Simple Summary
The NLR family pyrin domain-containing 5 (NLRP5) and NLRP9 genes are two important reproductive genes; however, their effects on litter size in sheep are unknown. In this study, we conducted population genetic and association analyses on five NLRP5 and NLRP9 loci of sheep. Our results suggested that a mutation in g.60495363G > A may decrease interactions of NLRP5 with proteins, such as the growth differentiation factor 9 (GDF9), whereas a mutation in g. g.59030623T > C may enhance the NLRP9-combining capacity with these proteins. Consequently, these mutations may lead to differences in ovulation rate and even litter size. Overall, this study provided useful genetic markers that can be used to improve sheep breeding.
Abstract
Previous studies showed that the NLR family pyrin domain-containing 5 (NLRP5) and NLRP9 genes are two important reproductive genes; however, their effects on sheep litter size are unknown. Therefore, in this study, we first genotyped seven sheep breeds via the MassARRAY® SNP system at the loci g.60495375A > G, g.60495363G > A, and g.60499690C > A in NLRP5, and g.59030623T > C and g.59043397A > C in NLRP9. Our results revealed that each locus in most sheep breeds contained three genotypes. Then, we conducted population genetic analysis of single nucleotide polymorphisms in NLRP5 and NLRP9, and we found that the polymorphism information content value in all sheep breeds ranged from 0 to 0.36, and most sheep breeds were under Hardy–Weinberg equilibrium (p > 0.05). Furthermore, association analysis in Small Tail Han sheep indicated that two loci, g.60495363G > A in NLRP5 and g.59030623T > C in NLRP9, were highly associated with litter size. The mutation in g.60495363G > A may decrease interactions of NLRP5 with proteins, such as GDF9, whereas the mutation in g.59030623T > C may enhance the combining capacity of NLRP9 with these proteins; consequently, these mutations may influence the ovulation rate and even litter size. The findings of our study provide valuable genetic markers that can be used to improve the breeding of sheep and even other mammals.
1. Introduction
Reproduction, a key process in sheep production, is an extremely complex process that is controlled by many genes. NLR family pyrin domain-containing 5 (NLRP5) is an important member of the NLR family and was reported to participate in reproduction in many species. A mutation of NLRP5 in humans was discovered to be a key factor in maternal reproductive fitness and early zygotic development [1]. Additionally, a lack of NLRP5 in mouse oocytes resulted in premature activation of the mitochondrial pool, which results in mitochondrial damage that cannot be recovered by BCL2 associated X (Bax) inactivation [2], and NLRP5 knockout in mice led to female infertility [3]. Furthermore, preovulatory aging in mouse oocyte maturation decreased NLRP5 abundance, which indicated that NLRP5 has critical roles in oocyte development [4]. Notably, NLRP5, as a member of the sub-cortical maternal complex, was also found only expressed in ovine ovary and especially in the ovine oocytes in the germinal vesicle and metaphase II stage [5], which suggested their important roles in the oocyte developmental potential of sheep. However, its effects on sheep litter size are poorly understood.
NLRP9 is also an important member of the NLR family. NLRP9 is highly detectable during in vitro maturation of bovine oocytes from small follicles, and may be a factor that affects the follicle diameter [6]. Moreover, NLRP9 is also highly expressed in the ovine ovary [7]; however, the effects of NLRP9 on sheep reproduction, such as litter size, remain largely unknown.
Significantly, point mutations of several genes affecting the litter size in sheep were discovered. Several were found to be major genes in reproduction; the most prominent gene, FecB (Fec = Fecundity, B = Booroola), was identified as a missense mutation in bone morphogenetic protein receptor, type 1B (BMPR1B) at base A746G, and this mutation results in an amino acid change from glutamine to arginine [8]. The effects of this mutation on ovulation and litter size mainly depend on the copy carried. Normally, ewes with one copy of the FecB mutation increase the rate of ovulation by 1.5 and litter size by 1, and ewes with two copies can significantly increase the rate of ovulation and litter size by 3 and 1.5 (summarized by Liu et al. [9]). In addition, several other mutations in genes, including the growth differentiation factor 9 gene (GDF9) [10,11], bone morphogenetic protein 15 (BMP15) [12], BMP2, and BMP7 [13], were also reported to participate in sheep reproduction and lead to differences in litter size; all of these point mutations could be important genetic markers for sheep breeding.
