L-OPU in Goat and Sheep—Different Variants of the Oocyte Recovery Method
Abstract
Simple summary
Abstract
1. Introduction
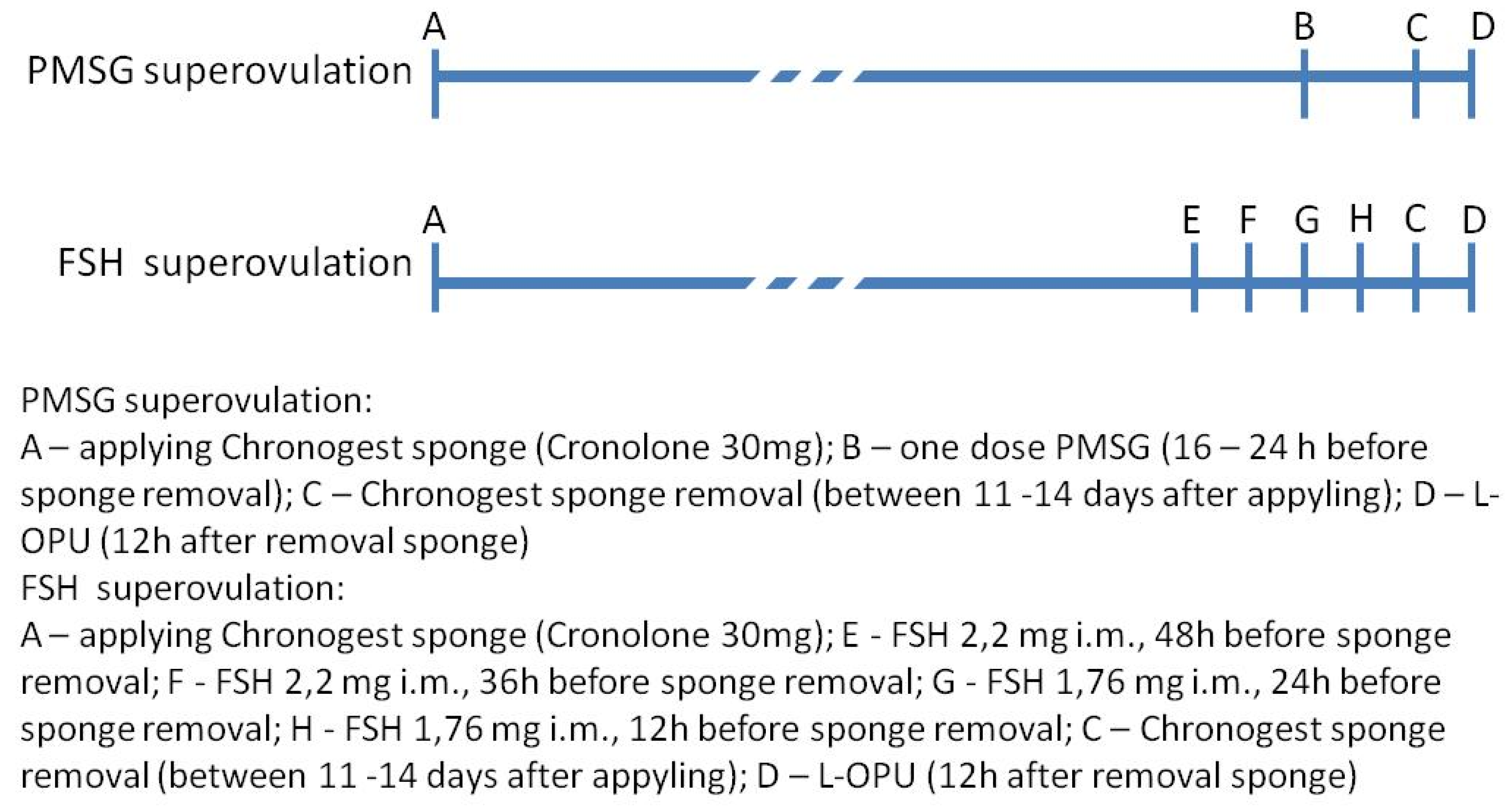
2. Materials and Methods
2.1. Animals
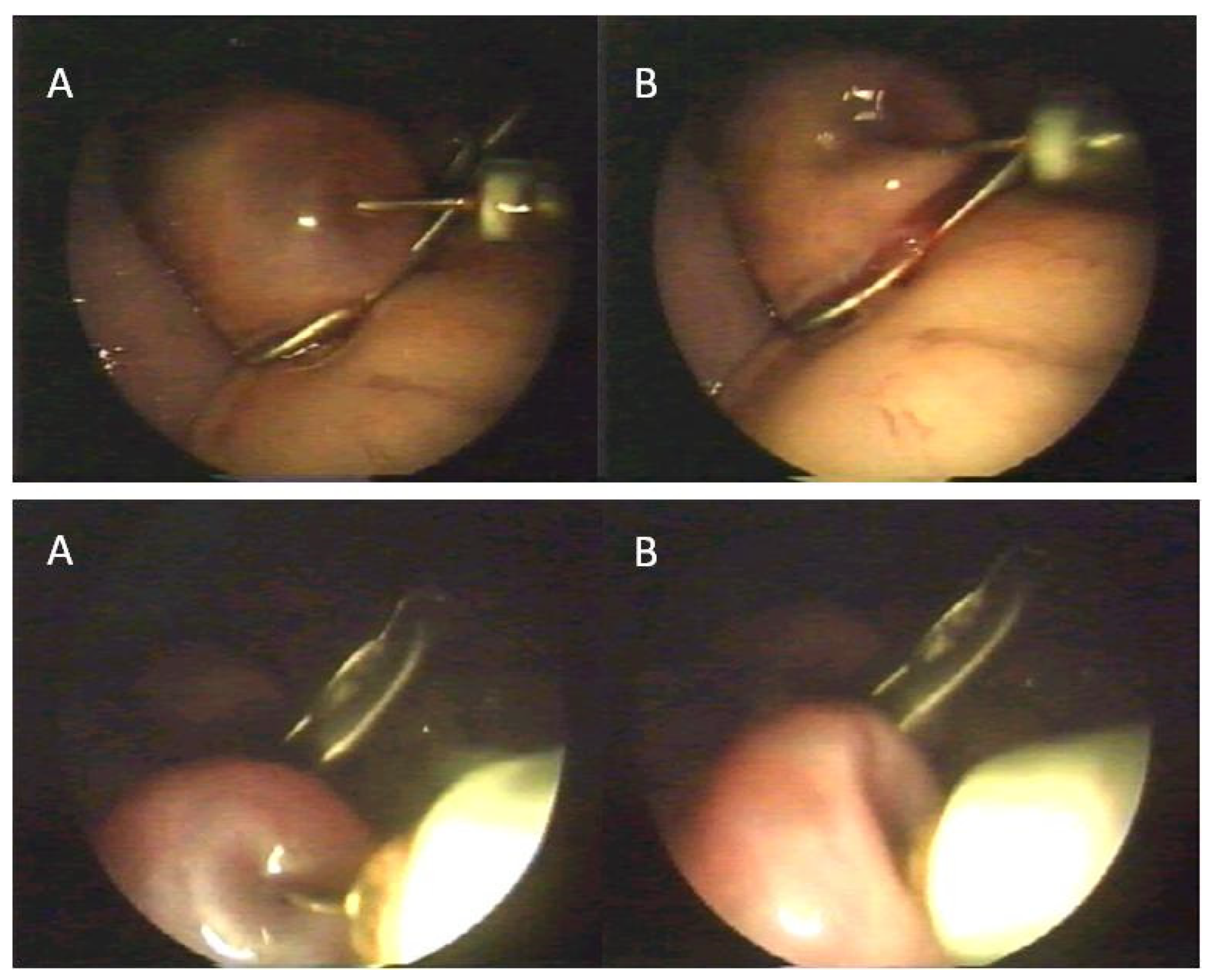
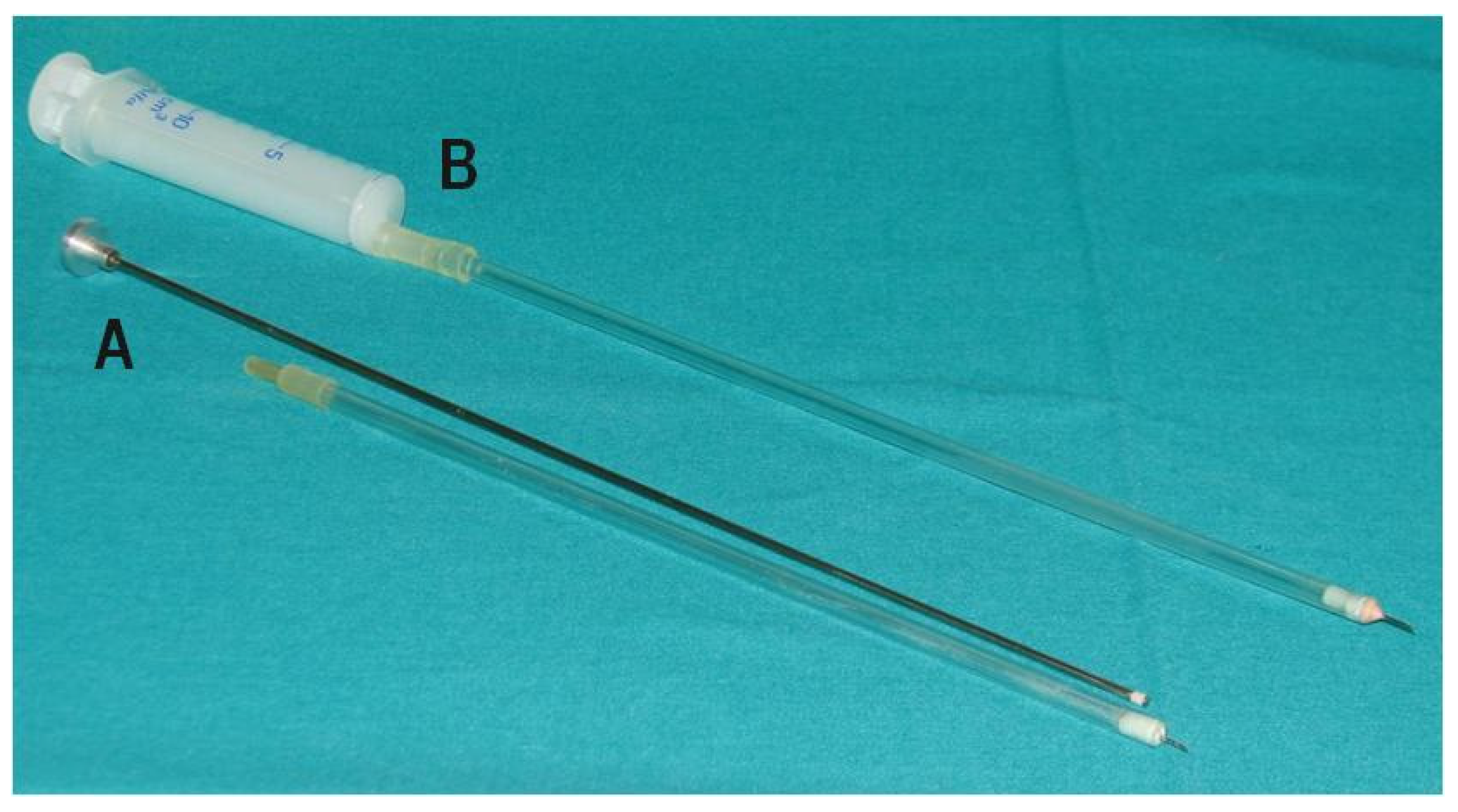
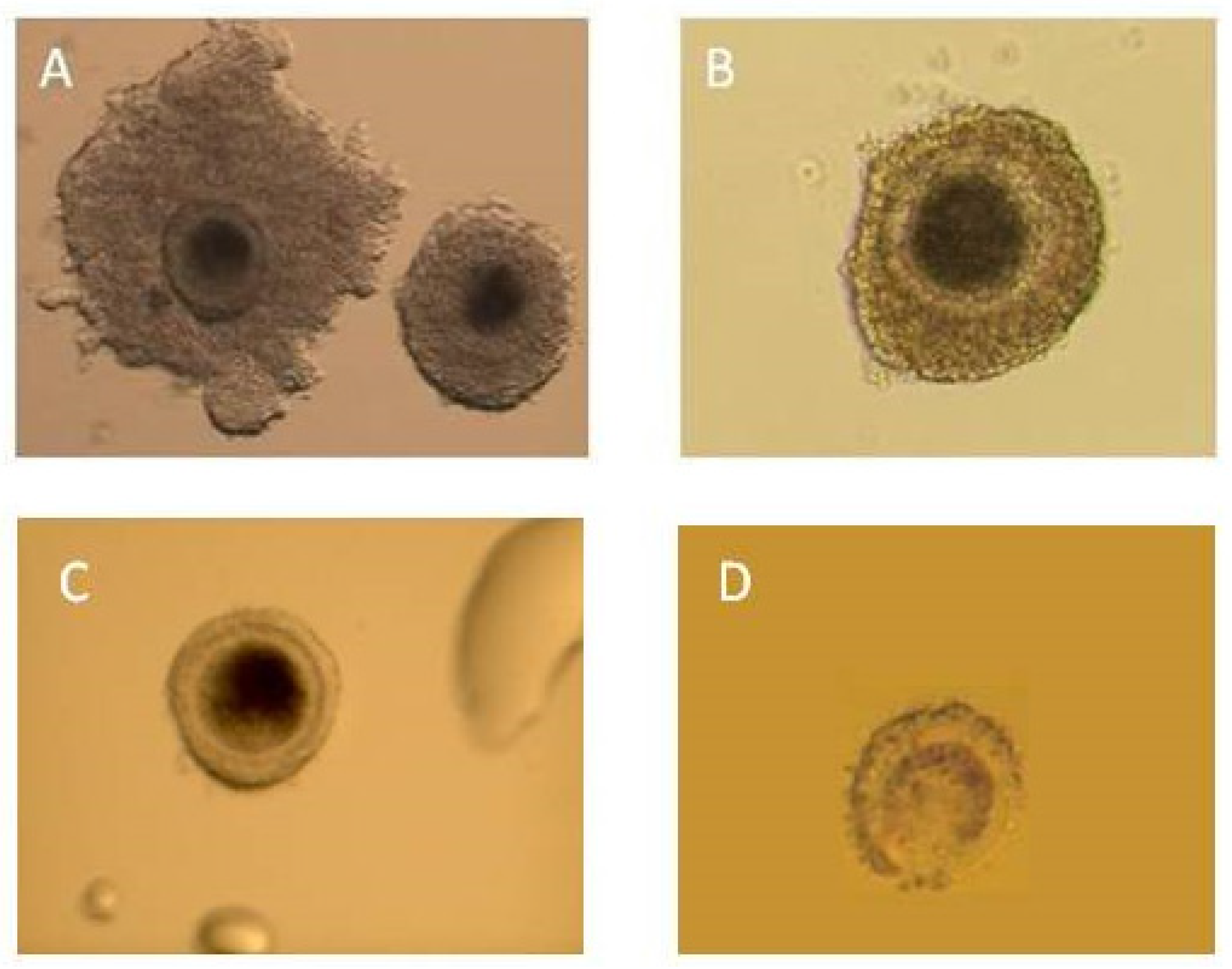
2.2. Procedure
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baldassarre, H.; Wang, B.; Kafidi, N.; Gauthier, M.; Neveu, N.; Lapointe, J.; Sneek, L.; Leduc, M.; Duguay, F.; Zhou, J.F.; et al. Production of transgenic goats by pronuclear microinjection of in vitro produced zygotes derived from oocytes recovered by laparoscopy. Theriogenology 2003, 59, 831–839. [Google Scholar] [CrossRef]
- Brandão, F.A.S.; Alves, B.G.; Alves, K.A.; Souza, S.S.; Silva, Y.P.; Freitas, V.J.F.; Teixeira, D.I.A.; Gastal, E.L. Laparoscopic ovarian biopsy pick-up method for goats. Theriogenology 2018, 107, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, W.; Sun, S. Research advances in reproduction for dairy goats. Asian-Australas J. Anim. Sci. 2019, 32, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Menchaca, A.; Barrera, N.; dos Santos Neto, P.C.; Cuadro, F.; Crispo, M. Advances and limitations of in vitro embryo production in sheep and goats. Anim. Reprod. 2016, 13, 273–278. [Google Scholar] [CrossRef]
- Fonseca, J.F.; Souza-Fabjan, J.M.; Oliveira, M.E.; Leite, C.R.; Nascimento-Penido, P.M.; Brandão, F.Z.; Lehloenya, K.C. Nonsurgical embryo recovery and transfer in sheep and goats. Theriogenology 2016, 86, 144–151. [Google Scholar] [CrossRef]
- Galli, C.; Crotti, G.; Notari, C.; Turini, P.; Duchi, R.; Lazzari, G. Embryo production by ovum pick up from live donors. Theriogenology 2000, 55, 1341–1357. [Google Scholar] [CrossRef]
- Cogine, Y. State of the art in sheep—Goat embryo transfer. Theriogenology 1999, 51, 105–116. [Google Scholar] [CrossRef]
- Menchaca, A.; Vilariño, M.; Crispo, M.; de Castro, T.; Rubianes, E. New approaches to superovulation and embryo transfer in small ruminants. Reprod. Fertil. Dev. 2010, 22, 113–118. [Google Scholar] [CrossRef]
