Effects of Different Selenium Sources on Meat Quality and Shelf Life of Fattening Pigs
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design and Feeding Management
2.2. Determination of ADG, ADFI and F/G
2.3. Determination of Carcass Performance and Meat Quality
2.4. Determination of TVB-N, Lactobacillus and E. coli of Longissimus thoracis
2.5. Data Calculations and Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Carcass Performance
3.3. Meat Quality
3.4. Gross Composition of Longissimus Thoracis
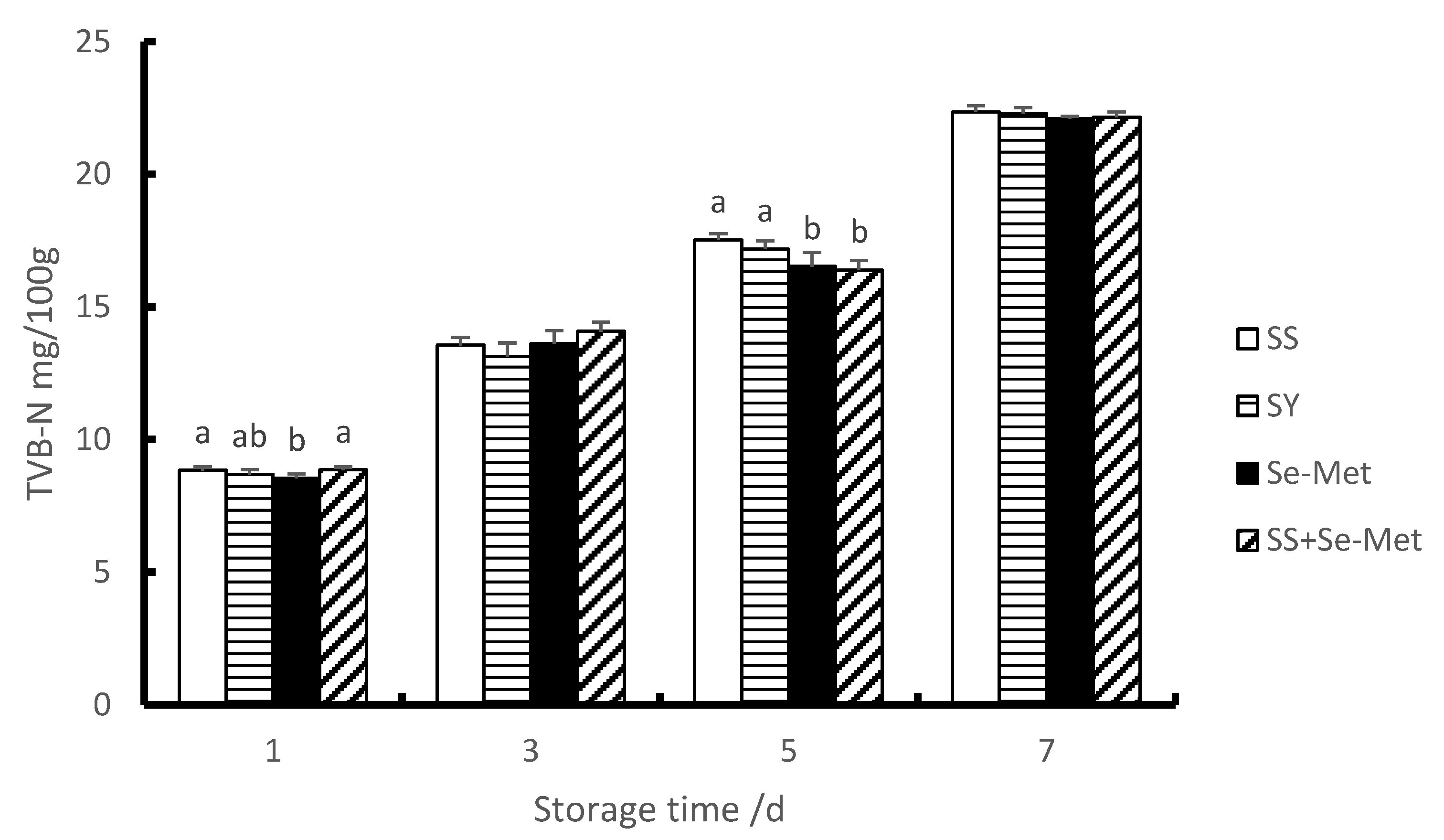
3.5. TVB-N of Longissimus Thoracis
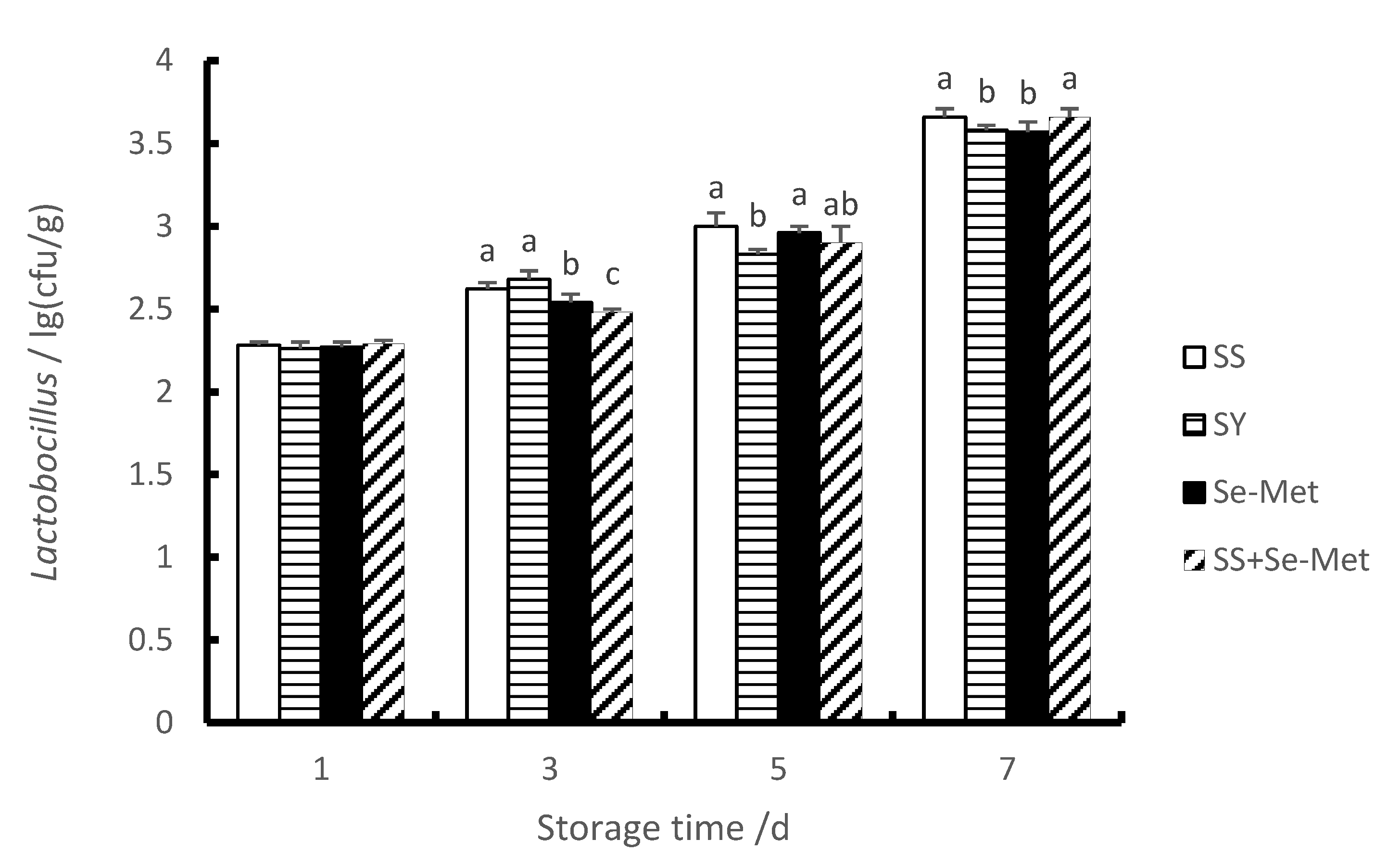
3.6. The Lactobacillus Contents of Longissimus Thoracis
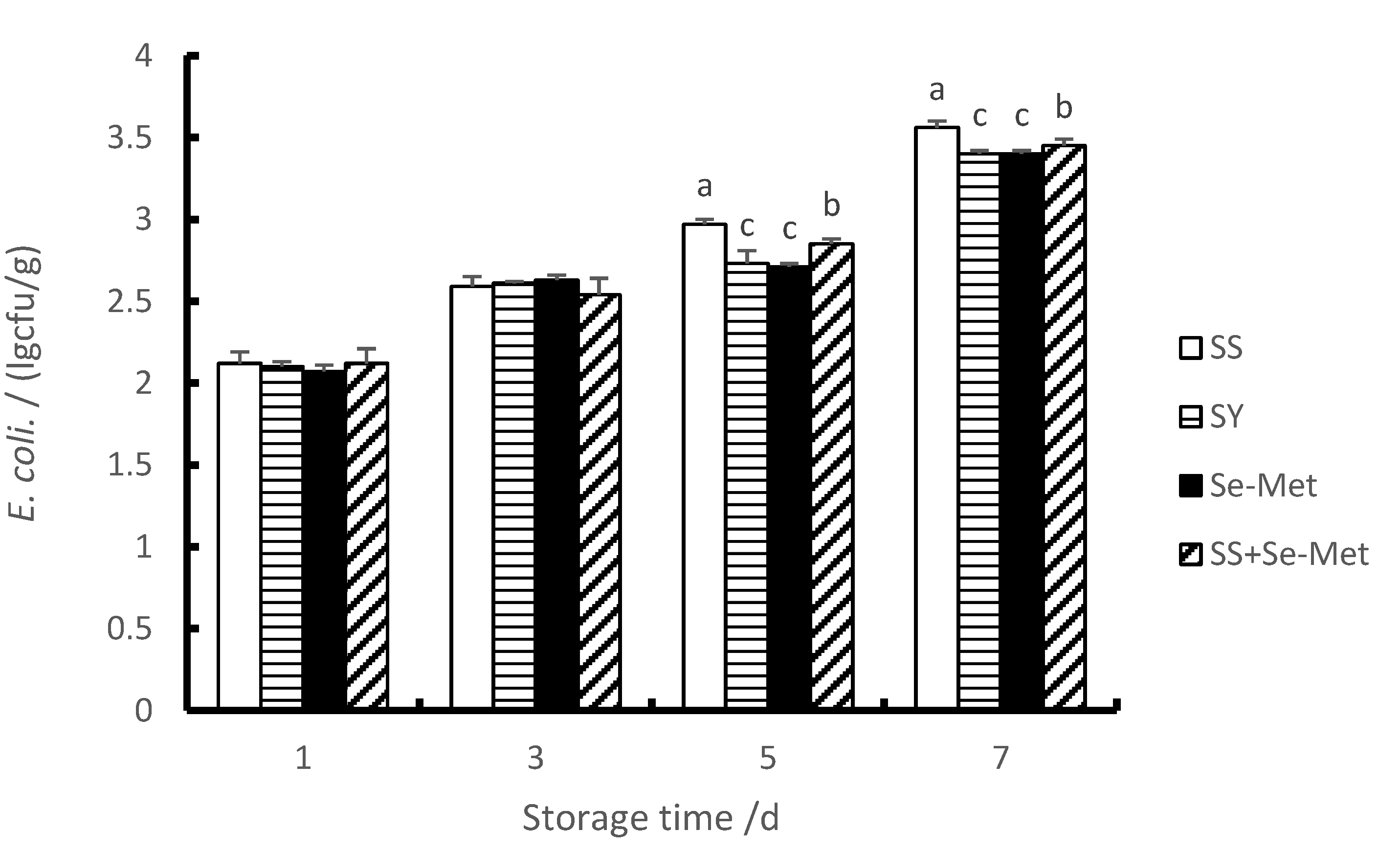
3.7. The E. coli Contents of Longissimus Thoracis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Surai, P.F.; Fisinin, V.I. Selenium in poultry breeder nutrition: An update. Anim. Feed Sci. Technol. 2014, 191, 1–15. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Mahan, D.C. Effect of dietary selenium source, level, and pig hair color on various selenium indices. J. Anim. Sci. 2001, 79, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Goehring, T.B.; Palmer, I.S.; Olson, O.E.; Libal, G.W.; Wahlstrom, R.C. Effects of seleniferous grains and inorganic selenium on tissue and blood composition and growth performance of rats and swine. J. Anim. Sci. 1984, 59, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.; Thompson, H.; Ganther, H.; Ip, C. Se-methylselenocysteine: A new compound for chemoprevention of breast cancer. Nutr. Cancer 2001, 40, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhang, J.S.; Wang, X.F.; Han-Qing, Y.U. Study on bioavailability and toxicity of selenium from methylselenocysteine. Acta Nutr. Sin. 2007, 29, 544–546. [Google Scholar]
- Ganther, H.E.; Lawrence, J.R. Chemical transformations of selenium in living organisms. Improved forms of selenium for cancer prevention. Tetrahedron 1997, 53, 12299–12310. [Google Scholar] [CrossRef]
