Supplementation of Lactobacillus plantarum or Macleaya cordata Extract Alleviates Oxidative Damage Induced by Weaning in the Lower Gut of Young Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Managements
2.2. Collection Procedures and Sampling
2.3. Measurements of Antioxidant Activities and Inflammation
2.4. Statistical Analysis
3. Results
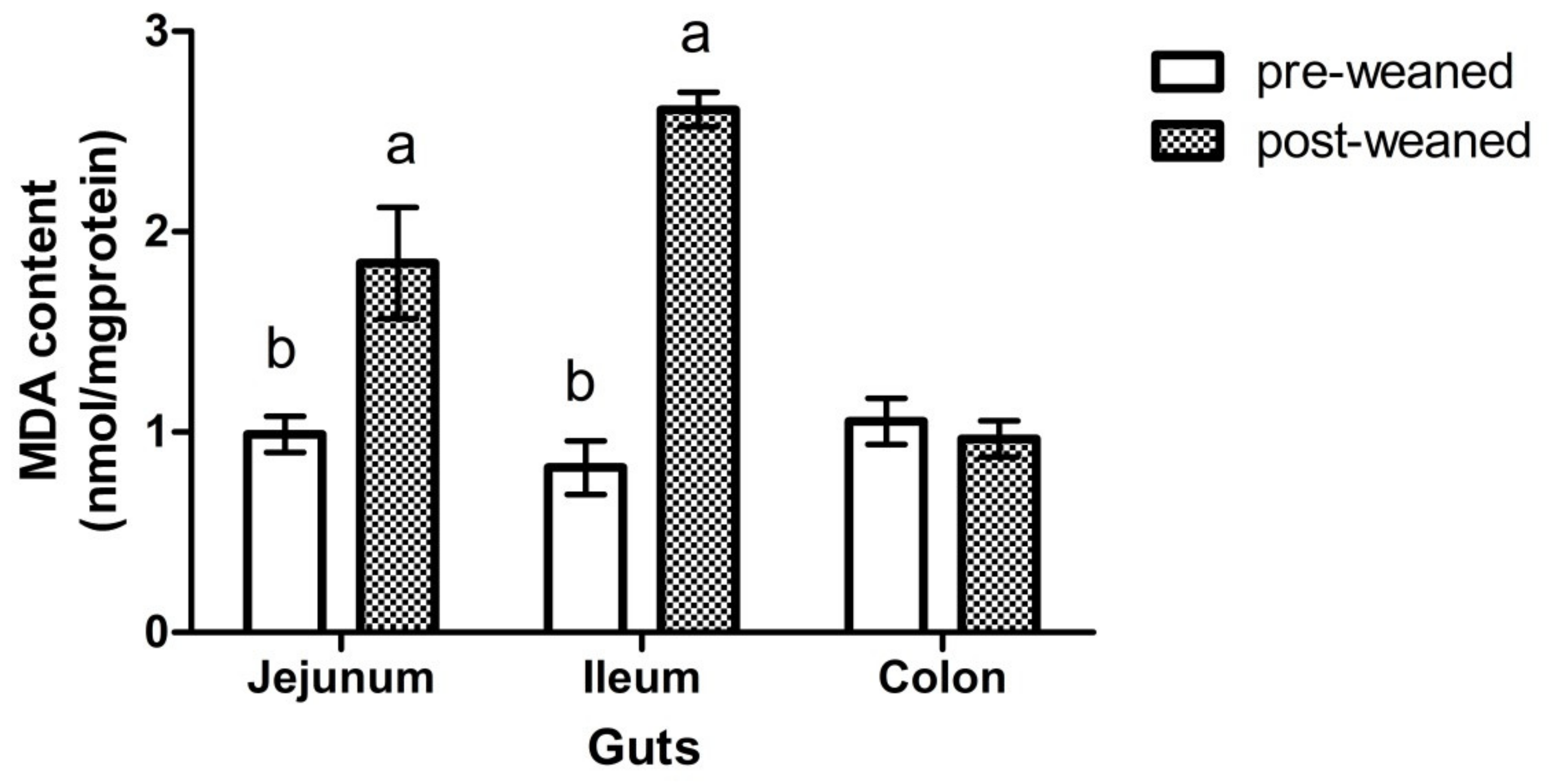
3.1. Weaning Effects on Activities of Antioxidant Enzyme and Inflammatory Factor
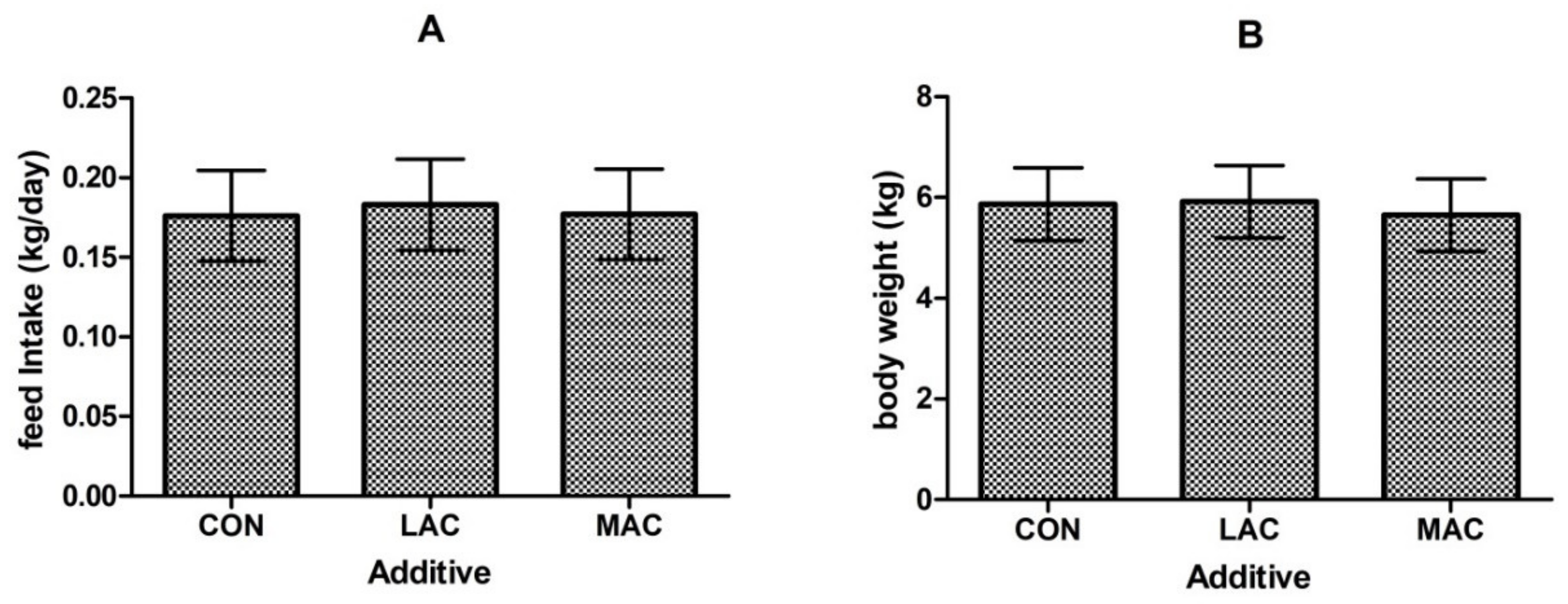
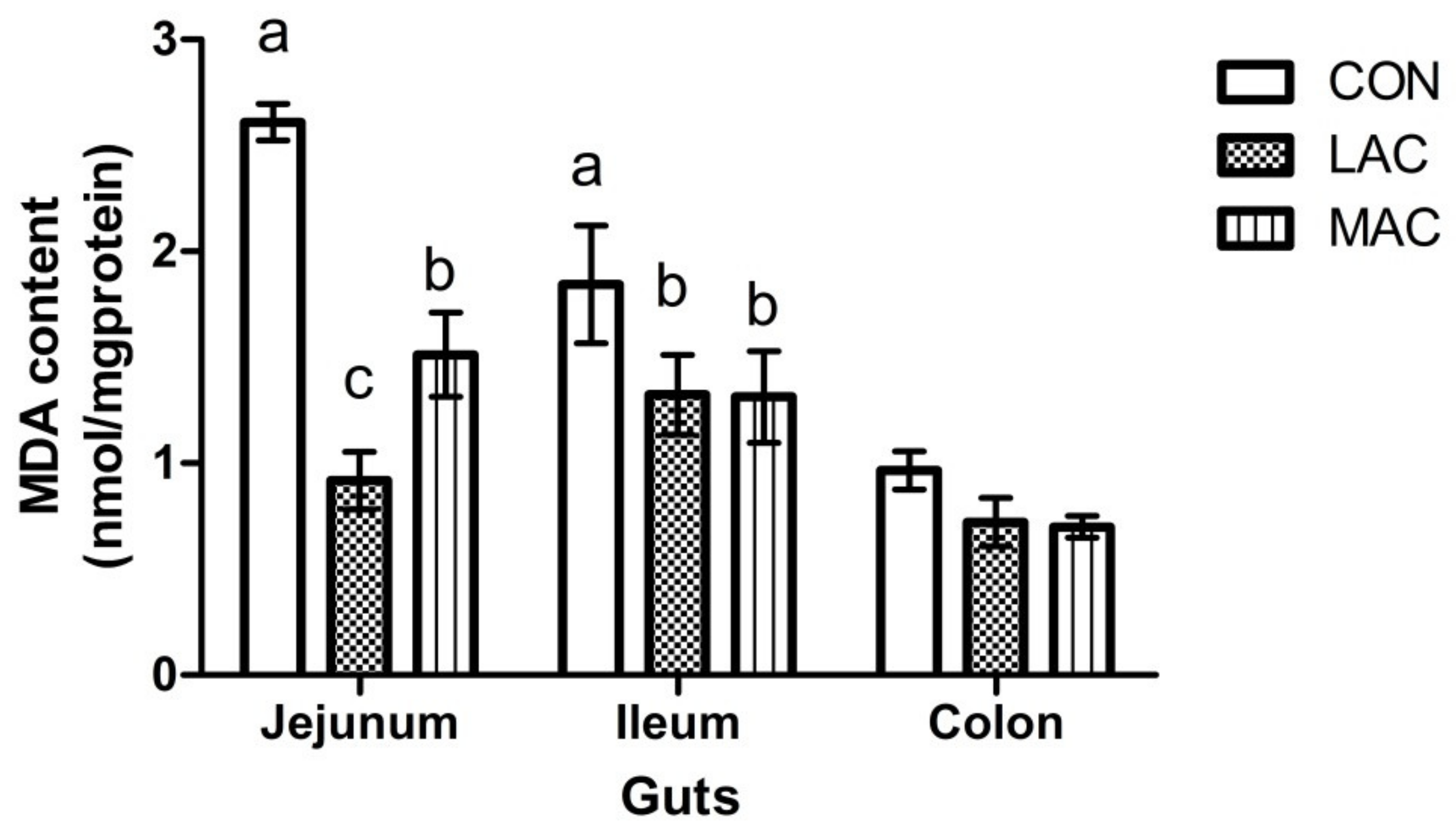
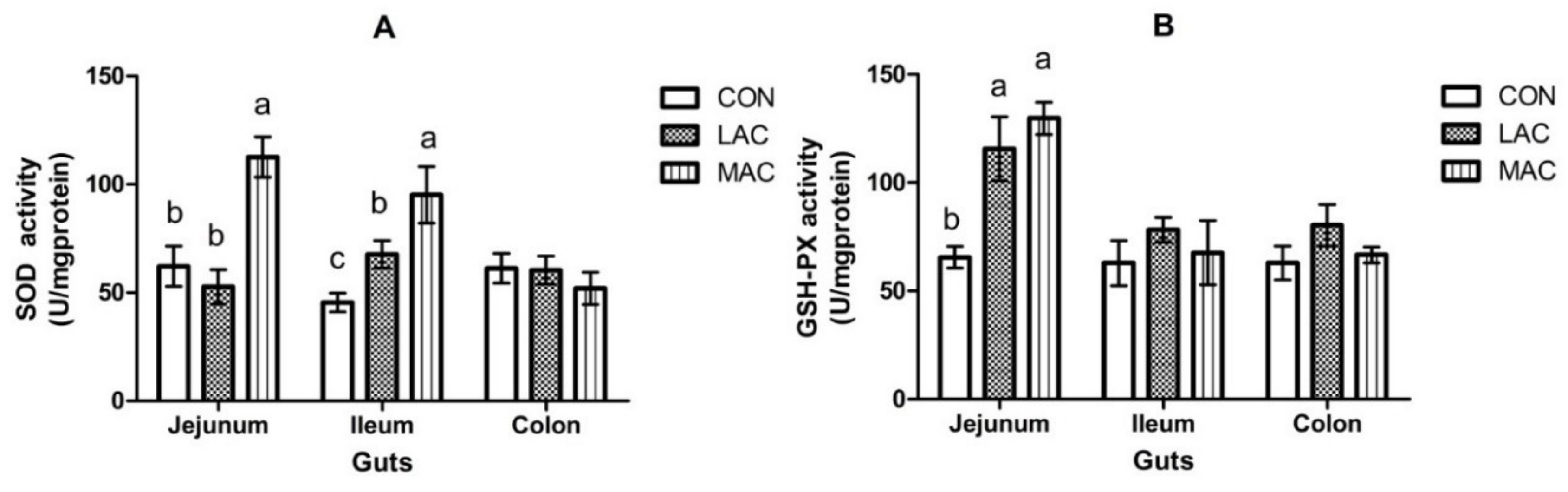
3.2. Effects of Supplementing Probiotics and Prebiotics on Antioxidant Enzyme, Inflammatory Factor, and Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alugongo, G.M.; Xiao, J.X.; Chung, Y.H.; Dong, S.Z.; Li, S.L.; Yoon, I.; Wu, Z.H.; Cao, Z.J. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: Performance and health. J. Dairy Sci. 2017, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, Y.; Wu, X.; Wan, D.; Yin, Y. Effects of Dietary Serine Supplementation on Intestinal Integrity, Inflammation and Oxidative Status in Early-Weaned Piglets. Cell Physiol. Biochem. 2018, 48, 993–1002. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning Induced Hepatic Oxidative Stress, Apoptosis, and Aminotransferases through MAPK Signaling Pathways in Piglets. Oxid. Med. Cell Longev. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, Y.; Wen, Z.; Jiang, X.; Ma, X.; Han, X. Weaning Stress Perturbs Gut Microbiome and Its Metabolic Profile in Piglets. