Individuals with Psychopathic Traits and Poor Attitudes towards Animals Can Recognise Infant Features But Give Them Reduced Attentional Priority
Simple Summary
Abstract
1. Introduction
2. Study 1 Materials and Methods
2.1. Ethical Statement
2.2. Demographic Information and Questionnaires
2.3. Cuteness Forced-Choice Task
2.4. Statistical Analysis
3. Results
3.1. Demographic Information
3.2. Associations between Psychopathic Traits and Attitudes towards Animals
3.3. Predictors of Cuteness Forced-Choice Score
3.4. Time to Complete
4. Study 2 Materials and Methods
4.1. Screening Process
4.2. Design
4.3. Free-Viewing Task: Image Characteristics
4.3.1. Subjective Rating of Image Characteristics
Image ‘Cuteness’
Image Complexity
4.4. Free-Viewing Task: Apparatus
4.5. Free-Viewing Task: Data Reduction
4.6. Free-Viewing Task: Procedure
4.7. Free-Viewing Task: Gaze Data
4.8. Statistical Analysis
5. Results
5.1. Associations between Psychopathic Traits and Attitudes towards Animals before Participant Screening
5.2. Eye-Tracking Variables
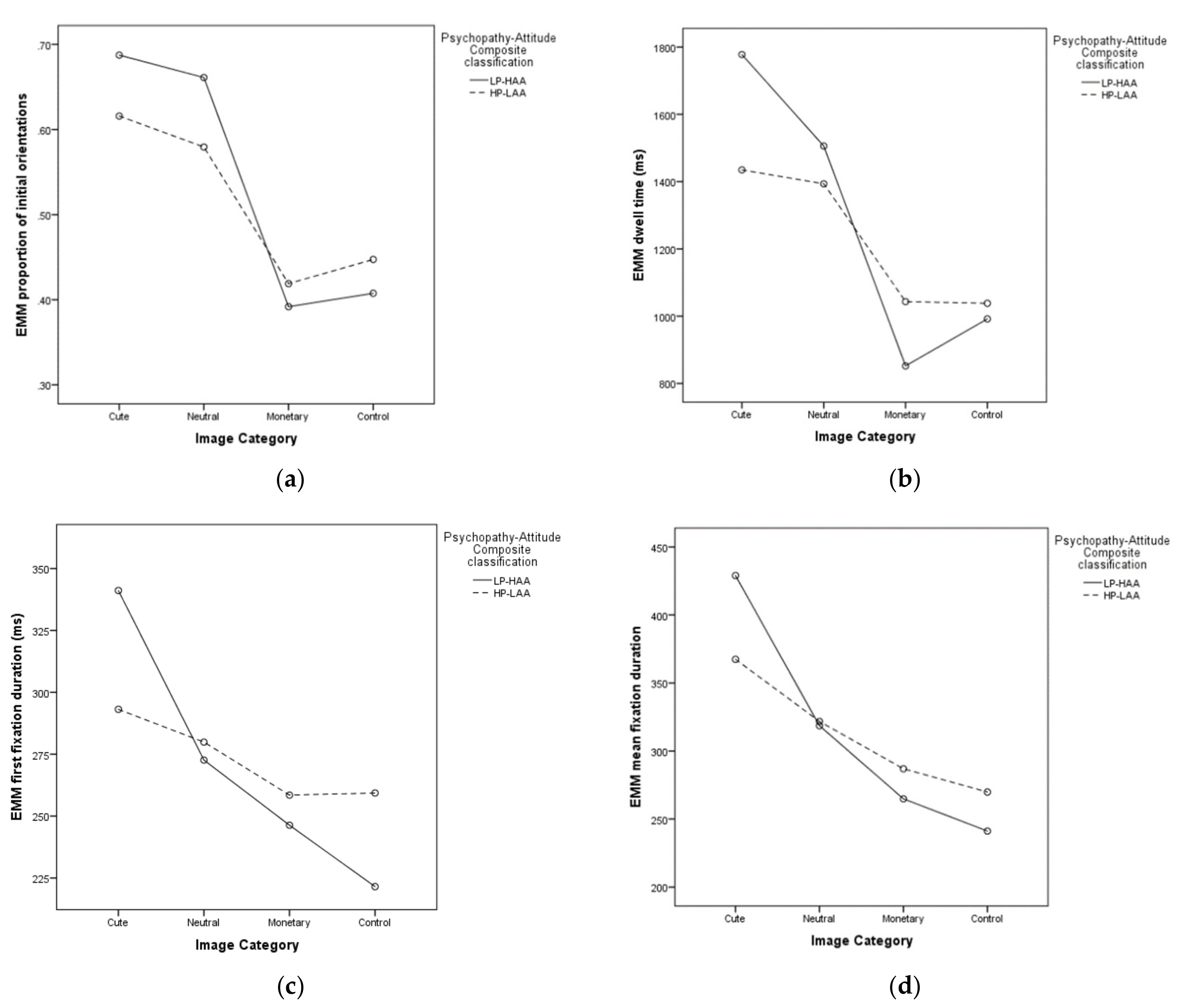
5.2.1. Proportion of Initial Orientations
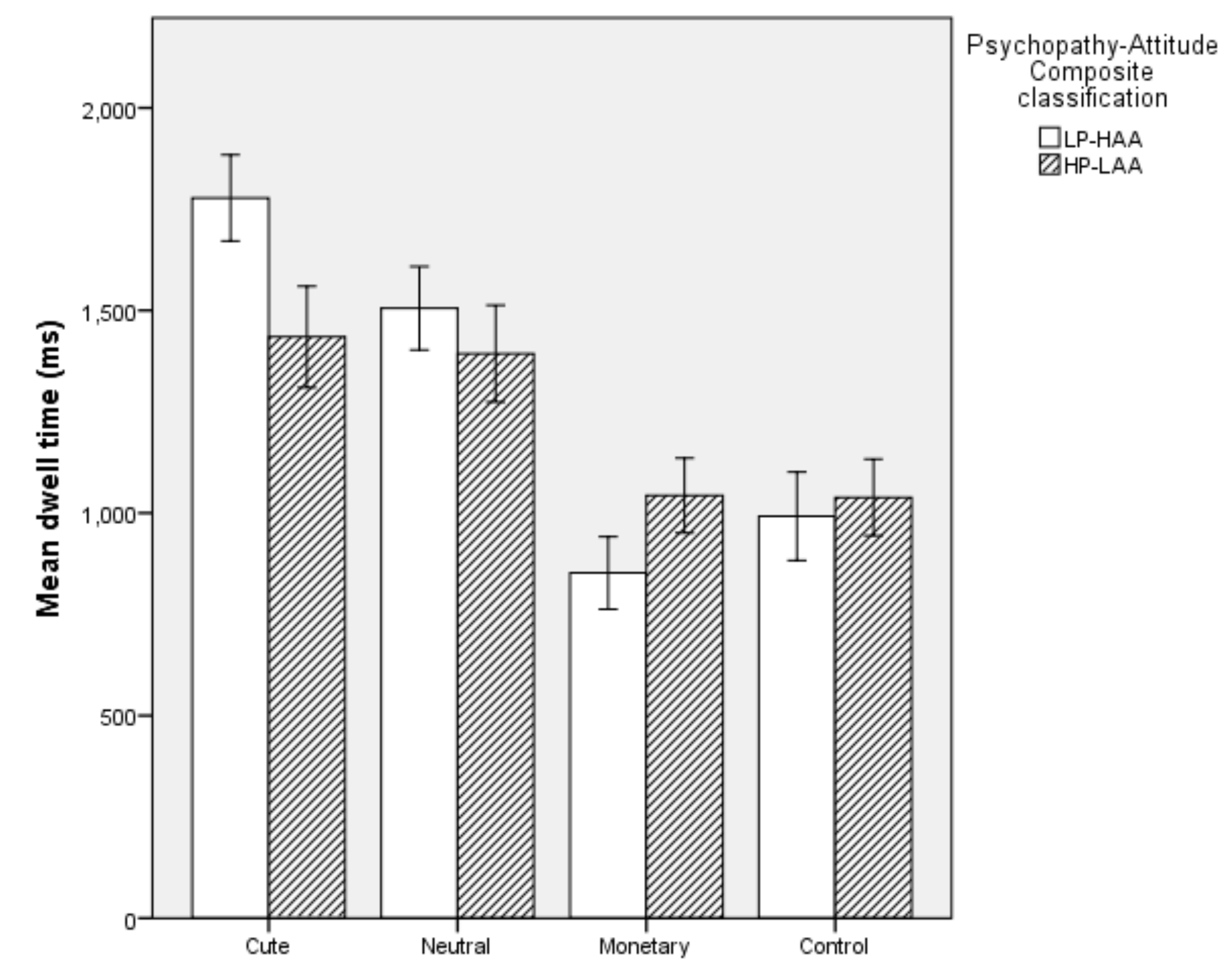
5.2.2. Dwell Time
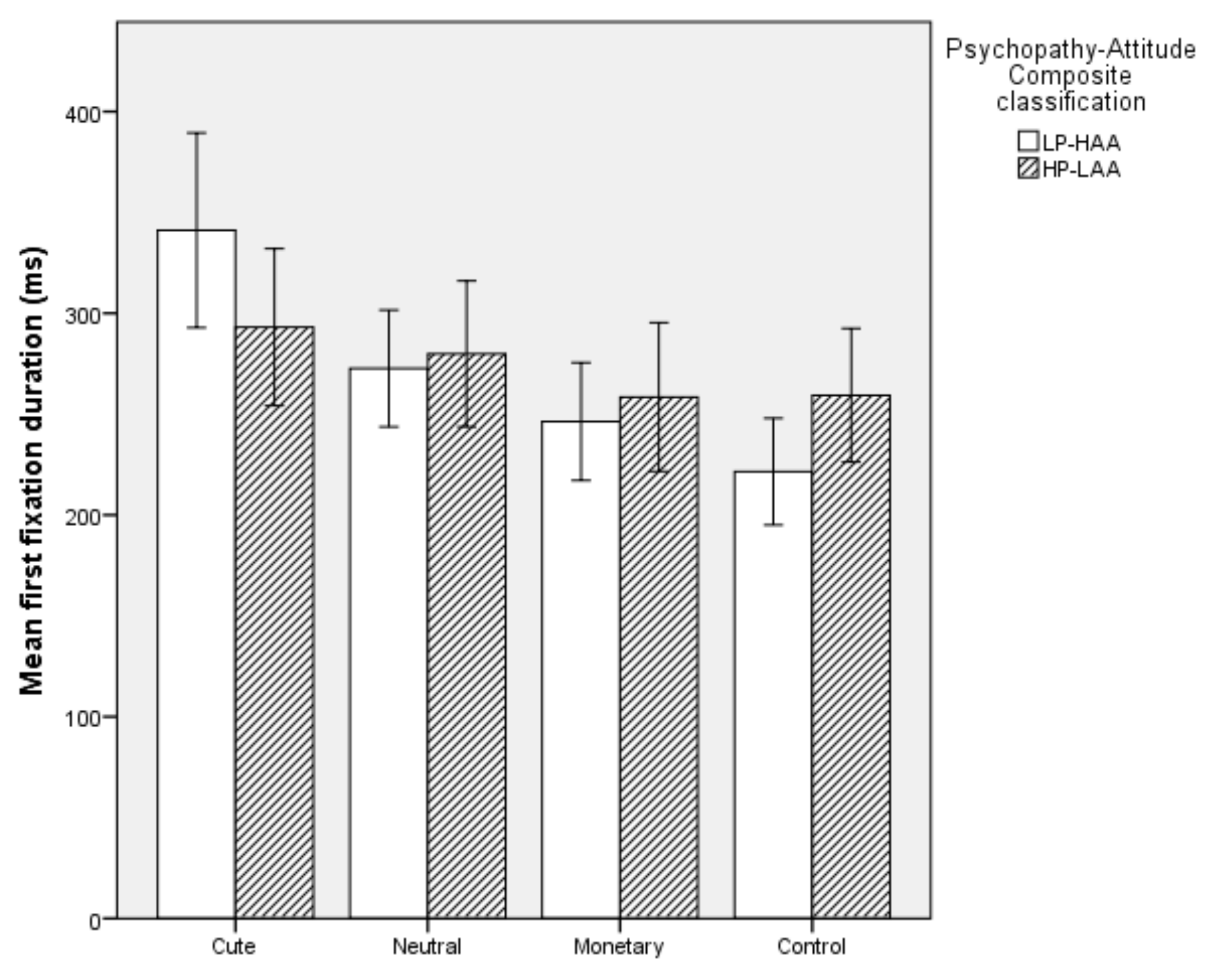
5.2.3. First Fixation Duration
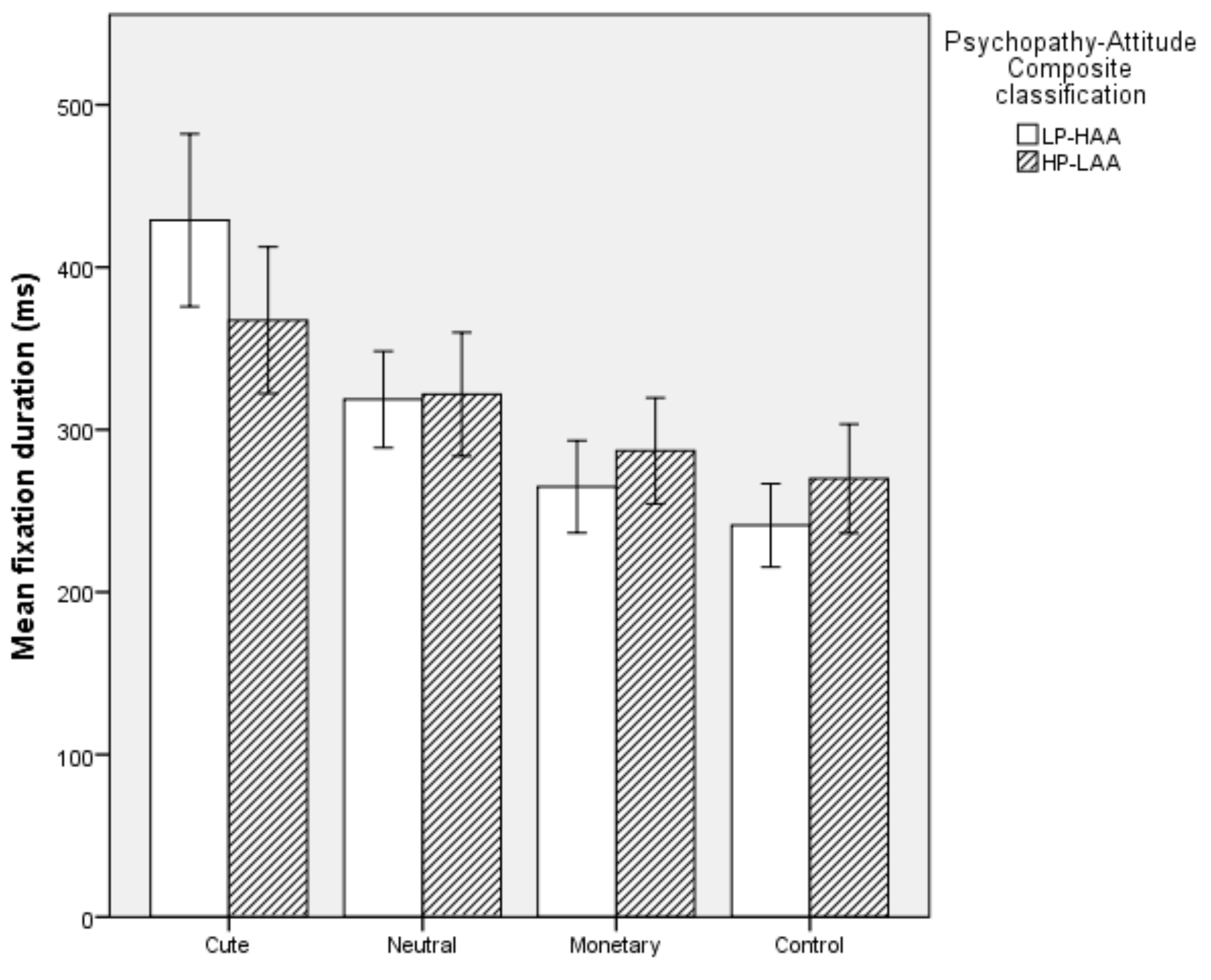
5.2.4. Mean Fixation Duration
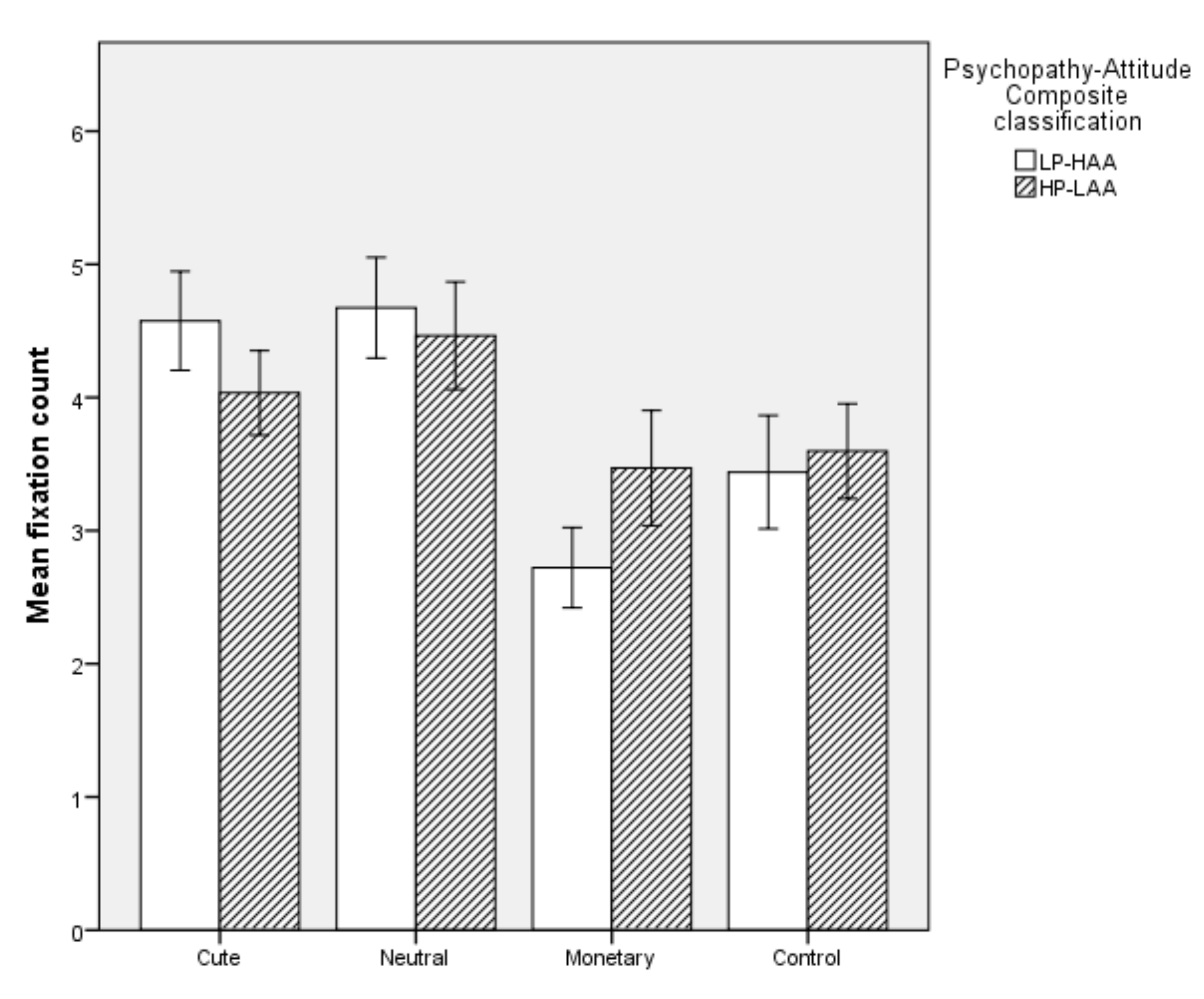
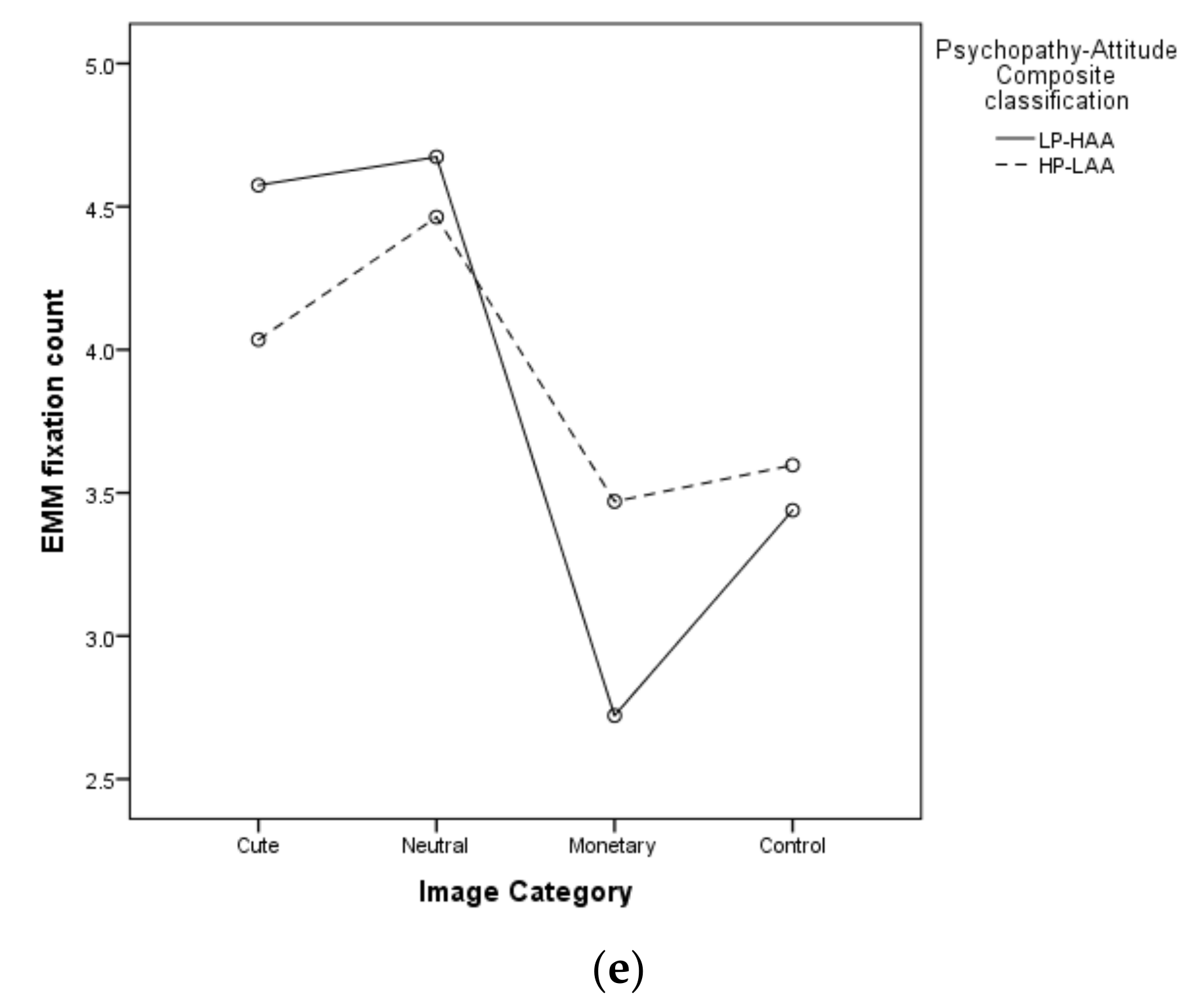
5.2.5. Mean Fixation Count
5.3. Interactions between Psychopathy-Attitude Composite Classification and Image Category
6. Discussion
6.1. Relationships between Psychopathic Traits and Animal Attitudes
6.2. Cuteness Forced-Choice Task Scores and Attention to ‘Cute’ Stimuli
6.3. Limitations and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenz, K. Die angeborenen formen müglicher erfahrung (“The innate forms of potential experience”). Z. Tierpsychol. 1943, 5, 235–409. [Google Scholar] [CrossRef]
- Borgi, M.; Cogliati-Dezza, I.; Brelsford, V.; Meints, K.; Cirulli, F. Baby schema in human and animal faces induces cuteness perception and gaze allocation in children. Front. Psychol. 2014, 5, 411. [Google Scholar] [CrossRef] [PubMed]
- Archer, J. Why do people love their pets? Evol. Hum. Behav. 1997, 18, 237–259. [Google Scholar] [CrossRef]
- Prguda, E.; Neumann, D.L. Inter-human and animal-directed empathy: A test for evolutionary biases in empathetic responding. Behav. Process. 2014, 108, 80–86. [Google Scholar] [CrossRef]
- Archer, J.; Monton, S. Preferences for Infant Facial Features in Pet Dogs and Cats. Ethology 2011, 117, 217–226. [Google Scholar] [CrossRef]