- Lin, C.G. The effect of selenium on swine production and the health and the key technology of selenium-rich pork production. Master’s Thesis, Fujian Agriculture and Forestry University, Fujian, China, 2013. [Google Scholar]
- Bierla, K.; Szpunar, J.; Yiannikouris, A.; Lobinski, R. Comprehensive speciation of selenium in selenium-Rich yeast. TrAC Trends Anal. Chem. 2012, 41, 122–132. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Cheng, G.M.; He, M.L.; Xu, X.T. Effects of selenium yeast on growth performance, blood routine and serum biochemical indexes of Dorper × Thin-Tailed Han crossbred sheep. Chin. J. Anim. Nutr. 2019, 31, 2907–2914. [Google Scholar]
- Silva, V.A.; Bertechini, A.G.; Clemente, A.H.; de Freitas, L.F.; Nogueira, B.R.; de Oliveira, B.L.; Ramos, A.D. Different levels of selenomethionine on the meat quality and selenium deposition in tissue of finishing pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1866–1874. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Q.; Zhan, T.; Han, Y.; Tang, C.; Zhang, J. Effect of different selenium sources on growth performance, tissue selenium content, meat quality, and selenoprotein gene expression in finishing pigs. Biol. Trace Elem. Res. 2019, 191, 1–9. [Google Scholar] [CrossRef]
- Surai, P.F. Selenium in poultry nutrition. 2. Reproduction, egg and meat quality and practical applications. J. World’s Poult. Sci. J. 2002, 58, 431–450. [Google Scholar] [CrossRef]
- Mahan, D.C.; Cline, T.R.; Richert, B. Effects of dietary levels of selenium-Enriched yeast and sodium selenite as selenium sources fed to growing-finishing pigs on performance, tissue selenium, serum glutathione peroxidase activity, carcass characteristics, and loin quality. Anim. Sci. 1999, 77, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Choct, M.; Naylor, A.J. The effect of dietary selenium source and vitamin e levels on performance of male broilers. Asian Austral. J. Anim. Sci. 2004, 17, 1000–1006. [Google Scholar] [CrossRef]
- Choct, M.; Naylor, A.J.; Reinke, N. Selenium supplementation affects broiler growth performance, meat yield and feather coverage. Br. Poult. Sci. 2004, 45, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.A.; Xu, Z.R. Effects of different selenium source on fresh meat color and drip loss in finishing pigs. Chin. J. Anim. Vet. Sci. 2004, 35, 505–509. [Google Scholar]