Therefore, in this study, we aim to explore the association between NLRP5 and NLRP9 single nucleotide polymorphisms (SNPs) with litter size. This information has not previously been elucidated but is expected to provide valuable genetic markers for sheep breeding.
2. Materials and Methods
2.1. Animal Preparation and Sample Collection
All experimental procedures involving animals used in this study were approved by the Science Research Department (in charge of animal welfare issues) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS; Beijing, China). In addition, ethics approval was given by the animal ethics committee of IAS-CAAS (no. IASCAAS-AE-03, 12 December 2016).
Blood samples from 768 ewes were obtained from the jugular vein, and all samples were processed with the phenol–chloroform method for DNA isolation. Among the seven sheep breeds used in this study, three are higher prolificacy breeds (Cele Black sheep, n = 68; Hu sheep, n = 83; and Small Tail Han sheep, n = 384) and four breeds are lower prolificacy breeds (Prairie Tibetan sheep, n = 80; Suffolk sheep, n = 60; Sunite sheep, n = 70; and Tan sheep, n = 23) (Table 1).

Table 1.
Basic information on ewes used in this study.
2.2. Genotyping
First, single-base extended primers for g.60495375A > G, g.60495363G >A, and g.60499690C > A in NLRP5 and g.59030623T > C and g.59043397A > C in NLRP9 were designed via MassARRAY Assay Design v. 3.1 based on the sheep sequences of NLRP5 and NLRP9 available in GenBank (accession no.: NC_019471.1, NLRP5; NC_019471.1, NLRP9). Then, these primers were synthesized by Beijing Compass Biotechnology Co., Ltd. (Beijing, China). The genotyping was conducted in a MassARRAY® SNP system; detailed information about the system and procedures has been previously described [6,14].
2.3. Statistical Analysis
Allele and genotype frequency, polymorphism information content (PIC), heterozygosity (HE), and number of effective alleles (NE), and p values (Chi-Square test) were calculated using the data after genotyping, and ewe populations with p > 0.05 (Chi-Square test) were considered to be under Hardy–Weinberg equilibrium. To determine the association between genotypes and litter size, the adjusted linear model, yijn = μ+ Pi+Gj + IPG + eijn, was applied, in which yijn is the phenotypic value (litter size); μ represents the population mean; Pi indicates the fixed effect of the ith parity (i = 1, 2, or 3); Gj represents the effect of the jth genotypes (j = 1, 2, or 3); IPG is the interactive effect of parity and genotype; and eijn indicates random error.
2.4. Protein Interaction Networks Predicted by STRING Database
To primarily explore the mechanisms by which NLRP5 and NLRP9 affect litter size, we predicted the protein interactions involving NLRP5 and NLRP9 via the STRING database v. 11.0 [15] (https://string-db.org), which is a useful tool to predict protein interactions. We firstly typed the protein name, then selected the organism sheep (Ovis aries), then carried out the prediction according to the default settings (meaning of network edges: evidence).