- Almanza-sepúlveda, M.L.; Dudin, A.; Wonch, K.E.; Steiner, M.; Feinberg, D.R.; Fleming, A.S.; Hall, B. Infant Behavior and Development Exploring the morphological and emotional correlates of infant cuteness. Infant Behav. Dev. 2018, 53, 90–100. [Google Scholar] [CrossRef]
- Parsons, C.E.; Young, K.S.; Bhandari, R.; Marinus, H.; Ijzendoorn, V.; Bakermanskranenburg, M.J.; Kringelbach, M.L. The bonnie baby: Experimentally manipulated temperament affects perceived cuteness and motivation to view infant faces. Dev. Sci. 2014, 2, 257–269. [Google Scholar] [CrossRef]
- Lehmann, V.; Huis in’t Veld, E.M.J.; Vingerhoets, A.J.J.M. The human and animal baby schema effect: Correlates of individual differences. Behav. Process. 2013, 94, 99–108. [Google Scholar] [CrossRef]
- Nittono, H.; Fukushima, M.; Yano, A.; Moriya, H. The Power of Kawaii: Viewing Cute Images Promotes a Careful Behavior and Narrows Attentional Focus. PLoS ONE 2012, 7, e46362. [Google Scholar] [CrossRef]
- DeLeeuw, J.L. Animal Shelter Dogs: Factors Predicting Adoption Versus Euthanasia. Ph.D. Thesis, Wichita State University, Wichita, KS, USA, December 2010. Available online: https://pdfs.semanticscholar.org/6fe9/e225839ca5510baf8293bfd58ddca5a513a3.pdf (accessed on 10 January 2020).
- Waller, B.M.; Peirce, K.; Caeiro, C.C.; Scheider, L.; Burrows, A.M.; McCune, S.; Kaminski, J. Paedomorphic facial expressions give dogs a selective advantage. PLoS ONE 2013, 8, 1013–1021. [Google Scholar] [CrossRef]
- Shipman, P. The animal connection and human evolution. Curr. Anthropol. 2010, 51, 519–538. [Google Scholar] [CrossRef]
- Royal Society for the Prevention of Cruelty to Animals (RSPCA). Available online: https://www.rspca.org.uk/whatwedo/latest/facts (accessed on 10 March 2020).
- Vaughn, M.G.; Fu, Q.; DeLisi, M.; Beaver, K.M.; Perron, B.E.; Terrell, K.; Howard, M.O. Correlates of cruelty to animals in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Psychiatr. Res. 2009, 43, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Baldry, A.C. Animal abuse and exposure to interparental violence in Italian youth. J. Interpers. Violence 2003, 18, 258–281. [Google Scholar] [CrossRef]
- Parfitt, C.H.; Alleyne, E. Animal abuse proclivity: Behavioral, personality and regulatory factors associated with varying levels of severity. Psychol. Crime Law 2018, 24, 538–557. [Google Scholar] [CrossRef]
- Henry, B.C. Empathy, home environment, and attitudes toward animals in relation to animal abuse. Anthrozoös 2006, 19, 17–34. [Google Scholar] [CrossRef]
- Oleson, J.C.; Henry, B.C. Relations among need for power, affect and attitudes toward animal Cruelty. Anthrozoös 2009, 22, 255–265. [Google Scholar] [CrossRef]