- Zhan, X.A.; Wang, M.; Zhao, R.Q.; Li, W.F.; Xu, Z.R. Effects of different selenium source on selenium distribution, loin quality and antioxidant status in finishing pigs. Anim. Feed Sci. Technol. 2007, 132, 202–211. [Google Scholar] [CrossRef]
- Ripoll, G.; Joy, M.; Muñoz, F. Use of dietary vitamin E and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat Sci. 2011, 87, 88–93. [Google Scholar] [CrossRef]
- Pi, Z.K.; Wu, Y.M.; Liu, J.X. Effect of pretreatment and pelletization on nutritive value of rice straw-based total mixed ration, and growth performance and meat quality of growing Boer goats fed on TMR. J. Small Ruminant Res. 2005, 56, 81–88. [Google Scholar] [CrossRef]
- Chao, Y.M.; Chen, D.W.; Yu, B.; He, J.; Yu, J.; Mao, X.B.; Luo, Y.H.; Huang, Z.Q.; Luo, J.Q.; Wang, S.H.; et al. Effects of tea polyphenol on growth performance, antioxidant capacity, carcass performance and meat quality of finishing pigs. Chin. J. Anim. Nutr. 2016, 28, 3996–4005. [Google Scholar]
- Li, Z.T.; Lin, T.; Shen, J.X.; Chen, W.Y.; Sun, C. Effect of rosemary on antibacterial and freshness of cold meat. Food. Res. Dev. 2017, 38, 181–186. [Google Scholar]
- Yin, A.W.; Gao, P.F.; Jiang, J.J. Fresh-Keeping effect of thorn tea polyphenols on chilled pork. J. Hunan Inst. Eng. 2017, 27, 47–52. [Google Scholar]
- Lin, J.; Lin, C.; Zhan, G.; Lin, Z.; Liu, Y.; Zheng, J. Effects of different selenium sources on production performance, thyroid level and immune function in sows during lactation. J. South China Agric. Univ. 2015, 36, 1–8. [Google Scholar]
- Zhang, H.J.; Xu, D.; Tang, M.; Yu, G.H.; Song, C.Y. Effects of nutrient level and yeast selenium on growth performance, nutrient apparent digestibility and serum antioxidant indexes of Yantai black pigs. Chin. J. Anim. Nutr. 2018, 30, 902–909. [Google Scholar]
- Jang, Y.D.; Choi, H.B.; Durosoy, S.; Schlegel, P.; Choi, B.R.; Kim, Y.Y. Comparison of bioavailability of organic selenium sources in finishing pigs. Asian Austral. J. Anim. 2010, 23, 931–936. [Google Scholar] [CrossRef]