3. Results
3.1. Population Genetic Analysis of SNPs in NLRP5 and NLRP9
Three SNPs in NLPR5 (g.60495375A > G, g.60495363G>A, and g.60499690C > A) and two SNPs in NLRP9 (g.59030623T > C and g.59043397A > C) were detected (Table 2 and Table 3). The g.60495375A > G locus had low polymorphism (PIC < 0.25) in Tan, Small Tail Han, and Suffolk sheep but had moderate polymorphism (0.25 < PIC < 0.5) in the Prairie Tibetan, Cele Black, Hu, and Sunite sheep; in addition, the Chi-square test revealed that this locus was under Hardy–Weinberg equilibrium in Cele Black, Hu, Sunite, Small Tail Han, and Suffolk sheep (p > 0.05) but not in Prairie Tibetan and Tan sheep (p < 0.05). The g.60495363G > A locus was moderately polymorphic (0.25 < PIC < 0.5) in all seven sheep breeds, and this SNP was under Hardy–Weinberg equilibrium in all seven sheep breeds (p > 0.05). In all seven sheep breeds, the g.60499690C > A locus was moderately polymorphic (0.25 < PIC < 0.5) and under Hardy–Weinberg equilibrium (p > 0.05). The g.59030623T > C locus had relatively low polymorphism (PIC < 0.25) in Prairie Tibetan, Cele Black, Hu, Small Tail Han, and Tan sheep but had moderate polymorphism (0.25 < PIC < 0.5) in Suffolk and Sunite sheep; additionally, this SNP was under Hardy–Weinberg equilibrium in all seven sheep breeds (p > 0.05). The g.59043397A > C locus had relatively low polymorphism (PIC < 0.25) in Prairie Tibetan, Suffolk, Sunite, Small Tail Han, and Tan sheep but had moderate polymorphism (0.25 < PIC < 0.5) in Cele Black and Hu sheep; furthermore, this locus was under Hardy–Weinberg equilibrium (p > 0.05) in all sheep breeds except Small Tail Han sheep (p < 0.05).

Table 2.
Population genetic analysis of three NLR family pyrin domain-containing 5 (NLRP5) loci in seven sheep breeds.

Table 3.
Population genetic analysis of two loci of NLRP9 in seven sheep breeds.
3.2. Associations between Five Loci in NLRP5 and NLRP9 with Litter Size in Small Tail Han Sheep
The results (Table 4) revealed that the g.60495363G > A locus in NLRP5 was highly associated with litter size in Small Tail Han sheep; the litter size of ewes with the GG genotype was higher than that of ewes with AA and GA genotypes. Additionally, the g.59030623T > C locus in NLRP9 was highly associated with litter size in Small Tail Han sheep; the litter size of ewes with the CC genotype was higher than that of ewes with TT and TC genotypes (Table 5). The remaining three loci had no significant association with litter size in Small Tail Han sheep.

Table 4.
Least squares mean and standard error of litter size in Small Tail Han sheep with different genotypes of g.60495375A > G, g.60495363G > A, and g.60499690C > A.

Table 5.
Least squares mean and standard error of litter size in Small Tail Han sheep with different genotypes of g.59030623T > C and g.59043397A > C.
3.3. Predicted Protein Interaction Networks Involving NLRP5 and NLRP9
As Figure 1 shows, NLRP5 was predicted to interact with 10 proteins, including GDF9, which has been proven to be a key factor that affects litter size [16,17]. Interestingly, NLRP9 was also predicted to interact with 10 proteins, including GDF9.
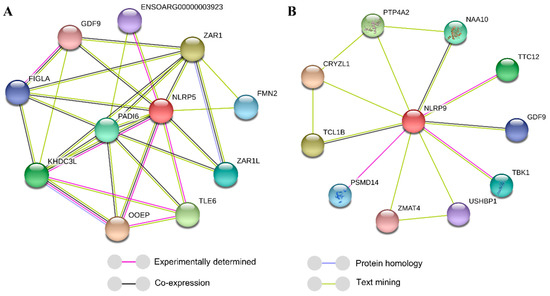
Figure 1.
Interactions between proteins including NLRP5 (A) and NLRP9 (B), as predicted by the STRING database.