- Kühholzer, B.; Müller, S.; Prokofiev, M.I.; Ernst, L.K.; Besenfelder, U.; Brem, G. Laparoscopic techniques for the recovery and transfer of microinjected goat zygotes. Theriogenology 1998, 49, 245. [Google Scholar] [CrossRef]
- Gonźalez-Bulnes, A.; Baird, D.T.; Campbell, B.K.; Cocero, M.J.; García-García, R.M.; Inskeep, E.K.; López-Sebastián, A.; McNeilly, A.S.; Santiago-Moreno, J.; Souza, C.J.; et al. Multiple factors affecting the efficiency of multiple ovulation and embryo transfer in sheep and goats. Reprod. Fertil. Dev. 2004, 16, 421–435. [Google Scholar] [CrossRef]
- Paramio, M.T.; Izquierdo, D. Recent advances in in vitro embryo production in small ruminants. Theriogenology 2016, 86, 152–159. [Google Scholar] [CrossRef] [PubMed]
- de Souza-Fabjan, J.M.; Panneau, B.; Duffard, N.; Locatelli, Y.; de Figueiredo, J.R.; Freitas, V.J.; Mermillod, P. In vitro production of small ruminant embryos: Late improvements and further research. Theriogenology 2014, 81, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, J.; Koseniuk, J.; Cegla, M. The repeatable method of laparoscopic ovum pick-up (OPU) in sheep: Clinical aspects and efficiency of the method. Pol. J. Vet. Sci. 2018, 21, 803–810. [Google Scholar] [PubMed]
- Stangl, M.; Kuhholzer, B.; Besenfelder, U.; Brem, G. Repeated endoscopic ovum pick-up in sheep. Theriogenology 1999, 52, 709–716. [Google Scholar] [CrossRef]
- Graff, K.J.; Meintjes, M.; Dyer, V.W.; Paul, J.B.; Denniston, R.S.; Ziomek, C.; Godke, R.A. Transvaginal ultrasound-guided oocyte retriveal following FSH stimulation of domestic goats. Theriogenology 1998, 51, 1099–1199. [Google Scholar] [CrossRef]
- Cognië, Y.; Baril, G.; Poulin, N.; Mermillod, P. Current status of embryo technologies in sheep and goat. Theriogenology 2003, 59, 171–188. [Google Scholar] [CrossRef]
- Cox, J.F.; Alfaro, V. In vitro fertilization and development of OPU derived goat and sheep oocytes. Reprod. Domest. Anim. 2007, 42, 83–87. [Google Scholar] [CrossRef]