- Batt, S. Human attitudes towards animals in relation to species similarity to humans: A multivariate approach. Biosci. Horiz. 2009, 2, 180–190. [Google Scholar] [CrossRef]
- Herzog, H.A., Jr.; Betchart, N.S.; Pittman, R.B. Gender, sex role orientation, and attitudes toward animals. Anthrozoös 1991, 4, 184–191. [Google Scholar] [CrossRef]
- Henry, B. The relation between animal cruelty, delinquency, and attitudes toward the treatment of animals. Soc. Anim. 2004, 12, 185–207. [Google Scholar] [CrossRef]
- Henry, B. Exposure to animal abuse and group context: Two factors affecting participation in animal abuse. Anthrozoös 2004, 17, 290–305. [Google Scholar] [CrossRef]
- Corrigan, R.H.; Farrell, M.E. Ethics: A University Guide; Progressive Frontiers Pubs: Gloucester, UK, 2010. [Google Scholar]
- Johansson-Stenman, O. Animal Welfare and Social Decisions: Is It Time to Take Bentham Seriously? Ecol. Econ. 2018, 145, 90–103. [Google Scholar] [CrossRef]
- Hawkins, R.D.; Hawkins, E.L.; Williams, J.M. Psychological Risk Factors for Childhood Nonhuman Animal Cruelty: A Systematic Review. Soc. Anim. 2017, 25, 280–312. [Google Scholar] [CrossRef]
- Morton, L.; Daly, B. Empathic Correlates of Witnessing the Inhumane Killing of an Animal: An Investigation of Single and Multiple Exposures. Soc. Anim. 2017, 16, 243–255. [Google Scholar] [CrossRef]
- Signal, T.D.; Taylor, N. Attitude to animals and empathy: Comparing animal protection and general community samples. Anthrozoös 2007, 20, 125–130. [Google Scholar] [CrossRef]
- Hare, R.D.; Hart, S.D.; Harpur, T.J. Psychopathy and the DSM-IV Criteria for Antisocial Personality Disorder. J. Abnorm. Psychol. 1991, 100, 391–398. [Google Scholar] [CrossRef]
- Lee, Z.; Salekin, R.T. Psychopathy in a noninstitutional sample: Differences in primary and secondary subtypes. Personal. Disord. 2010, 1, 153–169. [Google Scholar] [CrossRef]
- Del Gaizo, A.L.; Falkenbach, D.M. Primary and secondary psychopathic-traits and their relationship to perception and experience of emotion. Personal. Individ. Differ. 2008, 45, 206–212. [Google Scholar] [CrossRef]
- Falkenbach, D.; Poythress, N.; Creevy, C. The exploration of subclinical psychopathic subtypes and the relationship with types of aggression. Personal. Individ. Differ. 2008, 44, 821–832. [Google Scholar] [CrossRef]
- Wilkowski, B.M.; Robinson, M.D. Putting the brakes on antisocial behavior: Secondary psychopathy and post-error adjustments in reaction time. Personal. Individ. Differ. 2008, 44, 1807–1818. [Google Scholar] [CrossRef]
- Sethi, A.; McCrory, E.; Puetz, V.; Hoffmann, F.; Knodt, A.R.; Radtke, S.R.; Brigidi, B.D.; Hariri, A.R.; Viding, E. Primary and Secondary Variants of Psychopathy in a Volunteer Sample Are Associated With Different Neurocognitive Mechanisms. Biol. Psychiatry Cogn. Neurosci. 2018, 3, 1013–1021. [Google Scholar] [CrossRef]