4. Discussion
4.1. NLRP5 and NLRP9 Polymorphisms
As two important members of the NLR family, NLRP5 and NLRP9 play important roles in mammal reproduction [1,11,12]. However, little is known about their effects on litter size in ewes. Therefore, in this study, we first conducted population genetic analysis of five loci in NLRP5 and NLRP9. The results suggested that the loci in some sheep breeds exhibited relatively low polymorphism (PIC < 0.25), such as g.60495375A > G, g.59030623T > C, and g.59043397A > C in Tan sheep, and g.59030623T > C and g.59043397A > C in Prairie Tibetan sheep; this may result from the examination of a limited number of ewes, and increasing the number of examined ewes may increase the PIC value. Additionally, many ewe populations contained three genotypes, which indicated that those SNPs were widely distributed in sheep herds that include sheep breeds with different fecundity. However, only two genotypes were detected in some cases, such as for g.59030623T > C in Tan and Hu sheep, and three genotypes may be detected by increasing the number of examined ewes. In addition, several loci, such as g.60495375A > G in Tan sheep and g.59043397A > C in Small Tail Han sheep, were not in Hardy–Weinberg equilibrium (p < 0.05), which may result from natural and artificial selection.
4.2. Association Analyses of NLRP5 and NLRP9
Early studies grouped NLRP5 and NLRP9 as two members of the reproduction-related NLRP cluster in mammals [18], which indicates that they have potential functions in reproduction. Docherty et al. [1] reported that some NLRP5 variants in women were associated with a period of infertility, miscarriage, and molar pregnancy, which may be consequences of reproductive problems. In our results, three SNPs were detected; two SNPs showed an increasing litter size trend but failed to reach significance (p > 0.05), whereas the wildtype homozygous genotype of g.60495363G > A (GG) was associated with significantly greater litter size in Small Tail Han sheep compared with GA and AA genotypes; therefore, this mutation seems to be harmful.
Previous studies found that several variants in NLRP9 may influence the disease course in multiple sclerosis patients, as determined by an exome sequencing study [19], and may be associated with familial late-onset Alzheimer’s disease, as determined by a genome-wide association study [20]. However, little has been found regarding its effects on litter size. In our study, two detected SNPs showed increasing trends in litter size; in particular, a mutation in the g.59030623T > C locus could highly increase litter size in Small Tail Han sheep, which may be a useful genetic marker for increasing sheep litter size.
4.3. Reproductive Functions of GDF9 and Its Interactions with NLRP5 and NLRP9
GDF9 has been proven to be highly associated with litter size in sheep (Small Tail Han sheep) [10,21], goats [22,23], and even dogs [24]. Ongoing research has revealed some potential mechanisms underlying this association. GDF9 was reported to enhance mitochondrial activity, meiotic resumption, and secondary follicle development in sheep [25], and GDF9 was also shown to enhance granulosa cell proliferation [26]. Further study indicated that GDF9 is required for normal folliculogenesis in many mammal species [17]; therefore, GDF9 may be a direct factor that influences litter size in ewes by affecting functions of granulosa cells and folliculogenesis.
NLRP5 in ovine species was demonstrated to cooperate with oocyte expressed protein (OOEP), TLE family member 6 (TLE6), and KH domain containing 3 (KHDC3) to function in oocyte development [5], and it was also proven to meditate the mitochondrial function in mice, which is indispensable for oocyte development [2]. Moreover, NLRP5, also called MATER, could mediate follicular maturation in humans by acting as a substrate of protein kinase C epsilon in cumulus cells [27]. It is possible that a missense mutation from G to A at the g.60495363G > A locus may decrease its positive effects on oocyte development and follicular maturation. Little is known about the effects of NLRP9 on oocyte development or follicular maturation; however, researches demonstrated that NLRP9 was highly expressed in adult bovine oocytes rather than prepubertal animals [12] and was particularly highly expressed in sheep ovaries [7]. Additionally, two oocyte-specific NLRP genes in mammals, NLRP5 and NLRP9, were only expressed in mouse oocytes [28], which indicated that they have critical roles in ovary functions, such as oocyte development and follicular maturation. Therefore, by considering the key roles of NLRP5 and NLRP9 in ovine ovary functions, such as oocyte development and follicular maturation, predicted protein interactions, and point mutation effects on the protein-binding capacity [29,30], we conclude that the missense mutation in g.60495363G > A of NLRP5 may decrease the interactions of NLRP5, such as the combining capacity, with proteins, such as GDF9; this may decrease the litter size. Conversely, the missense mutation in g.59030623T > C of NLRP9 may enhance the NLRP9 combining capacity with proteins, such as GDF9, which would promote the ovulation rate and even increase the litter size.