- Wieczorek, J.; Cegla, M.; Kareta, W. Factors affecting the oocyte recovery rate from ovarian follicles of gilts. Ann. Anim. Sci. 2005, 5, 85–89. [Google Scholar]
- Baldassarre, H.; de Matos, D.G.; Furnus, C.C.; Castro, T.E.; Cabrera Fischer, E.I. Technique for efficient recovery of sheep oocytes by laparoscopic folliculocentesis. Anim. Reprod. Sci. 1994, 359, 145–150. [Google Scholar] [CrossRef]
- Rodriguez, C.; Anel, L.; Alvarez, M.; Anel, E.; Boixo, J.C.; Chamorro, C.A.; de Paz, P. Ovum pick-up in sheep: A comparison between different aspiration devices for optimal oocyte retrieval. Reprod. Domest. Anim. 2006, 41, 106–113. [Google Scholar] [CrossRef]
- Wang, B.; Baldassarre, H.; Tao, T.; Gauthier, M.; Neveu, N.; Zhou, J.F.; Leduc, M.; Duguay, F.; Bilodeau, A.S.; Lazaris, A.; et al. Transgenic goats produced by DNA pronuclear microinjection of in vitro derived zygotes. Mol. Reprod. Dev. 2002, 63, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Rahman, M.M.; Wan Khadijah, W.E.; Abdullah, R.B. Follicle Stimulating Hormone (FSH) Dosage Based on Body Weight Enhances Ovulatory Responses and Subsequent Embryo Production in Goats. Asian-Australas J. Anim. Sci. 2014, 27, 1270–1274. [Google Scholar]
- Armstrong, D.T.; Pfitzner, A.P.; Warnes, G.M.; Ralph, M.M.; Seamark, R.F. Endocrine responses of goats after induction of superovulation with PMSG and FSH. J. Reprod. Fertil. 1983, 67, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, R.J.; Youngs, C.R.; Rorie, R.W.; Pool, S.H.; Memon, M.A.; Godke, R.A. Follicle stimulating hormone versus pregnant mare serum gonadotropin for superovulation of dairy goats. Small Rumin. Res. 1992, 8, 217–224. [Google Scholar] [CrossRef]
- Rahman, M.R.; Rahman, M.M.; Wan Khadijah, W.E.; Abdullah, R.B. Comparison of Superovulatory Effect of Equine Chorionic Gonadotrophin and Follicle Stimulating Hormone on Embryo Production in Crossbred (Boer × Katjang) Goats. Pak. J. Zool. 2014, 46, 819–826. [Google Scholar]
- Kühholzer, B.; Müller, S.; Treuer, A.; Seregi, J.; Besenfelder, U.; Brem, G. Repeated endoscopic ovum pick-up in hormonally untreated ewes: A new technique. Theriogenology 1997, 48, 545–550. [Google Scholar] [CrossRef]