- Wang, J.H.; Ge, X.; Zhang, B.X.; Li, P.P.; Zheng, T.J.; Luan, H.Y.; Liu, Z.C. Effects of methionine selenium on growth, carcass traits and meat quality of commercial pigs. Feed Res. 2010, 5, 6–7. [Google Scholar]
- El, R.R.; Berri, C.; Le, B.D.E.; Babile, R.; Fernandez., X. Breed differences in the biochemical determinism of ultimate pH in breast muscles of broiler chickens--A key role of AMP deaminase? Poult. Sci. 2004, 83, 1445–1451. [Google Scholar]
- Bihan-Duval, E.L.; Debut, M.; Berri, C.M.; Sellier, N.; Beaumont, C. Chicken meat quality: Genetic variability and relationship with growth and muscle characteristics. BMC Genet. 2008, 9, 53. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931. [Google Scholar] [CrossRef]
- Wang, R.R.; Pan, X.J.; Peng, Z.Q. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 2009, 88, 1078. [Google Scholar] [CrossRef]
- Yang, H. Effect of Organic SE Fed to Duroc×Yorkshire×Landrace and Yorkshire×Landrace Growing-Finishing Pigs on Production Performance Carcass Trait Meat Quality and Mechanism of Action; Zhejiang University: Hang Zhou, China, 2002. [Google Scholar]
- Wu, F.G. Effect of Traditional Chinese Medicine Praparation of Selenium-Rich Lactobacillus on Groth Performance, Serum Indexes and Carcass Quality of Finishing Pigs; Yanbian University: Yanji, China, 2019. [Google Scholar]
- Mikulski, D.; Jankowski, J.; Zdun Czyk, Z.; Wróblewska, M.; Sartowska, K.; Majewska, T. The effect of selenium source on performance, carcass traits, oxidative status of the organism, and meat quality of turkeys. J. Anim. Feed Sci. 2009, 18, 518–530. [Google Scholar] [CrossRef]
- Zou, X.T.; Zheng, G.H.; Yin, Z.Z.; Lu, L.Z. Effects of different selenium sources on growth performance, carcass composition and meat quality in broilers. J. Zhejiang Univ. 2005, 6, 773–776. [Google Scholar]
- Jiang, Z. Effects of dietary selenomethionine supplementation on performance and meat quality of finishing pigs. Chin. J. Anim. Nutr. 2010, 14, 201–204. [Google Scholar]
