Flexible Foraging Response of Wintering Hooded Cranes (Grus monacha) to Food Availability in the Lakes of the Yangtze River Floodplain, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
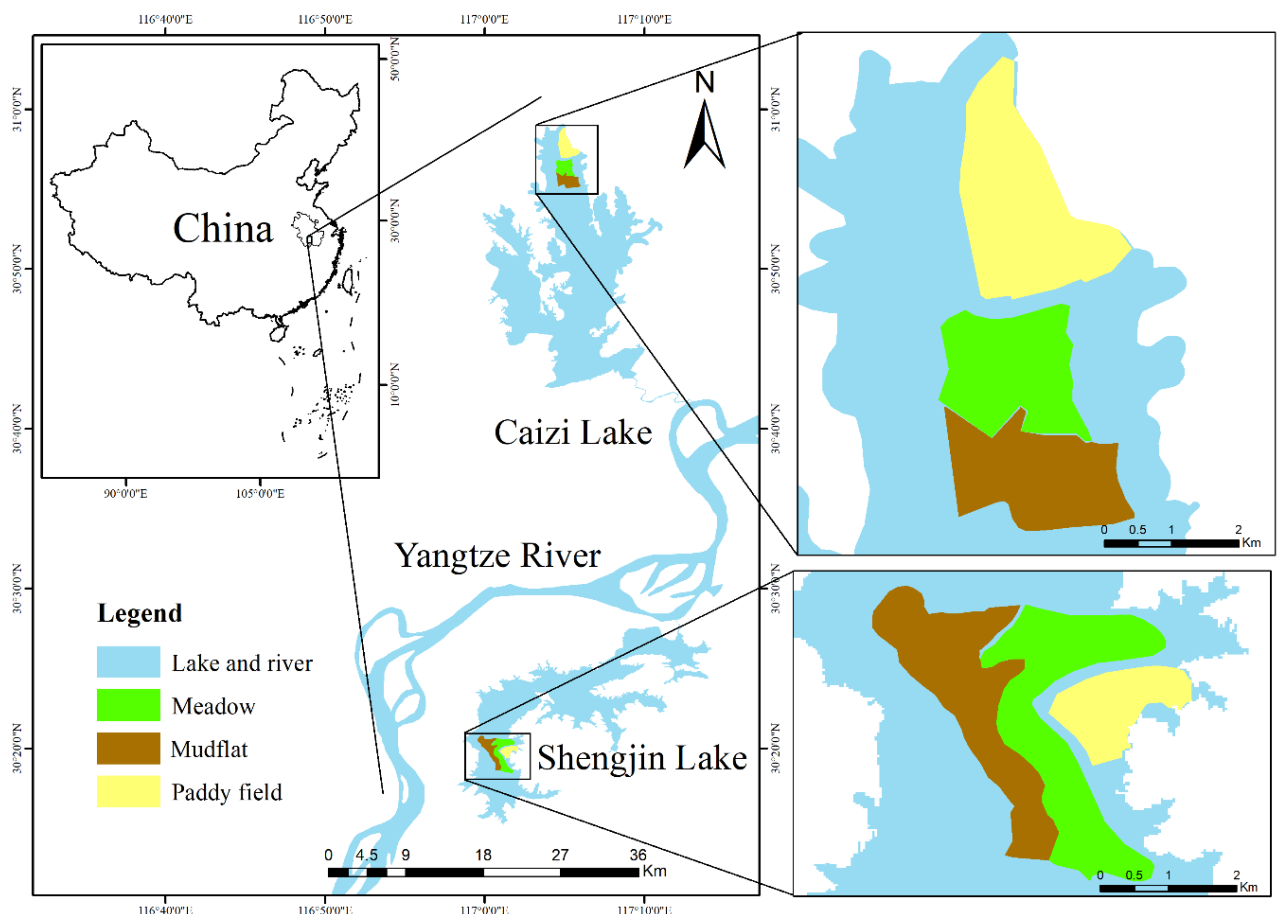
2.1. Study Sites
2.2. Food Characteristics
2.3. Observations of Cranes Behavior
2.4. Data Analysis
3. Results
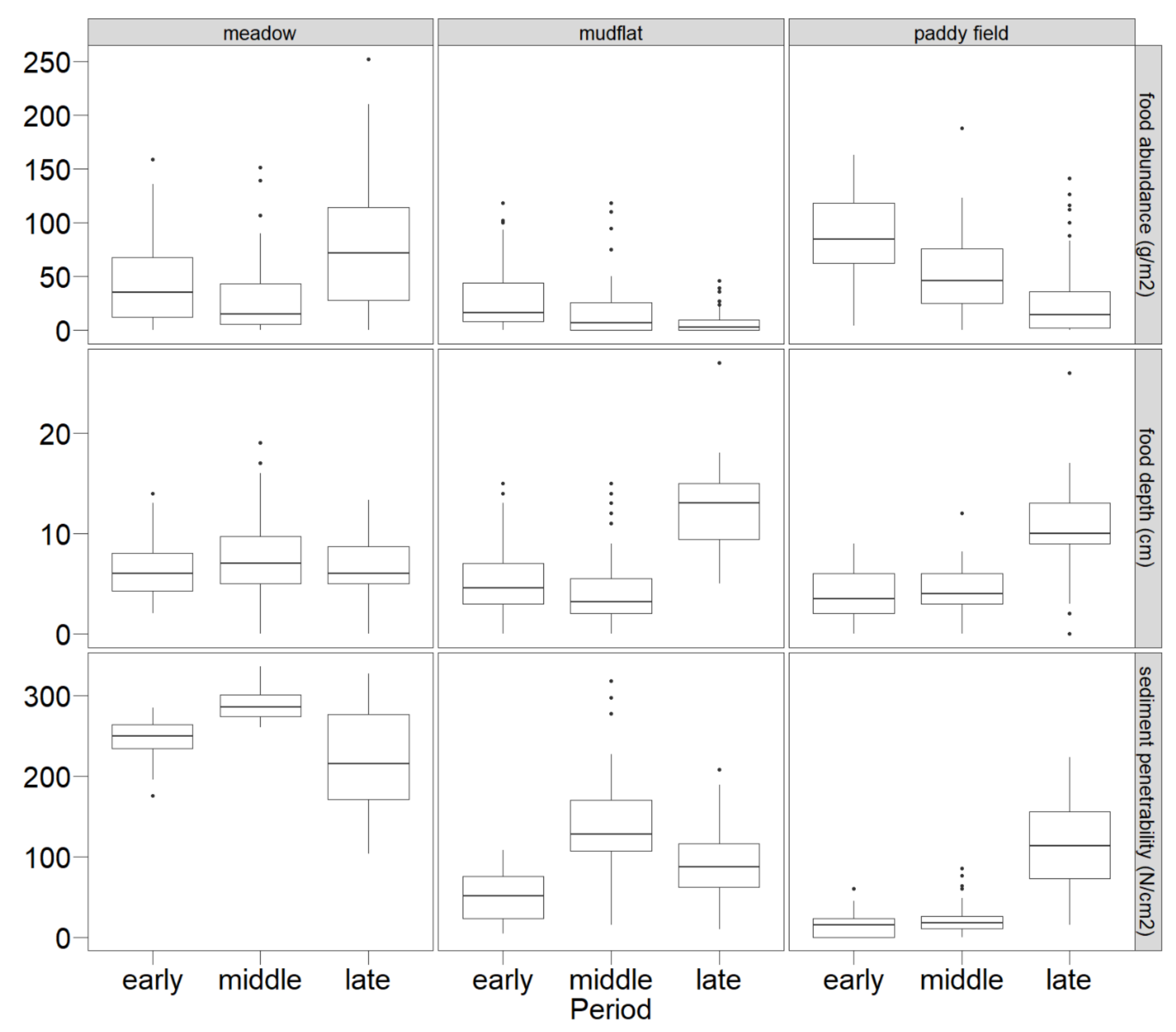
3.1. Food Characteristics
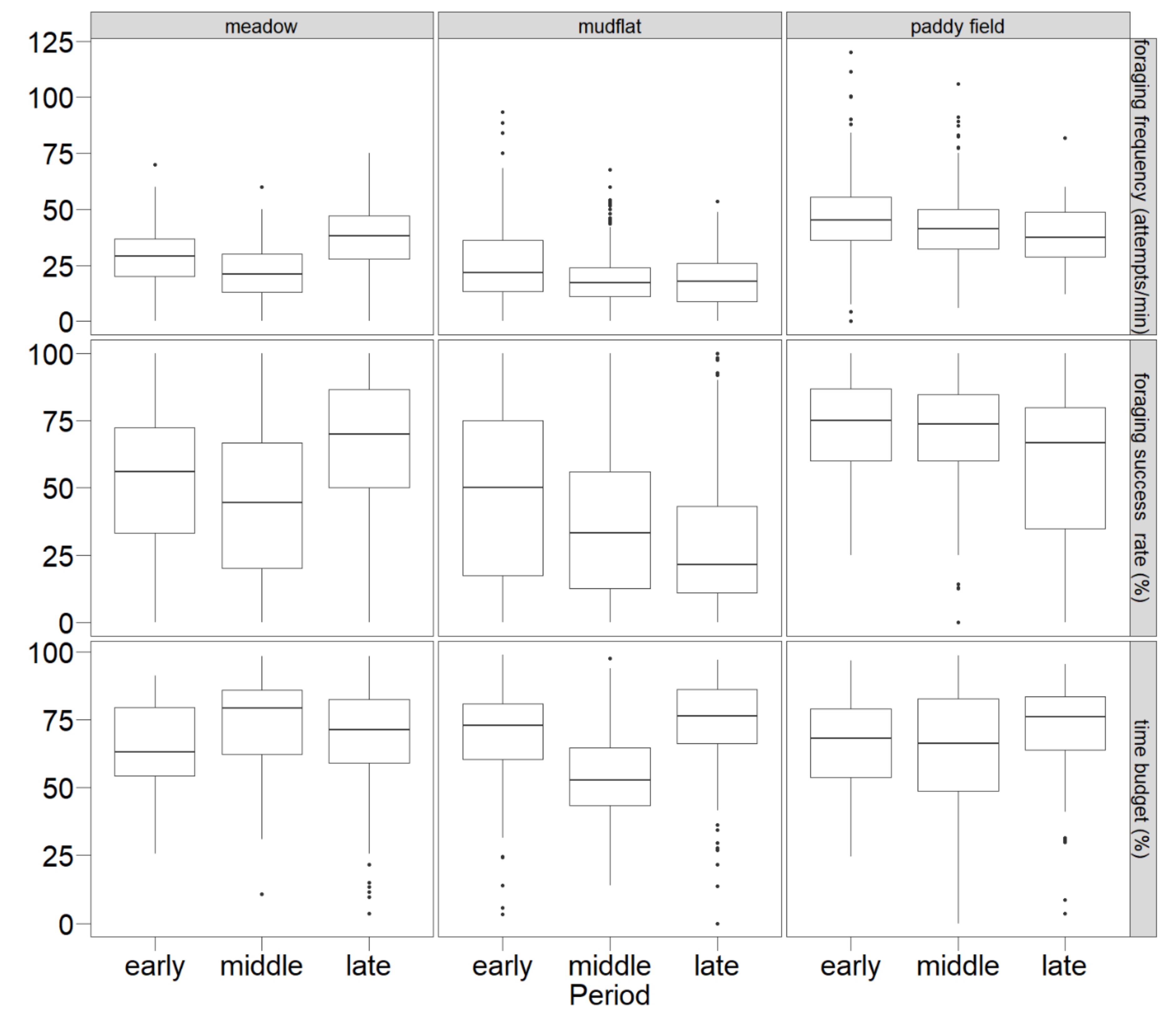
3.2. Foraging Behaviors
3.3. Behavioral Models of Foraging
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weimerskirch, H.; Ancel, A.; Caloin, M.; Zahariev, A.; Spagiari, J.; Kersten, M.; Chastel, O. Foraging efficiency and adjustment of energy expenditure in a pelagic seabird provisioning its chick. J. Anim. Ecol. 2003, 72, 500–508. [Google Scholar] [CrossRef]
- Heath, J.P.; Montevecchi, W.A.; Robertson, G.J. Allocating foraging effort across multiple time scales: Behavioral responses to environmental conditions by Harlequin ducks wintering at Cape St. Mary’s, Newfoundland. Waterbirds 2008, 31, 71–80. [Google Scholar] [CrossRef]
- Rayner, M.J.; Hartill, B.W.; Hauber, M.E.; Phillips, R.A. Central place foraging by breeding Cook’s petrel Pterodroma cookii: Foraging duration reflects range, diet and chick meal mass. Mar. Biol. 2010, 157, 2187–2194. [Google Scholar] [CrossRef]
- Jakubas, D.; Wojczulanis-Jakubas, K.; Walkusz, W. Response of dovekie to changes in food availability. Waterbirds 2007, 30, 421–428. [Google Scholar] [CrossRef]
- Macarthur, R.H.; Pianka, E.R. On optimal use of a patchy environment. Am. Nat. 1966, 100, 603–609. [Google Scholar] [CrossRef]
- Brown, J.S.; Kotler, B.P. Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 2004, 7, 999–1014. [Google Scholar] [CrossRef]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar] [CrossRef]
- Charnov, E.L.; Orians, G.H.; Hyatt, K. Ecological implications of resource depression. Am. Nat. 1976, 110, 247–259. [Google Scholar] [CrossRef]