- Cordeiro, M.F.; Teixeira, P.P.M.; Oliveira, M.E.F.; Di Filippo, P.A.; Dias, D.P.M.; Beretta, C.A.G.; Dória, R.S.G.; Feliciano, M.A.R.; Coutinho, L.N.; Vicente, W.R.R. Reproductive efficiency of adult and prepubertal goats subjected to repeated follicular aspiration. Arq. Bras. Med. Vet. Zootec. 2014, 66, 137–144. [Google Scholar] [CrossRef]
- Teixeira, P.P.M.; Padilha, L.C.; Oliviera, M.E.; Motheo, T.F.; da Silva, A.S.; Barros, F.F.; Coutinho, L.N.; Flôres, F.N.; Lopes, M.C.; Bandarra, M.B.; et al. Laparoscopic ovum collection in sheep: Gross and microscopic evaluation of the ovary and influence on oocyte production. Anim. Rep. Sci. 2011, 127, 169–175. [Google Scholar] [CrossRef]
- Katska, L.; Smorag, Z. Number and quality of oocytes in relation to age of cattle. Anim. Reprod. Sci. 1984, 7, 451–460. [Google Scholar] [CrossRef]
- Locatelli, Y.; Vallet, J.C.; Huyghe, F.P.; Cognié, Y.; Legendre, X.; Mermillod, P. Laparoscopic ovum pick-up and in vitro production of sika deer embryos: Effect of season and culture conditions. Theriogenology 2006, 66, 1334–1342. [Google Scholar] [CrossRef]
- Koeman, J.; Keefer, C.L.; Baldassarre, H.; Downey, B.R. Developmental competence of prepubertal and adult goat oocytes cultured in semi-defined media following laparoscopic recovery. Theriogenology 2003, 60, 879–889. [Google Scholar] [CrossRef]
- Pieterse, M.C.; Kappen, K.A.; Kruip, T.A.; Tayerne, M.A. Aspiration of bovine oocytes during transvaginal ultrasound scanning of the ovaries. Theriogenology 1988, 30, 751–762. [Google Scholar] [CrossRef]
- Rátky, J.; Torner, H.; Egerszegi, I.; Schneider, F.; Sarlos, P.; Manebe NBrüssow, K.P. Ovarian activity and oocyte development during follicular development in pigs at different reproductive phases estimated by the repeated endoscopic method. J. Reprod. Dev. 2005, 51, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Rátky, J.; Brüssow, K.P.; Egerszegi, I.; Torner, H.; Schneider, F.; Solti, L.; Manabe, N. Comparison of follicular and oocyte development and reproductive hormone secretion during the ovulatory period in Hungarian native breed, Mangalica, and Landrace gilts. J. Reprod. Dev. 2005, 51, 427–432. [Google Scholar] [CrossRef]