- Dean, A.C.; Altstein, L.L.; Berman, M.E.; Constans, J.I.; Sugar, C.A.; McCloskey, M.S. Secondary psychopathy, but not primary psychopathy, is associated with risky decision-making in noninstitutionalized young adults. Personal. Individ. Differ. 2013, 54, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Karpman, B. On the need for separating psychopathy into two distinct clinical types: Symptomatic and idiopathic. J. Crim. Psychopathol. 1941, 3, 112–137. [Google Scholar]
- Johnson, S.A. Understanding the violent personality: Antisocial personality disorder, psychopathy, & sociopathy explored. Forensic Res. Criminol. Int. J. 2019, 7, 76–88. [Google Scholar] [CrossRef]
- Stupperich, A.; Strack, M. Among a German Sample of Forensic Patients, Previous Animal Abuse Mediates Between Psychopathy and Sadistic Actions. J. Forensic Sci. 2016, 61, 699–705. [Google Scholar] [CrossRef]
- Gao, Y.; Raine, A. Successful and unsuccessful psychopaths: A neurobiological model. Behav. Sci. Law 2010, 28, 194–210. [Google Scholar] [CrossRef]
- Humane Society. 2020. Available online: https://www.humanesociety.org/resources/animal-cruelty-facts-and-stats (accessed on 10 March 2020).
- Blair, R.J.R.; Peschardt, K.S.; Budhani, S.; Mitchell, D.G.V.; Pine, D.S. The development of psychopathy. J. Child Psychol. Psychiatry 2006, 47, 262–275. [Google Scholar] [CrossRef]
- Blair, R.J.R. Dysfunctional neurocognition in individuals with clinically significant psychopathic traits. Dialogues Clin. Neurosci. 2019, 21, 291–299. [Google Scholar] [CrossRef]
- McComb, K.; Taylor, A.M.; Wilson, C.; Charlton, B.D. The cry embedded within the purr. Curr. Biol. 2019, 19, R507–R508. [Google Scholar] [CrossRef]
- Parsons, C.E.; Young, K.S.; Craske, M.G.; Stein, A.L.; Kringelbach, M.L. Introducing the Oxford Vocal (OxVoc) Sounds database: A validated set of non-acted affective sounds from human infants, adults, and domestic animals. Front. Psychol. 2014, 5, 562. [Google Scholar] [CrossRef]
- Trut, L.; Oskina, I.; Kharlamova, A. Animal evolution during domestication: The domesticated fox as a model. Bioessays 2009, 31, 349–360. [Google Scholar] [CrossRef]
- Krupp, D.B.; Sewall, L.A.; Lalumière, M.L.; Sheriff, C.; Harris, G.T. Nepotistic patterns of violent psychopathy: Evidence for adaptation? Front. Psychol. 2012, 3, 305. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.; Book, A.; Costello, K. Psychopathic traits and perceptions of victim vulnerability. Crim. Justice Behav. 2009, 36, 635–648. [Google Scholar] [CrossRef]