- Wood, K.A.; Hilton, G.M.; Newth, J.L.; Rees, E.C. Seasonal variation in energy gain explains patterns of resource use by avian herbivores in an agricultural landscape: Insights from a mechanistic model. Ecol. Model. 2019, 409, 108762. [Google Scholar] [CrossRef]
- Fryxell, J.M. Forage quality and aggregation by large herbivores. Am. Nat. 1991, 138, 478–498. [Google Scholar] [CrossRef]
- Hansen, B.B.; Aanes, R.; Herfindal, I.; Sæther, B.-E.; Henriksen, S. Winter habitat–space use in a large arctic herbivore facing contrasting forage abundance. Polar Biol. 2009, 32, 971–984. [Google Scholar] [CrossRef]
- Perry, G.; Pianka, E.R. Animal foraging: Past, present and future. Trends Ecol. Evol. 1997, 12, 360–364. [Google Scholar] [CrossRef]
- Wan, W.J.; Zhou, L.Z.; Song, Y.W. Shifts in foraging behavior of wintering Hooded cranes (Grus monacha) in three different habitats at Shengjin Lake, China. Avian Res. 2016, 7. [Google Scholar] [CrossRef]
- Jia, Y.F.; Jiao, S.W.; Zhang, Y.M.; Zhou, Y.; Lei, G.C.; Liu, G.H. Diet shift and its impact on foraging behavior of Siberian crane (Grus Leucogeranus) in Poyang Lake. PLoS ONE 2013, 8, e65843. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.A.; Stillman, R.A.; Wheeler, D.; Groves, S.; Hambly, C.; Speakman, J.R.; Daunt, F.; O’Hare, M.T. Go with the flow: Water velocity regulates herbivore foraging decisions in river catchments. Oikos 2013, 122, 1720–1729. [Google Scholar] [CrossRef]
- DesGranges, J.L.; Ingram, J.; Drolet, B.; Morin, J.; Savage, C.; Borcard, D. Modelling wetland bird response to water level changes in the Lake Ontario—St. Lawrence River hydrosystem. Environ. Monit. Assess. 2006, 113, 329–365. [Google Scholar] [CrossRef]
- Holm, T.E.; Clausen, P. Effects of water level management on autumn staging waterbird and macrophyte diversity in three Danish coastal lagoons. Biodivers. Conserv. 2006, 15, 4399–4423. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Sebastian, P.; Wilder, S.M.; Rypstra, A.L. The nutritional content of prey affects the foraging of a generalist arthropod predator. PLoS ONE 2012, 7, e49223. [Google Scholar] [CrossRef]