- Smorąg, Z.; Gogol, P.; Kuznetsov, V. Transwaginalna metoda uzyskiwania oocytów bydlęcych: Efektywność aspiracji, możliwości rozwojowe oocytów oraz zastosowanie w hodowli. Biotechnologia 1998, 2, 153–160. [Google Scholar]
- Wieczorek, J.; Kosenyuk, Y.; Kareta, W.; Cegla, M. Oocyte recovery rate with application of laparoscopic ovum pick-up in pigs. Ann. Anim. Sci. 2007, 7, 89–97. [Google Scholar]
- Brüssow, K.P.; Torner, H.; Rátky, J.; Hunter, M.G.; Nurnberg, G. Ovum pick up in swine: The influence of aspiration vacuum pressure on oocyte recovery from preovulatory follicles. Acta. Vet. Hung. 1997, 45, 189–196. [Google Scholar]
- Baldassarre, H.; Wang, B.; Kafidi, N.; Keefer, C.; Lazaris, A.; Karatzas, C.N. Advances in the production and propagation of transgenic goats using laparoscopic ovum pick-up and in vitro embryo production technologies. Theriogenology 2002, 57, 275–284. [Google Scholar] [CrossRef]
- Baldassarre, H.; Rao, K.M.; Neveu, N.; Brochu, E.; Begin, I.; Behboodi, E.; Hockley, D.K. Laparoscopic ovum pick-up followed by in vitro embryo production for the reproductive rescue of aged goats of high genetic value. Reprod. Fertil. Dev. 2007, 19, 612–616. [Google Scholar] [CrossRef]
- Souza-Fabjan, J.M.; Pereira, A.F.; Melo, C.H.; Sanchez, D.J.; Oba, E.; Mermillod, P.; Melo, L.M.; Teixeira, D.I.; Freitas, V.J. Assessment of the reproductive parameters, laparoscopic oocyte recovery and the first embryos produced in vitro from endangered Caninde goats (Capra hircus). Reprod. Biol. 2013, 13, 325–332. [Google Scholar] [CrossRef]
- Nor Farizah, A.H.; Rahman, M.M.; Wan Khadijah, W.E.; Abdullah, R.B. Effect of repeated laparoscopic ovum pick-up on yield and quality of oocytes in goats. Malays. J. Anim. Sci. 2015, 18, 55–59. [Google Scholar]