- Li, J.G.; Zhou, J.C.; Zhao, H.; Lei, X.G.; Xia, X.J.; Gao, G.; Wang, K.N. Enhanced water-Holding capacity of meat was associated with increased Sepw1 gene expression in pigs fed selenium-enriched yeast. Meat Sci. 2011, 87, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Vignola, G.; Lambertini, L.; Mazzone, G.; Giammarco, M.; Tassinari, M.; Martelli, G.; Bertin, G. Effects of selenium source and level of supplementation on the performance and meat quality of lambs. Meat Sci. 2009, 81, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Wolter, B.; Ellis, M.; McKeith, F.K.; Miller, K.D.; Mahan, D.C. Influence of dietary selenium source on growth performance, and carcass and meat quality characteristics in pigs. Can. J. Anim. Sci. 1999, 79, 119–121. [Google Scholar] [CrossRef]
- Boiago, M.M.; Borba, H.; Leonel, F.R.; Giampietro-Ganeco, A.; Ferrari, F.B.; Stefani, L.M.; Souza, P.A.D. Sources and levels of selenium on breast meat quality of broilers. Ciência Rural 2014, 44, 1692–1698. [Google Scholar] [CrossRef]
- Khan, A.Z.; Kumbhar, S.; Liu, Y.; Hamid, M.; Huang, K. Dietary Supplementation of Selenium-Enriched Probiotics Enhances Meat Quality of Broiler Chickens (Gallus gallus domesticus) Raised under High Ambient Temperature. Biol. Trace Elem. Res. 2018, 182, 328–338. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Chen, Q.; Zhang, Y. Nondestructive measurement of total volatile basic nitrogen (TVB-N) in pork meat by integrating near infrared spectroscopy, computer vision and electronic nose techniques. Food Chem. 2014, 145, 228–236. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Q.; Wan, X.; Zhao, J. Determination of total volatile basic nitrogen (TVB-N) content and Warner-Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chem. 2011, 126, 1354–1360. [Google Scholar] [CrossRef]
- Liu, W.W.; Chen, D.W.; Bing, Y.U. Effect of dietary supplementation of oxidative fish oil and selenium and vitamin E on growth performance and meat quality of finishing pigs. Chin. J. Anim. Sci. 2010, 46, 34–39. [Google Scholar]