- Li, C.L.; Yang, Y.; Wang, Z.; Yang, L.; Zhou, L.Z. The relationship between seasonal water level fluctuation and habitat availability for wintering waterbirds at Shengjin Lake, China. Bird Conserv. Int. 2018, 1–15. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Wang, X.; Cao, L.; Fox, A.D. Why Chinese wintering geese hesitate to exploit farmland. IBIS 2018, 160, 703–705. [Google Scholar] [CrossRef]
- Kuwae, T.; Miyoshi, E.; Sassa, S.; Watabe, Y. Foraging mode shift in varying environmental conditions by dunlin Calidris alpina. Mar. Ecol. Prog. Ser. 2010, 406, 281–289. [Google Scholar] [CrossRef]
- Zhang, S.D.; Ma, Z.J.; Choi, C.Y.; Peng, H.B.; Melville, D.S.; Zhao, T.T.; Bai, Q.Q.; Liu, W.L.; Chan, Y.C.; van Gils, J.A.; et al. Morphological and digestive adjustments buffer performance: How staging shorebirds cope with severe food declines. Ecol. Evol. 2019, 9, 3868–3878. [Google Scholar] [CrossRef] [PubMed]
- Naef-Daenzer, L.; Naef-Daenzer, B.; Nager, R.G. Prey selection and foraging performance of breeding Great tits Parus major in relation to food availability. J. Avian Biol. 2000, 31, 206–214. [Google Scholar] [CrossRef]
- Van Gils, J.A.; Spaans, B.; Dekinga, A.; Piersma, T. Foraging in a tidally structured environment by Red knots (Calidris canutus): Ideal, but not free. Ecology 2006, 87, 1189–1202. [Google Scholar] [CrossRef]
- Nolet, B.A.; Bevan, R.M.; Klaassen, M.; Langevoord, O.; Van Der Heijden, Y.G.J.T. Habitat switching by Bewick’s swans: Maximization of average long-term energy gain? J. Anim. Ecol. 2002, 71, 979–993. [Google Scholar] [CrossRef]
- Fox, A.D.; Cao, L.; Zhang, Y.; Barter, M.; Zhao, M.J.; Meng, F.J.; Wang, S.L. Declines in the tuber-feeding waterbird guild at Shengjin Lake National Nature Reserve, China—A barometer of submerged macrophyte collapse. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 82–91. [Google Scholar] [CrossRef]