- Islam, M.R.; Khandoker, M.A.; Afroz, S.; Rahman, M.G.; Khan, R.I. Qualitative and quantitative analysis of goat ovaries, follicles and oocytes in view of in vitro production of embryos. J. Zhejiang Univ. Sci. B 2007, 8, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Morton, K.M.; de Graaf, S.P.; Campbell, A.; Tomkins, L.M.; Maxwell, W.M.; Evans, G. Repeat ovum pick-up and in vitro embryo production from adult ewes with and without FSH treatment. Reprod. Domest. Anim. 2005, 40, 422–428. [Google Scholar] [CrossRef] [PubMed]
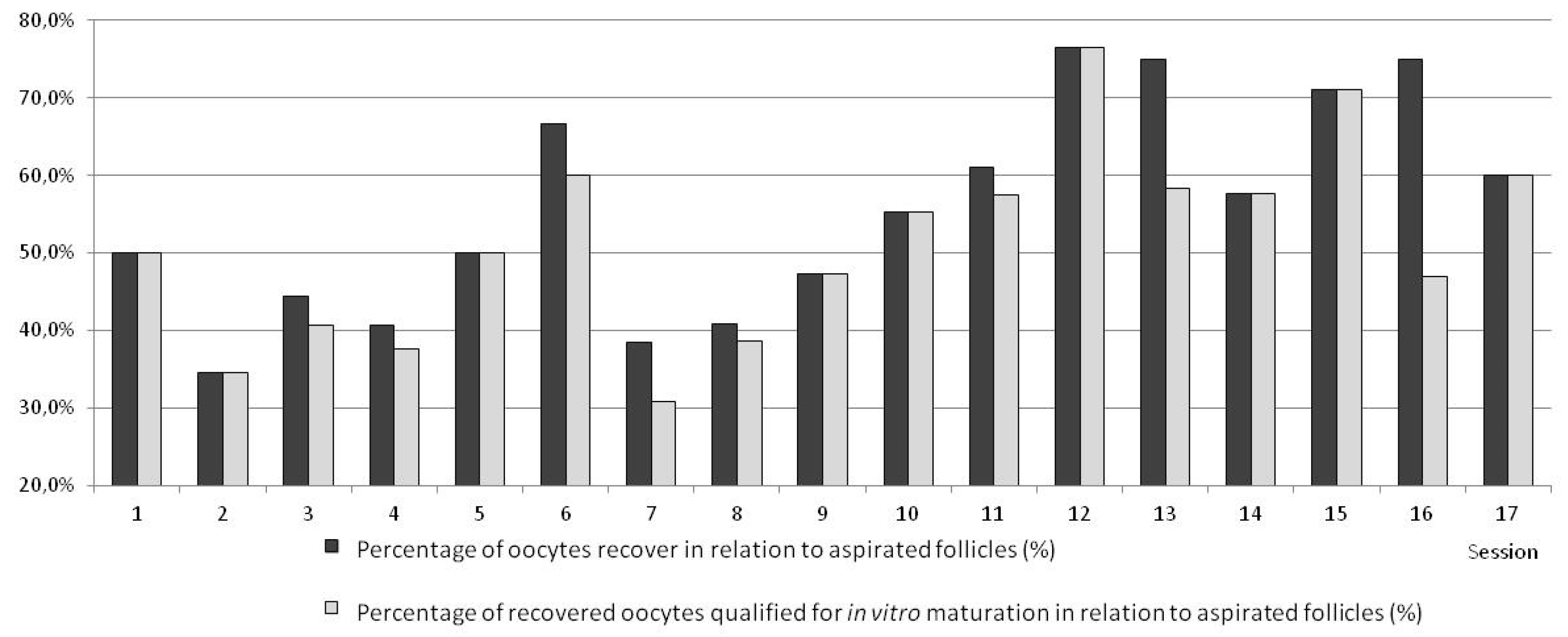
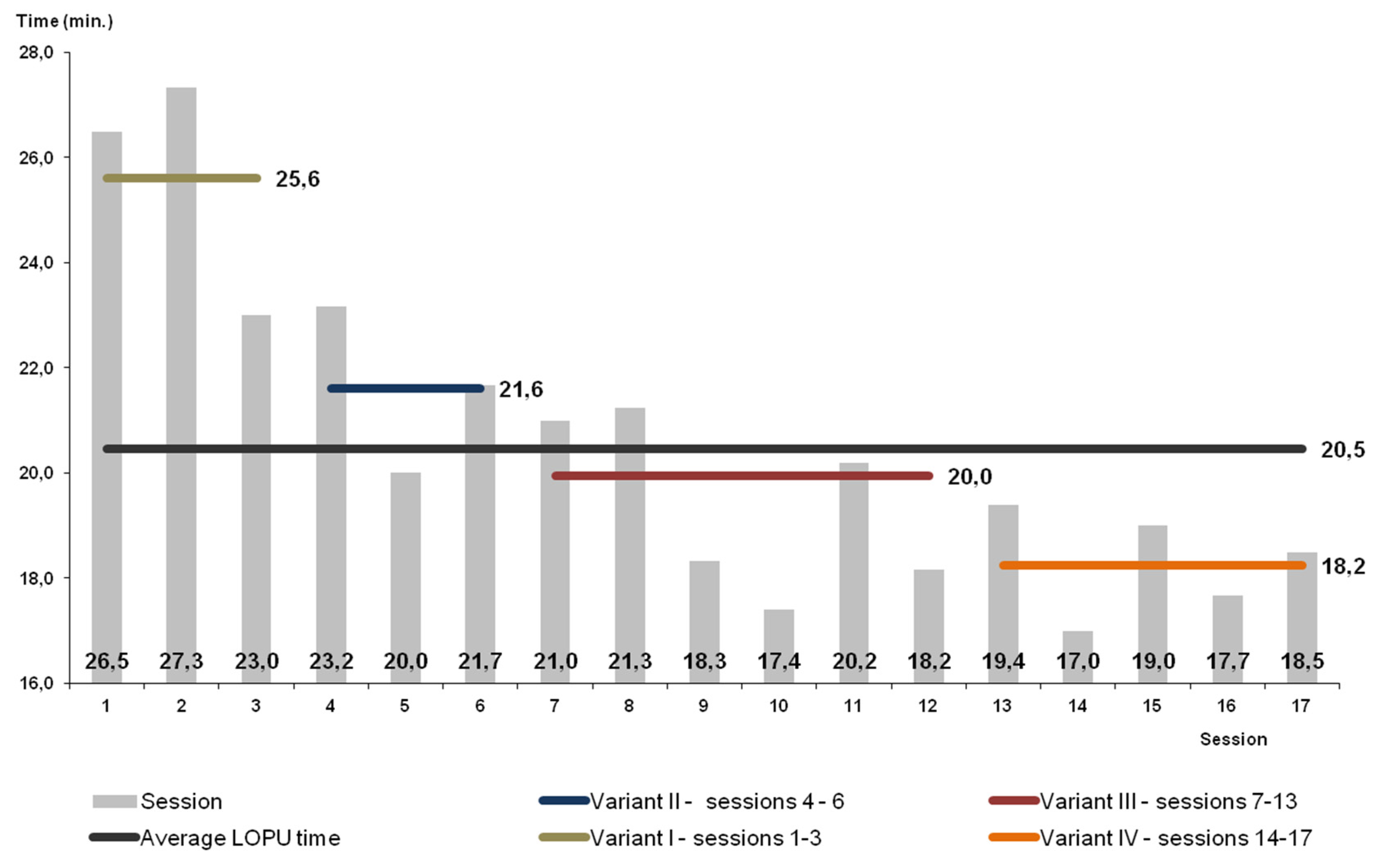
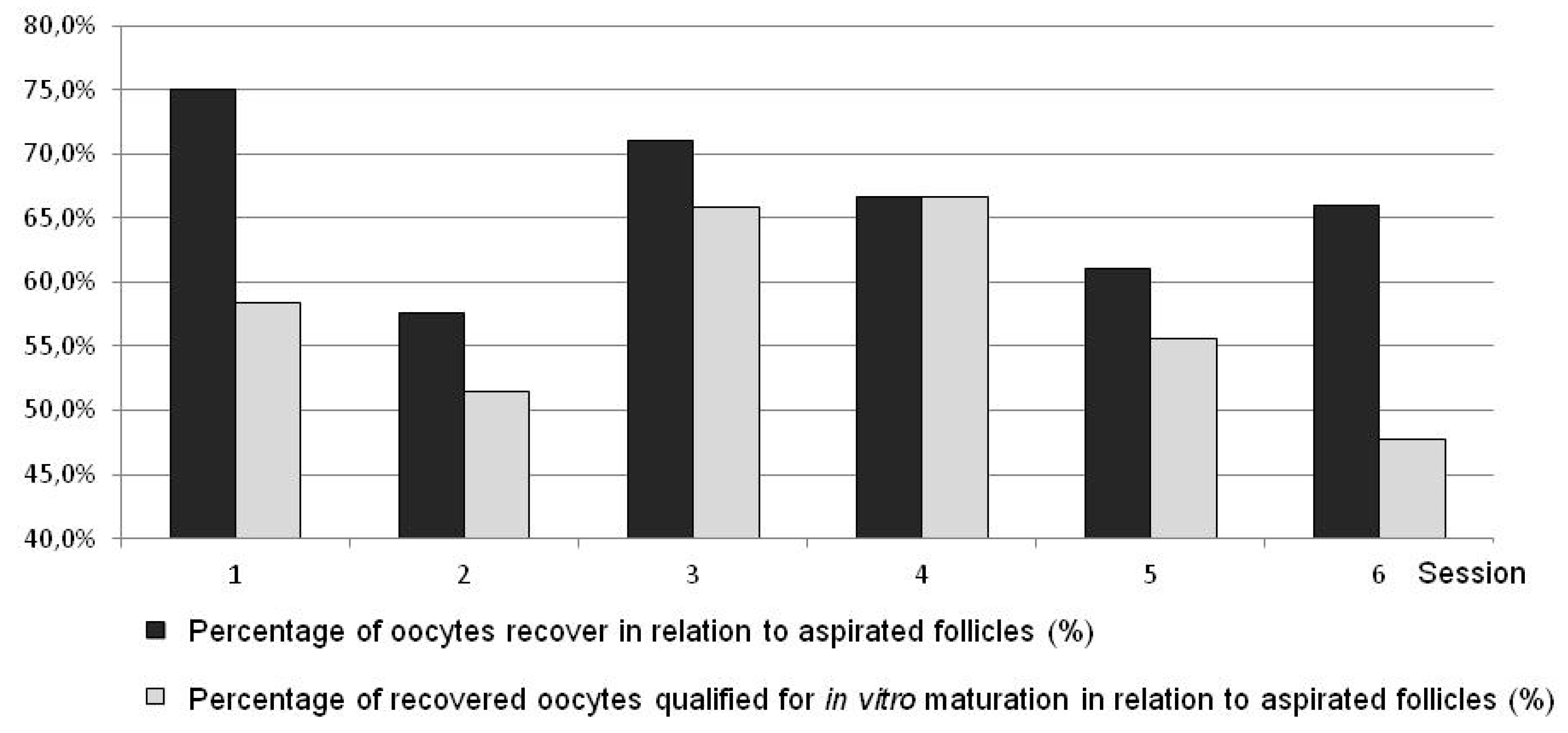
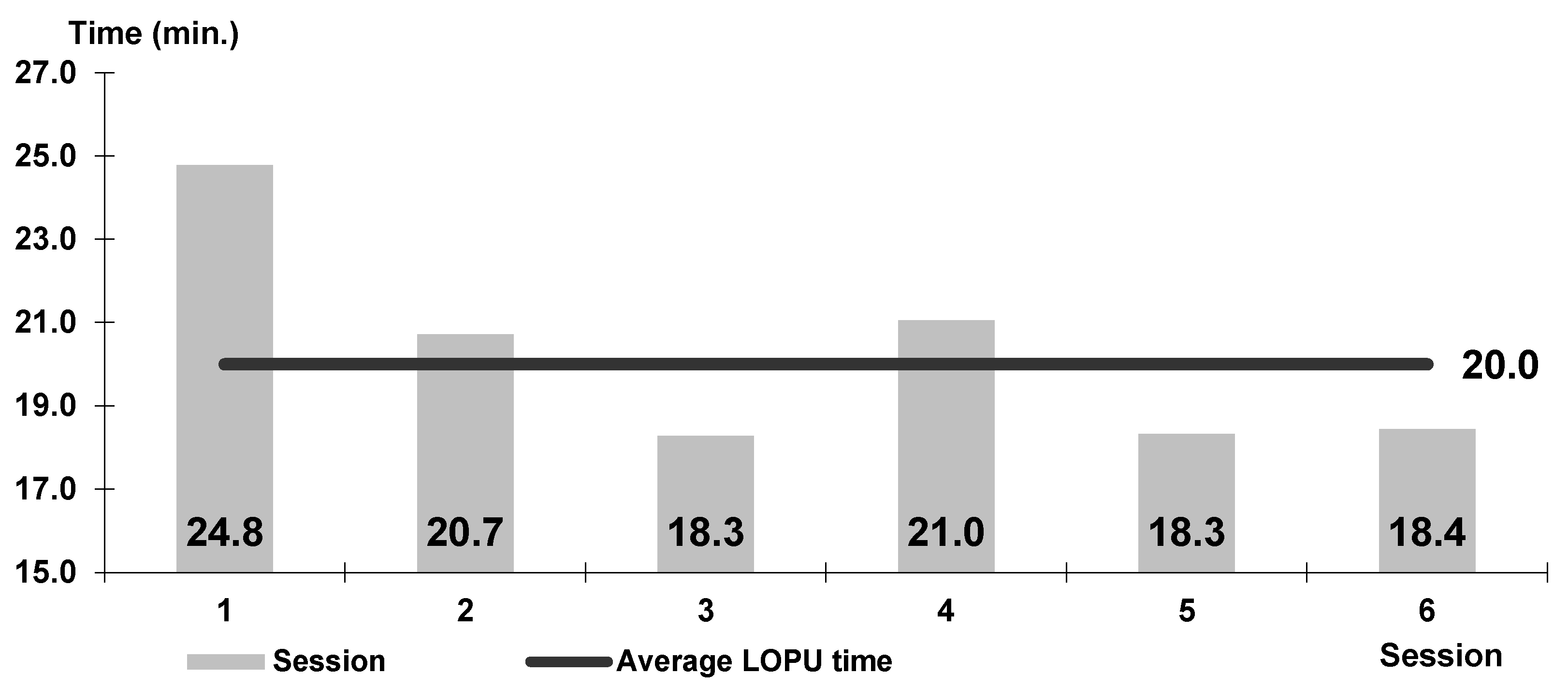
| Variant I (Goats) | Variant II (Goats) | Variant III (Goats) | Variants I to III in Total (Goats) | Variant IV (Goats) | Sheep | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | A | B | No. | A | B | No. | A | B | No. | A | B | No. | A | B | No. | A | B | ||
| Number of ovarian follicles | 98 | 66 | 246 | 410 | 202 | 212 | |||||||||||||
| Number of aspirated follicles | 96 | 65 | 238 | 399 | 195 | 205 | |||||||||||||
| Total number of recovered oocytes | 34 | 35.4 * ± 0.2 | 37 | 56.9 ± 0.3 | 139 | 72.1 * ± 0.2 | 210 | 52.6 ± 0.3 | 132 | 67.7 ± 0.2 | 137 | 66.8 ± 0.3 | |||||||
| Not qualified for IVM (Class IV) | 2 | 2.1 ± 0.04 | 5.9 ± 0.1 | 2 | 3.1 ±0.07 | 5.4 ± 0.1 | 10 | 4.2 ± 0.1 | 7.2 ± 0.3 | 14 | 3.5 ± 0.1 | 6.7 ± 0.2 | 20 | 10.3 * ± 0.1 | 15.2 * ± 0.2 | 19 | 9.3 * ± 0.2 | 13.9 * ± 0.4 | |
| Qualified for IVM Total (1 + 2 + 3) | 32 | 33.3 * ± 0.2 | 94 ± 0.1 | 35 | 53.8 ± 0.3 | 94.6 ± 0.1 | 129 | 54.2 ± 0.3 | 92.8 ± 0.3 | 196 | 49.1 ± 0.3 | 93.3 ± 0.2 | 112 | 57.4 ± 0.2 | 84.8 ± 0.2 | 118 | 57.6 ± 0.3 | 86.1 ± 0.3 | |
| Morphology oocytes qualified for IVM | Class I (1) | 12 | 12.5 * ± 1.4 | 35.3 * ± 0.4 | 11 | 16.9 ± 0.2 | 29.7 ± 0.3 | 55 | 23.1 ± 0.3 | 39.6 * ± 0.4 | 78 | 19.5 ± 0.3 | 37.1 * ± 0.4 | 58 | 29.7 ± 0.2 | 43.9 * ± 0.3 | 44 | 21.5 ± 0.3 | 32.1 ± 0.4 |
| Class II (2) | 17 | 17.7 ± 1.9 | 50.0 * ± 0.4 | 18 | 27.7 ± 2.0 | 48.6 * ± 0.4 | 52 | 21.8 ± 0.3 | 37.4 ± 0.4 | 87 | 21.8 ± 0.3 | 41.4* ± 0.4 | 16 | 8.2 * ± 0.2 | 12.1 * ± 0.3 | 58 | 28.3 ± 0.3 | 42.3 ± 0.4 | |