5. Conclusions
In this study, we first conducted population genetic analysis of SNPs in NLRP5 and NLRP9. We found two key loci (g.60495363G > A in NLRP5 and g.59030623T > C in NLRP9) via association analysis. Further analysis indicated that these detected missense mutations may influence litter size in ewes by affecting the interactions of NLRP5 and NLRP9 with proteins, such as GDF9.
Author Contributions
Conceptualization, Z.Z. and M.C.; Formal analysis, Z.Z.; Funding acquisition, R.D. and M.C.; Investigation, Z.Z., J.T. and X.H.; Project administration, R.D. and M.C.; Resources, X.H.; Software, Z.Z. and J.T.; Visualization, Z.Z. and J.T.; Writing-Original Draft Preparation, Z.Z.; Writing—Review and Editing, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31861143012 and 31772580), the Earmarked Fund for China Agriculture Research System (CARS-38), the Central Public-Interest Scientific Institution Basal Research Fund (Y2017JC24), the Agricultural Science and Technology Innovation Program of China (ASTIP-IAS13, CAAS-XTCX2016011-02-02), the China Agricultural Scientific Research Outstanding Talents and Their Innovative Teams Program, the China High-level Talents Special Support Plan Scientific and Technological Innovation Leading Talents Program (W02020274), and the Tianjin Agricultural Science and Technology Achievements Transformation and Popularization Program (201704020). The APC was funded by the National Natural Science Foundation of China (31861143012).
Conflicts of Interest
All authors declare no conflict of interest.
References
- Docherty, L.E.; Rezwan, F.I.; Poole, R.L.; Turner, C.L.S.; Kivuva, E.; Maher, E.R.; Smithson, S.F.; Hamilton-Shield, J.P.; Patalan, M.; Gizewska, M.; et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat. Commun. 2015, 6, 8086. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Tsuda, C.; Perumalsamy, A.L.; Naranian, T.; Chong, J.; Acton, B.M.; Tong, Z.-B.; Nelson, L.M.; Jurisicova, A. NLRP5 mediates mitochondrial function in mouse oocytes and embryos. Biol. Reprod. 2012, 86, 138. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.B.; Gold, L.; Pfeifer, K.E.; Dorward, H.; Nelson, L.M. Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet. 2000, 26, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Demond, H.; Trapphoff, T.; Dankert, D.; Heiligentag, M.; Grümmer, R.; Horsthemke, B.; Eichenlaub-Ritter, U. Preovulatory aging in vivo and in vitro affects maturation rates, abundance of selected proteins, histone methylation pattern and spindle integrity in murine oocytes. PLoS ONE 2016, 11, e0162722. [Google Scholar] [CrossRef]
- Bebbere, D.; Ariu, F.; Bogliolo, L.; Masala, L.; Murrone, O.; Fattorini, M.; Falchi, L.; Ledda, S. Expression of maternally derived KHDC3, NLRP5, OOEP and TLE6 is associated with oocyte developmental competence in the ovine species. BMC Dev. Biol. 2014, 14, 40. [Google Scholar] [CrossRef]