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Sun, M.Z.; Zhang, C.; Xin, Y. Antioxidant properties of lactobacillus and its protecting effects to oxidative stress caco-2 cells. J. Anim. Plant Sci. 2014, 24, 1766–1771. [Google Scholar]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.F.; Wang, S.X.; Zhang, D.Y.; Liu, H.; Shan, D.C.; Wang, Y.M. Lactobacillus plantarum ZLP001: In vitro Assessment of Antioxidant Capacity and Effect on Growth Performance and Antioxidant Status in Weaning Piglets. Asian Austral. J. Anim. 2012, 25, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszti-Gere, E.; Csibrik-Nemeth, E.; Szeker, K.; Csizinszky, R.; Palocz, O.; Farkas, O.; Galfi, P. Lactobacillus plantarum 2142 prevents intestinal oxidative stress in optimized in vitro systems. Acta Physiol. Hung. 2013, 100, 89–98. [Google Scholar] [CrossRef]
- Pena, J.A.; Rogers, A.B.; Ge, Z.M.; Ng, V.; Li, S.Y.; Fox, J.G.; Versalovic, J. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect. Immun. 2005, 73, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, T.; Ishijima, A.S.; Ida, M.; Izumo, T.; Ono, Y.; Shibata, H.; Abe, S. Prophylactic Effect of Lactobacillus pentosus strain S-PT84 on Candida Infection and Gastric Inflammation in a Murine Gastrointestinal Candidiasis Model Errata. Med. Mycol. 2016, 57, E81–E92. [Google Scholar]