| Class III (3) | 3 | 3.1 ± 0.1 | 8.8 ± 0.2 | 6 | 9.2 ± 0.2 | 16.2 * ± 0.9 | 22 | 9.2 ± 0.1 | 15.8 ± 0.3 | 31 | 7.8 ± 0.1 | 14.8 ± 0.3 | 38 | 19.5 ± 0.2 | 28.8 * ± 0.2 | 16 | 7.8 ± 0.05 | 11.7 ± 0.08 | |
| Variant I (Goat) | Variant II (Goat) | Variant III (Goat) | Variant I to III in Total(Goat) | Variant IV (Goat) | Sheep | |||
|---|---|---|---|---|---|---|---|---|
| Number of ovarian follicles | Mean ± SD | 9.8 * ± 4.2 | 6.6 ± 3.5 | 5.7 ± 5.4 | 6.5 ± 5.1 | 8.1 ± 4.1 | 4.7 ± 2.4 | |
| Min | 5 | 4 | 0 | 0 | 2 | 1 | ||
| Max | 20 | 11 | 25 | 25 | 21 | 11 | ||
| Number of aspirated follicles | Mean ± SD | 9.6 * ± 4.3 | 6.5 * ± 3.4 | 5.5 ± 5.4 | 6.3 ± 5.1 | 7.8 ± 4.3 | 4.6 ± 2.3 | |
| Min | 4 | 1 | 0 | 0 | 2 | 0 | ||
| Max | 20 | 9 | 25 | 25 | 21 | 11 | ||
| Total number of recovered oocytes (%) | Mean ± SD | 3.4 ± 1.2 | 3.7 ± 2.4 | 3.2 ± 3.4 | 3.3 ± 3.0 | 5.3 * ± 3.6 | 3.0 ± 1.8 | |
| Min | 2 | 0 | 0 | 0 | 2 | 0 | ||
| Max | 5 | 9 | 18 | 18 | 17 | 9 | ||
| Not qualified for IVM (Class IV) | Mean ± SD | 0.2 ± 0.41 | 0.2 ± 0.44 | 0.2 ± 0.5 | 0.2 ± 0.5 | 0.2 ± 0.4 | 0.4 ± 0.6 | |
| Min | 0 | 0 | 0 | 0 | 0 | |||
| Max | 1 | 1 | 2 | 2 | 2 | |||
| Qualified for IVM Total (1 + 2 + 3) | Mean ± SD | 3.2 ± 1.2 | 3.5 ± 2.4 | 3.0 ± 3.3 | 3.1 ± 2.9 | 4.5 * ± 1.7 | 2.62 ± 1.9 | |
| Min | 2 | 0 | 0 | 0 | 2 | 0 | ||
| Max | 5 | 9 | 17 | 17 | 6 | 9 | ||
| Morphology oocytes qualified for IVM | Class I (1) | Mean ± SD | 1.2 ± 1.3 | 1.1 ± 2.2 | 1.3 ± 2.0 | 1.2 ± 1.9 | 2.3 * ± 1.2 | 1.1 ± 1.3 |
| Min | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Max | 3 | 7 | 7 | 7 | 4 | 4 | ||
| Class II (2) | Mean ± SD | 1.7 ± 1.9 | 1.8 ± 1.6 | 1.2 ± 2.3 | 1.4 ± 2.1 | 0.6 ± 1.1 | 1.29 ± 1.7 | |
| Min | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Max | 5 | 5 | 13 | 13 | 3 | 7 | ||
| Class III (3) | Mean ± SD | 0.3 ± 0.5 | 0.6 ± 0.8 | 0.5 ± 0.9 | 0.5 ± 0.8 | 1.52 * ± 1.14 | 0.36 ± 0.9 | |
| Min | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Max | 1 | 2 | 2 | 2 | 3 | 4 | ||