- Romar, R.; Santis, T.D.; Papillier, P.; Perreau, C.; Thélie, A.; Dell’Aquila, M.; Mermillod, P.; Dalbiès-Tran, R. Expression of maternal transcripts during bovine oocyte in vitro maturation is affected by donor age. Reprod. Domest. Anim. 2011, 46, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xie, M.; Chen, W.; Talbot, R.; Maddox, J.F.; Faraut, T.; Wu, C.; Muzny, D.M.; Li, Y.; Zhang, W.; et al. The sheep genome illuminates biology of the rumen and lipid metabolism. Science 2014, 344, 1168–1173. [Google Scholar] [CrossRef]
- Fogarty, N.M. A review of the effects of the Booroola gene (FecB) on sheep production. Small Rumin. Res. 2009, 85, 75–84. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, Z.; Wang, X.; Hu, W.; Di, R.; Yao, Y.; Chu, M. Progress on major genes for high fecundity in ewes. Front. Agric. Sci. Eng. 2014, 1, 282–290. [Google Scholar] [CrossRef]
- Chu, M.X.; Yang, J.; Feng, T.; Cao, G.L.; Fang, L.; Di, R.; Huang, D.W.; Tang, Q.Q.; Ma, Y.H.; Li, K. GDF9 as a candidate gene for prolificacy of Small Tail Han sheep. Mol. Biol. Rep. 2011, 38, 5199–5204. [Google Scholar] [CrossRef]
- Wang, W.; La, Y.; Zhou, X.; Zhang, X.; Li, F.; Liu, B. The genetic polymorphisms of TGFβ superfamily genes are associated with litter size in a Chinese indigenous sheep breed (Hu sheep). Anim. Reprod. Sci. 2018, 189, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.H.; Chantepie, L.; Serrano, M.; Sarto, M.P.; Iguacel, L.P.; Jimenez, M.A.; Alabart, J.L.; Folch, J.; Fabre, S.; Lahoz, B. A new allele in the BMP15 gene (FecX(RA)) that affects prolificacy co-segregates with FecX(R) and FecX(GR) in Rasa Aragonesa sheep. Theriogenology 2020, 144, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Q.; Di, R.; Hu, W.; Wang, X.; He, X.; Ma, L.; Chu, M. Single nucleotide polymorphisms in BMP2 and BMP7 and the association with litter size in Small Tail Han sheep. Anim. Reprod. Sci. 2019, 204, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Pan, Z.Y.; Cao, X.H.; Guo, X.F.; He, X.Y.; Sun, Q.; Chu, M.X. Single nucleotide polymorphisms in the HIRA gene affect litter size in Small Tail Han Sheep. Animals 2018, 8, 71. [Google Scholar] [CrossRef]
- von Mering, C.; Jensen, L.J.; Snel, B.; Hooper, S.D.; Krupp, M.; Foglierini, M.; Jouffre, N.; Huynen, M.A.; Bork, P. STRING: Known and predicted protein–protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005, 33 (Suppl. S1), 433–437. [Google Scholar] [CrossRef]
- Ahmad, H.I.; Liu, G.; Jiang, X.; Edallew, S.G.; Wassie, T.; Tesema, B.; Yun, Y.; Pan, L.; Liu, C.; Chong, Y.; et al. Maximum-likelihood approaches reveal signatures of positive selection in BMP15 and GDF9 genes modulating ovarian function in mammalian female fertility. Ecol. Evol. 2017, 7, 8895–8902. [Google Scholar] [CrossRef]
- Christoforou, E.R.; Pitman, J.L. Intrafollicular growth differentiation factor 9: Bone morphogenetic 15 ratio determines litter size in mammals. Biol. Reprod. 2019, 100, 1333–1343. [Google Scholar] [CrossRef]
- Tian, X.; Pascal, G.; Monget, P. Evolution and functional divergence of NLRP genes in mammalian reproductive systems. BMC Evol. Biol. 2009, 9, 202. [Google Scholar] [CrossRef]
- Gil-Varea, E.; Urcelay, E.; Vilariño-Güell, C.; Costa, C.; Midaglia, L.; Matesanz, F.; Rodríguez-Antigüedad, A.; Oksenberg, J.; Espino-Paisan, L.; Dessa Sadovnick, A.; et al. Exome sequencing study in patients with multiple sclerosis reveals variants associated with disease course. J. Neuroinflamm. 2018, 15, 265. [Google Scholar] [CrossRef]