- Baschuk, M.S.; Koper, N.; Wrubleski, D.A.; Goldsborough, G. Effects of water depth, cover and food resources on habitat use of marsh birds and waterfowl in boreal wetlands of Manitoba, Canada. Waterbirds 2012, 35, 44–55. [Google Scholar] [CrossRef]
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 934. [Google Scholar] [CrossRef]
- Wood, K.A.; Newth, J.L.; Brides, K.; Burdekin, M.; Harrison, A.L.; Heaven, S.; Kitchin, C.; Marshall, L.; Mitchell, C.; Ponting, J.; et al. Are long-term trends in Bewick’s swan Cygnus columbianus bewickii numbers driven by changes in winter food resources? Bird Conserv. Int. 2018, 29, 479–496. [Google Scholar] [CrossRef]
- Beekman, J.; Koffijberg, K.; Wahl, J.; Kowallik, C.; Hall, C.; Devos, K.; Clausen, P.; Hornman, M.; Laubek, B.; Luigujõe, L.; et al. Long-term population trends and shifts in distribution of Bewick’s swans Cygnus columbianus bewickii wintering in northwest Europe. Wildfowl 2019, 5, 73–102. [Google Scholar]
- Peron, G.; Nicolai, C.A.; Koons, D.N. Demographic response to perturbations: The role of compensatory density dependence in a North American duck under variable harvest regulations and changing habitat. J. Anim. Ecol. 2012, 81, 960–969. [Google Scholar] [CrossRef]
- Ma, Z.J.; Li, B.; Jing, K.; Zhao, B.; Tang, S.M.; Chen, J.K. Effects of tidewater on the feeding ecology of Hooded crane (Grus monacha) and conservation of their wintering habitats at Chongming Dongtan, China. Ecol. Res. 2003, 18, 321–329. [Google Scholar] [CrossRef]
- Cai, T.L.; Huettmann, F.; Guo, Y.M. Using stochastic gradient boosting to infer stopover habitat selection and distribution of Hooded cranes Grus monacha during spring migration in Lindian, northeast China. PLoS ONE 2014, 9, e89913. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Su, L.Y.; Higuchi, H.; Ueta, M.; Zhang, Z.W.; Zhang, Y.Y.; Ni, X.J. Migratory stopover and wintering locations in eastern China used by White-naped cranes Grus vipio and Hooded cranes G. monacha as determined by satellite tracking. Forktail 2000, 16, 93–99. [Google Scholar]