- Book, A.; Costello, K.; Camilleri, J.A. Psychopathy and Victim Selection: The Use of Gait as a Cue to Vulnerability. J. Interpers. Violence 2013, 28, 2368–2383. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.B.; Blais, J.; Forth, A.E.; Book, A.S. Identifying vulnerability to violence: The role of psychopathy and gender. J. Crim. Psychol. 2018, 8, 125–137. [Google Scholar] [CrossRef]
- Dadds, M.R.; El Masry, Y.; Wimalaweera, S.; Guastella, A.J. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. J. Am. Acad. Child Psychiatry 2008, 47, 455–463. [Google Scholar] [CrossRef]
- Dadds, M.R.; Allen, J.L.; Oliver, B.R.; Faulkner, N.; Legge, K.; Moul, C.; Woolgar, M.; Scott, S. Love, eye contact and the developmental origins of empathy v. psychopathy. Br. J. Psychiatry 2012, 200, 191–196. [Google Scholar] [CrossRef]
- Viding, E.; McCrory, E. Towards understanding atypical social affiliation in psychopathy. Lancet Psychiatry 2019, 6, 437–444. [Google Scholar] [CrossRef]
- Glenn, A.L.; Kurzban, R.; Raine, A. Evolutionary theory and psychopathy. Aggress. Violent Behav. 2011, 16, 371–380. [Google Scholar] [CrossRef]
- Jonason, P.K.; Webster, G.D.; Schmitt, D.P.; Li, N.P.; Crysel, L. The antihero in popular culture: Life history theory and the dark triad personality traits. Rev. Gen. Psychol. 2012, 16, 192–199. [Google Scholar] [CrossRef]
- Lalumière, M.L.; Harris, G.T.; Rice, M.E. Psychopathy and developmental instability. Evol. Hum. Behav. 2001, 22, 75–92. [Google Scholar] [CrossRef]
- Volk, A.A.; Atkinson, J.A. Infant and child death in the human environment of evolutionary adaptation. Evol. Hum. Behav. 2013, 34, 182–192. [Google Scholar] [CrossRef]
- Herzog, H.; Grayson, S.; Mccord, D. Brief Measures of the Animal Attitude Scale. Anthrozoös 2015, 28, 145–152. [Google Scholar] [CrossRef]
- Levenson, M.R.; Kiehl, K.A.; Fitzpatrick, C.M. Assessing psychopathic attributes in a noninstitutionalized population. J. Pers. Soc. Psychol. 1995, 68, 151. [Google Scholar] [CrossRef] [PubMed]
- Lobmaier, J.S.; Sprengelmeyer, R.; Wiffen, B.; Perrett, D.I. Female and male responses to cuteness, age and emotion in infant faces. Evol. Hum. Behav. 2010, 31, 16–21. [Google Scholar] [CrossRef]
- Ferrigan, M.M.; Valentiner, D.P.; Berman, M.E. Psychopathy dimensions and awareness of negative and positive consequences of aggressive behavior in a nonforensic sample. Personal. Individ. Differ. 2000, 28, 527–538. [Google Scholar] [CrossRef]
- Open Psychometrics. Levenson Self-Report Psychopathy Scale. 2020. Available online: https://openpsychometrics.org/tests/LSRP.php (accessed on 20 February 2020).