- Tobita, K.; Watanabe, I.; Saito, M. Specific vaginal lactobacilli suppress the inflammation induced by lipopolysaccharide stimulation through downregulation of toll-like receptor 4 expression in human embryonic intestinal epithelial cells. Biosci. Microb. Food Health 2017, 36, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holowacz, S.; Blondeau, C.; Guinobert, I.; Guilbot, A.; Hidalgo, S.; Bisson, J.F. Lactobacillus salivarius LA307 and Lactobacillus rhamnosus LA305 attenuate skin inflammation in mice. Benef. Microbes 2018, 9, 299–309. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, L.; Li, M.; Hu, Y.; Zeng, B.; Yuan, H.; Zhao, L.; Zhang, C. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, Y.; Xiao, K.; Jiang, F.; Wang, H.; Tang, D.; Liu, D.; Liu, B.; Liu, Y.; He, X.; et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.M.; Yang, X.Y.; Zhong, Y.R.; Yu, J.P. Chemical composition, antioxidant and antimicrobial activity of the essential oil from the leaves of Macleaya cordata (Willd) R. Br. Nat. Prod. Res. 2016, 30, 438–442. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Li, H.; Liu, Y.; Wang, S.; Dong, X.; Su, F.; Yao, G.; Sun, T.; Zhang, H. In vitro screen of Lactobacillus plantarum as probiotic bacteria and their fermented characteristics in soymilk. Ann. Microbiol. 2012, 62, 1311–1320. [Google Scholar] [CrossRef]
- Girotti, M.J.; Khan, N.; McLellan, B.A. Early measurement of systemic lipid-peroxidation products in the plasma of major blunt trauma patients. J. Trauma 1991, 31, 32–35. [Google Scholar] [CrossRef]