- Yang, S.L.; Zhao, Q.Y.; Belkin, I.M. Temporal variation in the sediment load of the Yangtze River and the influences of human activities. J. Hydrol. 2002, 263, 56–71. [Google Scholar] [CrossRef]
- Fang, J.Y.; Wang, Z.H.; Zhao, S.Q.; Li, Y.K.; Tang, Z.Y.; Yu, D.; Ni, L.Y.; Liu, H.Z.; Xie, P.; Da, L.J.; et al. Biodiversity changes in the lakes of the central Yangtze. Front. Ecol. Environ. 2006, 4, 369–377. [Google Scholar] [CrossRef]
- Yang, X.D.; Anderson, N.J.; Dong, X.H.; Shen, J.I. Surface sediment diatom assemblages and epilimnetic total phosphorus in large, shallow lakes of the Yangtze floodplain: Their relationships and implications for assessing long-term eutrophication. Freshw. Biol. 2008, 53, 1273–1290. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Y.; Barter, M.; Lei, G. Anatidae in eastern China during the non-breeding season: Geographical distributions and protection status. Biol. Conserv. 2010, 143, 650–659. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, L.Z.; Chen, J.Y.; Cheng, Y.Q.; Xu, W.B. Diurnal time-activity budgets of wintering Hooded cranes (Grus monacha) in Shengjin Lake, China. Waterbirds 2010, 33, 110–115. [Google Scholar] [CrossRef]
- King, S.; Elphick, C.S.; Guadagnin, D.; Taft, O.; Amano, T. Effects of landscape features on waterbird use of rice fields. Waterbirds 2010, 33, 151–159. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhou, L.Z.; Zhou, B.; Xu, R.X.; Zhu, W.Z.; Xu, W.B. Seasonal dynamics of wintering waterbirds in two shallow lakes along Yangtze River in Anhui Province. Zool. Res. 2011, 32, 540–548. [Google Scholar] [CrossRef]