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; Technical Report A-8; University of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Calvo, M.G.; Lang, P.J. Gaze patterns when looking at emotional pictures: Attention and affect. Motiv. Emot. 2004, 28, 221–243. [Google Scholar] [CrossRef]
- Buodo, G.; Sarlo, M.; Palomba, D. Attentional resources measured by reaction times highlight differences within pleasant and unpleasant, high arousing stimuli. Motiv. Emot. 2002, 26, 123–138. [Google Scholar] [CrossRef]
- Yu, H.; Winkler, S. Image complexity and spatial information. Presented at the 5th International Workshop on Quality of Multimedia Experience, Institute of Electrical and Electronics Engineers, Klagenfurt, Austria, 3–5 July 2013. [Google Scholar]
- Holmqvist, K.; Nyström, M.; Andersson, R.; Dewhurst, R.; Jarodzka, H.; Van de Weijer, J. Eye Tracking: A Comprehensive Guide to Methods and Measures; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Giel, K.E.; Friederich, H.C.; Teufel, M.; Hautzinger, M.; Enck, P.; Zipfel, S. Attentional processing of food pictures in individuals with anorexia nervosa—An eye tracking study. Biol. Psychiatry 2011, 69, 661–667. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th ed.; American Psychiatric Pub: Arlington, VA, USA, 2013. [Google Scholar]
- Anderson, N.E.; Kiehl, K.A. Psychopathy: Developmental perspectives and their implications for treatment. Restor. Neurol. Neurosci. 2014, 32, 103–117. [Google Scholar] [CrossRef]
- Franklin, P.; Volk, A.A.; Wong, I. Are newborns’ faces less appealing? Evol. Hum. Behav. 2018, 39, 269–276. [Google Scholar] [CrossRef]
- Lewis, D.M.G.; Conroy-beam, D.; Asao, K.; Buss, D.M. Evolutionary Psychology: A How-To Guide. Am. Psychol. 2017, 72, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Serpell, J.A.; Paul, E.S. Pets in the Family: An Evolutionary Perspective. In The Oxford Handbook of Evolutionary Family Psychology; Salmon, C., Shackelford, T.K., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 297–307. [Google Scholar] [CrossRef]
- Golle, J.; Lisibach, S.; Mast, F.W.; Lobmaier, J.S. Sweet Puppies and Cute Babies: Perceptual Adaptation to Babyfacedness Transfers across Species. PLoS ONE 2013, 8, e58248. [Google Scholar] [CrossRef] [PubMed]
- Bedford, R.; Pickles, A.; Sharp, H.; Wright, N.; Hill, J. Reduced Face Preference in Infancy: A Developmental Precursor to Callous-Unemotional Traits? Biol. Psychiatry 2015, 78, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ramot, M.; Walsh, C.; Reimann, G.E.; Martin, A. Distinct neural mechanisms of social orienting and mentalizing revealed by independent measures of neural and eye movement typicality. Commun. Biol. 2020, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Prinzmetal, W.; Zvinyatskovskiy, A.; Gutierrez, P.; Dilem, L. Voluntary and involuntary attention have different consequences: The effect of perceptual difficulty. J. Exp. Psychol. 2009, 62, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.R.; Benning, S.D. The “successful” psychopath. In Handbook of Psychopathy; Patrick, C.J., Ed.; The Guilford Press: New York, NY, USA, 2006; pp. 459–478. [Google Scholar]
