Riboflavin/UV-A Corneal Phototherapy Efficacy in a Porcine Corneal Organ Culture Model. Animals 2020, 10, 730. https://doi.org/10.3390/ani10040730
Perazzi A, Gomiero C, Corain L, Iacopetti I, Grisan E, Lombardo M, Lombardo G, Salvalaio G, Contin R, Patruno M, et al. An Assay System to Evaluate Riboflavin/UV-A Corneal Phototherapy Efficacy in a Porcine Corneal Organ Culture Model. Animals. 2020; 10(4):730. https://doi.org/10.3390/ani10040730
Chicago/Turabian StylePerazzi, Anna, Chiara Gomiero, Livio Corain, Ilaria Iacopetti, Enrico Grisan, Marco Lombardo, Giuseppe Lombardo, Gianni Salvalaio, Roberta Contin, Marco Patruno, and et al. 2020. "An Assay System to Evaluate Riboflavin/UV-A Corneal Phototherapy Efficacy in a Porcine Corneal Organ Culture Model" Animals 10, no. 4: 730. https://doi.org/10.3390/ani10040730
APA StylePerazzi, A., Gomiero, C., Corain, L., Iacopetti, I., Grisan, E., Lombardo, M., Lombardo, G., Salvalaio, G., Contin, R., Patruno, M., Martinello, T., & Peruffo, A. (2020). An Assay System to Evaluate Riboflavin/UV-A Corneal Phototherapy Efficacy in a Porcine Corneal Organ Culture Model. Animals, 10(4), 730. https://doi.org/10.3390/ani10040730