- Zhao, F.T.; Zhou, L.Z.; Xu, W.B. Habitat utilization and resource partitioning of wintering Hooded cranes and three goose species at Shengjin Lake. Chin. Birds 2013, 4, 281–290. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Xiang, X.J.; Zhao, G.H.; Song, Y.W.; Zhou, L.Z. Variations in gut bacterial communities of hooded crane (Grus monacha) over spatial-temporal scales. PeerJ 2019, 7, e7045. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhou, L.Z.; Mahtab, N.; Fan, S.J.; Song, Y.W. The influence of food density, flock size, and disturbance on the functional response of Bewick’s swans (Cygnus columbianus bewickii) in wintering habitats. Animals 2019, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Li, H.F.; Zhang, Y.; Zha, D.D.; Zhao, B.B.; Yang, S.; Zhang, B.W.; de Boer, W.F. Predicting hydrological impacts of the Yangtze-to-Huaihe Water Diversion Project on habitat availability for wintering waterbirds at Caizi Lake. J. Environ. Manag. 2019, 249, 109251. [Google Scholar] [CrossRef]
- Fang, L.; Dong, B.; Wang, C.; Yang, F.; Cui, Y.; Xu, W.; Peng, L.; Wang, Y.; Li, H. Research on the influence of land use change to habitat of cranes in Shengjin Lake wetland. Environ. Sci. Pollut. Res. 2020, 27, 7515–7525. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Maslo, B.; Burger, J.; Handel, S.N. Modeling foraging behavior of piping plovers to evaluate habitat restoration success. J. Wildl. Manag. 2012, 76, 181–188. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer Science & Business Media: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Oppel, S.; Powell, A.N.; Butler, M.G. King eider foraging effort during the pre-breeding period in Alaska. Condor 2011, 113, 52–60. [Google Scholar] [CrossRef]
- Slobodkin, L.B. Prudent predation does not require group selection. Am. Nat. 1974, 108, 665–678. [Google Scholar] [CrossRef]
- Gawlik, D.E. The effects of prey availability on the numerical response of wading birds. Ecol. Monogr. 2002, 72, 329–346. [Google Scholar] [CrossRef]
- Turner, R.K.; van den Bergh, J.C.J.M.; Söderqvist, T.; Barendregt, A.; van der Straaten, J.; Maltby, E.; van Ierland, E.C. Ecological-economic analysis of wetlands: Scientific integration for management and policy. Ecol. Econ. 2000, 35, 7–23. [Google Scholar] [CrossRef]