- Zhao, W.Z.; Gao, Y.G.; Wang, Z.K.; Yu, S.; Li, G.H.; Xuan, Y.C. Effects of selenium-enriched germanium yeast on quality of Yanbian cattle beef meat during storage. Food. Mach. 2017, 33, 136–140. [Google Scholar]
- Dykes, G.A.; Marshall, L.A.; Meissner, D.; Holy, A.V. Acid treatment and pasteurization affect the shelf life and spoilage ecology of vacuum-Packaged Vienna sausages. Food Microbiol. 1996, 13, 69–74. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Holy, A.V. Bacterial populations associated with pasteurized vacuum-Packed Vienna sausages. Food Microbiol. 1996, 13, 165–174. [Google Scholar] [CrossRef]
- Chen, D.J.; Li, X.Y.; Zhang, Y.H.; Jiang, P.H.; Guo, F.J. Effect of different packaging on the quality and dominant spoilage bacteria of chill pork during storage. Sci. Technol. Food Ind. 2017, 2017 38, 281–285. [Google Scholar]
- Berruga, M.I.; Vergara, H.; Gallego, L. Influence of packaging conditions on microbial and lipid oxidation in lamb meat. J. Small Rumin. Res. 2005, 57, 257–264. [Google Scholar] [CrossRef]
- Huang, L.; Chen, Q.S.; Zhang, Y.H.; Bi, X.K.; Xu, H.; Zhao, J.W. Isolation, identification and spoilage capability of dominant spoilage bacteria in chilled pork. Food Sci. 2013, 34, 205–209. [Google Scholar]
- Su, P.; Song, S.Y.; Wu, Q.M.; Su, X.; Liu, Y. Effects of Flavonoids from Okra and Yeast microcapsules on Fresh-keeping of cold meat. J. Chin. Inst. Food Sci. Technol. 2016, 16, 139–144. [Google Scholar]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Yang, H.Y. Mechanism for the selenium and the effects for meat quality. Jilin J. Anim. Husb. Vet. Med. 2005, 11, 17–18. [Google Scholar]
| Item | Content |
|---|---|
| Ingredient, % | |
| Corn | 55.91 |
| Wheat middlings | 19 |
| Soybean meal | 19 |
| Soybean oil | 2.3 |
| CaH2PO4 | 0.4 |
| Limestone | 1.3 |
| Sodium chloride | 0.4 |
| DL-Met | 0.04 |
| L-Lys·HCl | 0.6 |
| Thr | 0.05 |
| Vitamin-trace mineral premix 1 | 1 |
| Total | 100 |
| Analyzed composition | |
| DE, MJ/kg | 13.84 |
| CP, % | 16.1 |
| Ca, % | 0.62 |
| Available P, % | 0.21 |
| Lys, % | 1.19 |
| Met, % | 0.39 |
| Met + Cys, % | 1 |
| Thr, % | 0.67 |
| Try, % | 0.19 |