- Pool, E.; Brosch, T.; Delplanque, S.; Sander, D. Attentional Bias for Positive Emotional Stimuli: A Meta-Analytic Investigation. Psychol. Bull. 2016, 142, 79–106. [Google Scholar] [CrossRef]
- Borgi, M.; Cirulli, F. Children’s preferences for infantile features in dogs and cats. Hum. Anim. Interact. Bull. 2013, 1, 1–15. [Google Scholar]
- Alleyne, E.; Tilston, L.; Parfitt, C.; Butcher, R. Adult-perpetrated animal abuse: Development of a proclivity scale. Psychol. Crime Law 2015, 21, 570–588. [Google Scholar] [CrossRef]
- Lobbestael, J.; van Teffelen, M.; Baumeister, R.F. Psychopathy subfactors distinctively predispose to dispositional and state-level of sadistic pleasure. J. Behav. Ther. Exp. Psychiatry 2019, 67, 101458. [Google Scholar] [CrossRef]


| Demographic Information | Percentage |
|---|---|
| Recruitment route | |
| Prolific® | 85 |
| Social media/posters | 15 |
| County of residence | |
| UK | 78.8 |
| USA | 13.2 |
| Other | 8.0 |
| Gender | |
| Female | 70.3 |
| Male | 27.4 |
| Other | 1.6 |
| Prefer not to say | 0.3 |
| Age range | |
| 18–24 | 24.3 |
| 25–34 | 35.9 |
| 35–44 | 23.0 |
| 45–54 | 9.6 |
| 55–64 | 6.2 |
| 65–74 | 1.0 |
| Employment status | |
| Employed | 63.8 |
| Unemployed | 7.2 |
| Student | 18.6 |
| Homemaker | 8.3 |
| Retired | 2.1 |
| Prefer not to say | 1.8 |
| Pet ownership | |
| Currently own a pet | 61.2 |
| Have never owner a pet | 8.8 |
| Previously owned a pet | 30.0 |
| Parental status | |
| Yes | 33.6 |
| No | 65.6 |
| Prefer not to say | 0.8 |
| Questionnaire | HP-LAA | LP-HAA | ||||
|---|---|---|---|---|---|---|
| Levenson SRPS | mean (±) | min | max | mean (±) | min | max |
| Primary Psychopathy | 2.59 (0.65) | 1.8 | 4.3 | 1.66 (0.37) | 1.1 | 2.6 |
| Secondary Psychopathy | 2.61 (0.65) | 1.8 | 3.9 | 2.07 (0.51) | 1.1 | 3.4 |
| Animal Attitude score | 60.9 (13.10) | 21 | 82 | 82.4 (8.39) | 61 | 98 |
| Eyetracking Variable | Treatment | Levenson SRPS | Animal Attitude | |
|---|---|---|---|---|
| Primary | Secondary | |||
| Cute | ||||
| p† of initial orientations | n.s. | n.s. | 0.48 ** | |
| no. of fixations | −0.46 * | n.s. | n.s. | |
| first fixation duration (ms) | n.s. | n.s. | n.s. | |
| mean dwell time (ms) | −0.58 ** | n.s. | n.s. | |
| mean fixation duration (ms) | n.s. | n.s. | n.s. | |
| Neutral | ||||