- Durell, S.E.A.L.V.D. Individual feeding specialisation in shorebirds: Population consequences and conservation implications. Biol. Rev. 2007, 75, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, J.X.; Yang, X.J.; Zhao, J.L.; Yu, H.Z. Foraging habitats and utilization distributions of Black-necked cranes wintering at the Napahai Wetland, China. J. Field Ornithol. 2010, 81, 21–30. [Google Scholar] [CrossRef]
- Lantz, S.M.; Gawlik, D.E.; Cook, M.I. The effects of water depth and submerged aquatic vegetation on the selection of foraging habitat and foraging success of wading birds. Condor 2010, 112, 460–469. [Google Scholar] [CrossRef]
- Tourenq, C.; Bennetts, R.E.; Kowalski, H.; Vialet, E.; Lucchesi, J.L.; Kayser, Y.; Isenmann, P. Are ricefields a good alternative to natural marshes for waterbird communities in the Camargue, southern France? Biol. Conserv. 2001, 100, 335–343. [Google Scholar] [CrossRef]
| Lake | Period | Food Quadrats | Foraging Behavior Samples | ||||
|---|---|---|---|---|---|---|---|
| Meadow | Mudflat | Paddy Field | Meadow | Mudflat | Paddy Field | ||
| Shengjin Lake | Early a | 25 | 25 | 20 | 11 | 18 | 35 |
| Middle b | 20 | 25 | 25 | 63 | 53 | 18 | |
| Late c | 20 | 20 | 30 | 120 | 35 | 26 | |
| Caizi Lake | Early a | 20 | 25 | 20 | 14 | 45 | 29 |
| Middle b | 20 | 30 | 20 | 6 | 49 | 50 | |
| Late c | 20 | 20 | 35 | 50 | 58 | 56 | |
| Total | 125 | 145 | 150 | 264 | 258 | 214 | |
| Model | AICC b | △AICC C | ML | K | Wi | R2adj d |
|---|---|---|---|---|---|---|
| Foraging time budget | ||||||
| Depth + penetrability | 118.38 | 0 | 1.00 | 3 | 0.27 | 0.43 |
| Penetrability | 119.25 | 0.87 | 0.62 | 2 | 0.18 | 0.13 |
| Depth | 119.85 | 1.47 | 0.53 | 2 | 0.13 | 0.38 |
| Null | 124.11 | 15.73 | 0 | 1 | 0 | |
| Foraging frequency | ||||||
| Habitat + abundance | 104.84 | 0 | 1.00 | 3 | 0.43 | 0.85 |
| Habitat + abundance + penetrability | 105.45 | 0.61 | 0.74 | 4 | 0.32 | 0.83 |
| Null | 130.47 | 25.63 | 0 | 1 | 0 | |