- Flohe, L.; Otting, F. superoxide-dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Mach, N.; Foury, A.; Kittelmann, S.; Reigner, F.; Moroldo, M.; Ballester, M.; Esquerre, D.; Riviere, J.; Salle, G.; Gerard, P.; et al. The Effects of Weaning Methods on Gut Microbiota Composition and Horse Physiology. Front. Physiol. 2017, 8, 535. [Google Scholar] [CrossRef] [Green Version]
- He, Z.X.; Sun, Z.H.; Tan, Z.L.; Tang, S.X.; Zhou, C.S.; Han, X.F.; Wang, M.; Wu, D.Q.; Kang, J.H.; Beauchemin, K.A. Effects of maternal protein or energy restriction during late gestation on antioxidant status of plasma and immune tissues in postnatal goats. J. Anim. Sci. 2012, 90, 4319–4326. [Google Scholar] [CrossRef]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Intracellular redox status and oxidative stress: Implications for cell proliferation, apoptosis, and carcinogenesis. Arch. Toxicol. 2008, 82, 273–299. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Hu, M.; Cao, J.; Chen, D.; Liu, Z. Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch. Toxicol. 2009, 83, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, L.F.; Titov, V.N. Lipid peroxidation in relation to ageing and the role of endogenous aldehydes in diabetes and other age-related diseases. Ageing Res. Rev. 2010, 9, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Verona, C.; Hackenhaar, F.S.; Teixeira, C.; Medeiros, T.M.; Alabarse, P.V.; Salomon, T.B.; Shüller, Á.K.; Maccari, J.A.G.; Condessa, R.L.; Oliveira, R.P.; et