| Se, mg/kg | 0.34 |
| Item | SS | SY | Se-Met | SS + Se-Met 2 | p-Value |
|---|---|---|---|---|---|
| 0.3 mg/kg | 0.3 mg/kg | 0.3 mg/kg | 0.15 + 0.15 mg/kg | ||
| Initial weight, kg | 62.03 ± 3.16 | 61.45 ± 0.97 | 61.83 ± 1.25 | 62.91 ± 2.55 | 0.810 |
| Final weight, kg | 92.80 b ± 1.29 | 99.43 a ± 1.31 | 99.50 a ± 3.44 | 101.68 a ± 4.31 | 0.007 |
| ADG, g | 615.41 b ± 18.03 | 759.46 a ± 17.01 | 753.40 a ± 22.97 | 775.36 a ± 36.59 | <0.001 |
| ADFI, kg | 2.30 ± 0.33 | 2.44 ± 0.19 | 2.47 ± 0.11 | 2.46 ± 0.12 | 0.644 |
| F/G 3 | 3.72 a ± 0.16 | 3.21 b ± 0.19 | 3.29 b ± 0.29 | 3.17 b ± 0.02 | 0.005 |
| Item | SS | SY | Se-Met | SS + Se-Met 2 | p-Value |
|---|---|---|---|---|---|
| 0.3 mg/kg | 0.3 mg/kg | 0.3 mg/kg | 0.15 + 0.15 mg/kg | ||
| Dressing percentage, % | 72.92 ± 2.11 | 75.28 ± 1.72 | 73.16 ± 1.14 | 72.87 ± 3.15 | 0.371 |
| Carcass straight length, mm | 101.92 b ± 1.32 | 104.35 a ± 1.39 | 101.50 b± 0.91 | 101.20 b ± 0.54 | 0.006 |
| Carcass sloping length, mm | 91.15 b ± 0.75 | 92.25 a ± 0.65 | 91.45 a, b ± 0.64 | 90.75 b ± 098 | <0.086 |
| Back fat thickness, mm | 10.63 c ± 0.19 | 14.32 a ± 0.31 | 10.53 c ± 0.49 | 11.91 b ± 0.68 | <0.001 |
| Skin thickness, mm | 8.72 b ± 0.21 | 9.25 a ± 0.15 | 8.56 b ± 0.22 | 9.48 a ± 0.26 | <0.001 |
| Belly fat rate, % | 10.77 b, c ± 0.08 | 12.86 a ± 0.48 | 9.99 c ± 0.40 | 11.11 b, c ± 1.09 | <0.001 |
| Eye muscle area, cm 2 | 61.86 ± 1.38 | 63.76 ± 1.90 | 61.58 ± 1.68 | 62.46 ± 2.18 | 0.370 |
| Item | SS | SY | Se-Met | SS + Se-Met 2 | p-Value |
|---|---|---|---|---|---|
| 0.3 mg/kg | 0.3 mg/kg | 0.3 mg/kg | 0.15 + 0.15 mg/kg | ||
| pH (45 min) | 6.30 ± 0.15 | 6.17 ± 0.10 | 6.18 ± 0.12 | 6.31 ± 0.20 | 0.414 |
| pH (24 h) | 5.89 ± 0.03 | 5.91 ± 0.08 | 5.94 ± 0.03 | 5.72 ± 0.34 | 0.310 |
| 24 h drip loss, % | 4.69 a ± 0.40 | 3.60 b ± 0.11 | 3.30 b, c ± 0.09 | 3.23 c ± 0.09 | <0.001 |
| 48 h drip loss, % | 8.81 a ± 0.05 | 7.80 b ± 0.10 | 6.98 c ± 0.13 | 7.02 c ± 0.18 | <0.001 |
| cooking loss, % | 31.99 a ± 0.69 | 27.90 c ± 1.68 | 31.55 a, b ± 2.71 | 29.25 b, c ± 0.36 | 0.013 |