| Foraging success rate | ||||||
| Habitat + abundance + depth + penetrability | 123.77 | 0 | 1.00 | 5 | 0.37 | 0.85 |
| Habitat + abundance | 124.29 | 0.52 | 0.76 | 3 | 0.28 | 0.82 |
| Habitat + abundance + penetrability | 125.54 | 1.77 | 0.41 | 4 | 0.15 | 0.81 |
| Null | 148.03 | 24.26 | 0 | 1 | 0 |
| Response Variable | Explanatory Parameter | Estimate | SE | RI a |
|---|---|---|---|---|
| Foraging time budget | Intercept | 59.948 | 2.337 | |
| Depth | 0.321 | 0.161 | 0.677 | |
| Penetrability | 0.027 | 0.014 | 0.741 | |
| Foraging frequency | Intercept | −3.370 | 6.858 | |
| abundance | 0.495 | 0.049 | 1.000 | |
| penetrability | −0.061 | 0.022 | 0.497 | |
| Habitat | 1.129 | 1.448 | 1.000 | |
| Foraging success rate | Intercept | 3.361 | 10.426 | |
| Abundance | 0.695 | 0.086 | 1.000 | |
| Depth | −0.460 | 0.195 | 0.498 | |
| Penetrability | −0.079 | 0.035 | 0.519 | |
| Habitat | 13.433 | 3.466 | 1.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Zheng, M.; Zhou, L.; Xu, W. Flexible Foraging Response of Wintering Hooded Cranes (Grus monacha) to Food Availability in the Lakes of the Yangtze River Floodplain, China. Animals 2020, 10, 568. https://doi.org/10.3390/ani10040568
Wei Z, Zheng M, Zhou L, Xu W. Flexible Foraging Response of Wintering Hooded Cranes (Grus monacha) to Food Availability in the Lakes of the Yangtze River Floodplain, China. Animals. 2020; 10(4):568. https://doi.org/10.3390/ani10040568
Chicago/Turabian StyleWei, Zhenhua, Meng Zheng, Lizhi Zhou, and Wenbin Xu. 2020. "Flexible Foraging Response of Wintering Hooded Cranes (Grus monacha) to Food Availability in the Lakes of the Yangtze River Floodplain, China" Animals 10, no. 4: 568. https://doi.org/10.3390/ani10040568
APA StyleWei, Z., Zheng, M., Zhou, L., & Xu, W. (2020). Flexible Foraging Response of Wintering Hooded Cranes (Grus monacha) to Food Availability in the Lakes of the Yangtze River Floodplain, China. Animals, 10(4), 568. https://doi.org/10.3390/ani10040568