| Method | Method Effectiveness (%) | Oocytes Qualified for In Vitro Maturation * | Author/Date |
|---|---|---|---|
| L-OPU ** | 71.8 | Graff 1998 [15] | |
| TUGA *** | 61 | ||
| L-OPU | 87.3 | Baldassarre 2002 [38] | |
| 82.1 | |||
| 73 | |||
| 83.7 | |||
| L-OPU | 85.7 | 92.5 | Baldassarre 2003 [1] |
| L-OPU | 69.3 | 93.6 | Cogniė 2003 [16] |
| 56.9 | 28.5 | ||
| L-OPU | 56.25 | 46.4 | Koeman 2003 [31] |
| 88.8 | 50.7 | ||
| L-OPU | 87.6 | 72 | Baldassarre 2007 [39] |
| L-OPU | 81.2 | Cox 2007 [17] | |
| L-OPU | 74.3 | 84 | Souza 2013 [40] |
| L-OPU | 47.4–86.5 | Cordeiro 2014 [27] | |
| L-OPU | 91.2 | 85.1 | Nor 2014 [41] |
| Post slaughter | 73.0 | 57.2 | Islam 2007 [42] |
| Method | Method Effectiveness (%) | Oocytes Qualified for in Vitro Maturation * | Author/Date |
|---|---|---|---|
| L-OPU | 79.5 | 60 | Baldassarre 1996 [19] |
| 77.3 | 57 | ||
| L-OPU | 67.9 | 83 | Kühholzer 1997 [26] |
| L-OPU | 55.5 | 65–70 | Stangl 1999 [14] |
| L-OPU | 56.2 | 85.7 | Morton 2005 [43] |
| 38.8 | 81.8 | ||
| L-OPU | 28.3–69.5 | Rodríguez 2006 [20] | |
| L-OPU | 85.6 | Cox 2007 [17] | |
| L-OPU | 51.7 | Teixeira 2011[28] | |
| L-OPU ** | 63.8 | 86.1 | Wieczorek 2018 [13] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, J.; Koseniuk, J.; Skrzyszowska, M.; Cegła, M. L-OPU in Goat and Sheep—Different Variants of the Oocyte Recovery Method. Animals 2020, 10, 658. https://doi.org/10.3390/ani10040658
Wieczorek J, Koseniuk J, Skrzyszowska M, Cegła M. L-OPU in Goat and Sheep—Different Variants of the Oocyte Recovery Method. Animals. 2020; 10(4):658. https://doi.org/10.3390/ani10040658
Chicago/Turabian StyleWieczorek, Jarosław, Jurij Koseniuk, Maria Skrzyszowska, and Mirosław Cegła. 2020. "L-OPU in Goat and Sheep—Different Variants of the Oocyte Recovery Method" Animals 10, no. 4: 658. https://doi.org/10.3390/ani10040658
APA StyleWieczorek, J., Koseniuk, J., Skrzyszowska, M., & Cegła, M. (2020). L-OPU in Goat and Sheep—Different Variants of the Oocyte Recovery Method. Animals, 10(4), 658. https://doi.org/10.3390/ani10040658