- Fernández, M.V.; John, B.L.; Del-Aguila, J.L.; Ibañez, L.; Deming, Y.; Harari, O.; Norton, J.; Morris, J.C.; Goate, A.M.; NIA-LOAD family study group; et al. Evaluation of gene-based family-based methods to detect novel genes associated with familial late onset Alzheimer disease. Front. Neurosci. 2018, 12, 209. [Google Scholar] [CrossRef]
- El Fiky, Z.A.; Hassan, G.M.; Nassar, M.I. Genetic polymorphism of growth differentiation factor 9 (GDF9) gene related to fecundity in two Egyptian sheep breeds. J. Assist. Reprod. Genet. 2017, 34, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Q.; Zhang, S.; Zhang, X.; Pan, C.; Chen, H.; Zhu, H.; Lan, X. Genetic effects of single nucleotide polymorphisms in the goat GDF9 gene on prolificacy: True or false positive? Animals 2019, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, P.; Rashidi, A.; Rostamzadeh, J.; Razmkabir, M. Association between c.1189G>A single nucleotide polymorphism of GDF9 gene and litter size in goats: A meta-analysis. Anim. Reprod. Sci. 2019, 209, 106140. [Google Scholar] [CrossRef] [PubMed]
- Torrecilha, R.B.P.; Milanesi, M.; Gallana, M.; Falbo, A.K.; Reichler, I.M.; Hug, P.; Jagannathan, V.; Trigo, B.B.; Paulan, S.C.; Bruno, D.B.; et al. Association of missense variants in GDF9 with litter size in Entlebucher Mountain dogs. Anim. Genet. 2020, 51, 78–86. [Google Scholar] [CrossRef]
- Monte, A.P.O.; Santos, J.M.; Menezes, V.G.; Gouveia, B.B.; Lins, T.L.B.G.; Barberino, R.S.; Oliveira, J.L.; Donfack, N.J.; Matos, M.H.T. Growth differentiation factor-9 improves development, mitochondrial activity and meiotic resumption of sheep oocytes after in vitro culture of secondary follicles. Reprod. Domest. Anim. 2019, 54, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Pitman-Crawford, J.L.; Bibby, A.H.; Hudson, N.I.L.; McIntosh, C.J.; Juengel, J.L.; McNatty, K.P. Effects of species differences on oocyte regulation of granulosa cell function. Reproduction 2012, 144, 557–567. [Google Scholar] [CrossRef]
- Maraldi, T.; Riccio, M.; Sena, P.; Marzona, L.; Nicoli, A.; La Marca, A.; Marmiroli, S.; Bertacchini, J.; La Sala, G.; De Pol, A. MATER protein as substrate of PKC in human cumulus cells. Mol. Hum. Reprod. 2009, 15, 499–506. [Google Scholar] [CrossRef]
- Hamatani, T.; Falco, G.; Carter, M.G.; Akutsu, H.; Stagg, C.A.; Sharov, A.A.; Dudekula, D.B.; VanBuren, V.; Ko, M.S. Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 2004, 13, 2263–2278. [Google Scholar] [CrossRef]
- Eroshkin, F.M.; Fedina, N.V.; Martynova, N.Y.; Bayramov, A.V.; Zaraisky, A.G. A point mutation of the Noggin2 protein increasing its binding capacity to activin. Bioorg. Khim. 2015, 41, 675–677. [Google Scholar] [CrossRef]
- Cauwe, B.; Tian, L.; Franckaert, D.; Pierson, W.; Staats, K.A.; Schlenner, S.M.; Liston, A. A novel Zap70 mutation with reduced protein stability demonstrates the rate-limiting threshold for Zap70 in T-cell receptor signaling. Immunology 2014, 141, 377–387. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).