| shearing stress, N | 33.31 a ± 0.95 | 33.27 a ± 1.34 | 27.07 b ± 0.76 | 32.04 a ± 1.44 | <0.001 |
| L value (45 min) | 55.20 a ± 0.34 | 50.56 b ± 0.51 | 54.82 a, b ± 0.47 | 54.25 b± 0.79 | <0.001 |
| a value (45 min) | 9.35 ± 0.13 | 9.58 ± 0.13 | 9.60 ± 0.18 | 9.47± 0.21 | 0.182 |
| b value (45 min) | 9.98 a ± 0.28 | 10.13 a ± 0.33 | 9.28 b ± 0.15 | 9.60 b ± 0.14 | 0.001 |
| L value (24 h) | 57.05 a ± 0.66 | 55.48 b ± 0.75 | 56.40 a ± 0.56 | 51.55 c ± 0.33 | <0.001 |
| a value (24 h) | 12.85 ± 0.28 | 13.15 ± 0.31 | 13.15 ± 0.21 | 12.95 ± 0.58 | 0.602 |
| b value (24 h) | 14.18 a ± 0.17 | 11.85 b ± 0.37 | 12.15 b ± 0.24 | 13.88 a ± 0.22 | <0.001 |
| Item | SS | SY | Se-Met | SS + Se-Met 2 | p-Value |
|---|---|---|---|---|---|
| 0.3 mg/kg | 0.3 mg/kg | 0.3 mg/kg | 0.15 + 0.15 mg/kg | ||
| Moisture, % | 73.43 a ± 0.63 | 72.05 b ± 0.70 | 72.74 a, b ± 0.44 | 72.22 b ± 0.67 | 0.031 |
| ASH, % | 1.20 ± 0.01 | 1.20 ± 0.02 | 1.20 ± 0.01 | 1.21 ± 0.01 | 0.644 |
| CP, % | 22.39 b ± 0.50 | 23.44 a ± 0.36 | 22.75 b ± 0.19 | 23.33 a ± 0.37 | 0.005 |
| EE, % | 2.98 b± 0.07 | 3.31 a± 0.08 | 3.31 a ± 0.07 | 3.24 a± 0.04 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xie, Y.; Li, M.; Yang, H.; Li, S.; Li, J.; Xu, Q.; Yang, W.; Jiang, S. Effects of Different Selenium Sources on Meat Quality and Shelf Life of Fattening Pigs. Animals 2020, 10, 615. https://doi.org/10.3390/ani10040615
Zhang S, Xie Y, Li M, Yang H, Li S, Li J, Xu Q, Yang W, Jiang S. Effects of Different Selenium Sources on Meat Quality and Shelf Life of Fattening Pigs. Animals. 2020; 10(4):615. https://doi.org/10.3390/ani10040615
Chicago/Turabian StyleZhang, Shaotao, Yuhuai Xie, Min Li, Haitao Yang, Shiyin Li, Junhui Li, Qingqing Xu, Weiren Yang, and Shuzhen Jiang. 2020. "Effects of Different Selenium Sources on Meat Quality and Shelf Life of Fattening Pigs" Animals 10, no. 4: 615. https://doi.org/10.3390/ani10040615
APA StyleZhang, S., Xie, Y., Li, M., Yang, H., Li, S., Li, J., Xu, Q., Yang, W., & Jiang, S. (2020). Effects of Different Selenium Sources on Meat Quality and Shelf Life of Fattening Pigs. Animals, 10(4), 615. https://doi.org/10.3390/ani10040615