| p† of initial orientations | n.s. | 0.46 * | 0.34 * | |
| no. of fixations | n.s. | n.s. | n.s. | |
| first fixation duration (ms) | n.s. | n.s. | n.s. | |
| mean dwell time (ms) | n.s. | n.s. | n.s. | |
| mean fixation duration (ms) | n.s. | n.s. | n.s. | |
| Monetary | ||||
| p† of initial orientations | n.s. | n.s. | n.s. | |
| no. of fixations | n.s. | n.s. | n.s. | |
| first fixation duration (ms) | n.s. | n.s. | −0.42 ** | |
| mean dwell time (ms) | n.s. | n.s. | −0.45 ** | |
| mean fixation duration (ms) | n.s. | n.s. | −0.33 * | |
| Control | ||||
| p† of initial orientations | n.s. | n.s. | n.s. | |
| no. of fixations | n.s. | n.s. | n.s. | |
| first fixation duration (ms) | n.s. | n.s. | n.s. | |
| mean dwell time (ms) | n.s. | n.s. | n.s. | |
| mean fixation duration (ms) | n.s. | n.s. | n.s. | |
| Demographic Information | HP-LAA | LP-HAA |
|---|---|---|
| Gender | ||
| Female | 61.9 | 72.4 |
| Male | 38.1 | 24.1 |
| Other | 0 | 3.4 |
| Age range | ||
| 18–24 | 71.4 | 51.7 |
| 25–34 | 19.0 | 37.9 |
| 35–44 | 4.8 | 10.3 |
| 45–54 | 0 | 0 |
| 55–64 | 4.8 | 0 |
| 65–74 | 0 | 0 |
| Employment status | ||
| Employed | 19 | 10.3 |
| Unemployed | 0 | 0 |
| Student | 81.0 | 89.7 |
| Homemaker | 0 | 0 |
| Retired | 0 | 0 |
| Pet ownership | ||
| Currently own a pet | 28.6 | 65.5 |
| Have never owned a pet | 23.8 | 17.2 |
| Previously owned a pet | 47.6 | 17.2 |
| Parental status | ||
| Yes | 4.8 | 10.3 |
| No | 95.2 | 89.7 |
| Prefer not to say | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carroll, G.A.; Cohen, L.R.; McParland, A.; Jack, S.; Montrose, V.T. Individuals with Psychopathic Traits and Poor Attitudes towards Animals Can Recognise Infant Features But Give Them Reduced Attentional Priority. Animals 2020, 10, 721. https://doi.org/10.3390/ani10040721
Carroll GA, Cohen LR, McParland A, Jack S, Montrose VT. Individuals with Psychopathic Traits and Poor Attitudes towards Animals Can Recognise Infant Features But Give Them Reduced Attentional Priority. Animals. 2020; 10(4):721. https://doi.org/10.3390/ani10040721
Chicago/Turabian StyleCarroll, Grace A., Leah R. Cohen, Aideen McParland, Sam Jack, and V. Tamara Montrose. 2020. "Individuals with Psychopathic Traits and Poor Attitudes towards Animals Can Recognise Infant Features But Give Them Reduced Attentional Priority" Animals 10, no. 4: 721. https://doi.org/10.3390/ani10040721
APA StyleCarroll, G. A., Cohen, L. R., McParland, A., Jack, S., & Montrose, V. T. (2020). Individuals with Psychopathic Traits and Poor Attitudes towards Animals Can Recognise Infant Features But Give Them Reduced Attentional Priority. Animals, 10(4), 721. https://doi.org/10.3390/ani10040721