al. Blood markers of oxidative stress predict weaning failure from mechanical ventilation. J. Cell Mol. Med. 2015, 19, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zhou, C.; Guan, L.L.; McSweeney, C.S.; Tang, S.; Wang, M.; Tan, Z. Shifts in Host Mucosal Innate Immune Function Are Associated with Ruminal Microbial Succession in Supplemental Feeding and Grazing Goats at Different Ages. Front. Microbiol. 2017, 8, 1655. [Google Scholar] [CrossRef]
- Wang, T.W.; Teng, K.L.; Liu, Y.Y.; Shi, W.X.; Zhang, J.; Dong, E.Q.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; Jiang, Q.; Huang, W.; Liu, N. Effect of supplemental lactic acid bacteria on growth performance, glutathione turnover and aflatoxin B1 removal in lambs. Czech J. Anim. Sci. 2019, 64, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Finamore, A.; Ambra, R.; Nobili, F.; Garaguso, I.; Raguzzini, A.; Serafini, M. Redox Role of Lactobacillus casei Shirota against the cellular damage induced by 2,2’-Azobis (2-Amidinopropane) dihydrochloride-induced oxidative and inflammatory stress in enterocytes-like epithelial cells. Front. Immunol. 2018, 9, 1131. [Google Scholar] [CrossRef] [Green Version]
- Raabis, S.; Li, W.; Cersosimo, L. Effects and immune responses of probiotic treatment in ruminants. Vet. Immunol. Immunopathol. 2018, 12, 006. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.F.; Wu, G.Y.; Liao, Y.P.; Hou, Z.P.; Liu, H.J.; Yin, F.G.; Li, T.J.; Huang, R.L.; Zhang, Y.M.; Deng, D.; et al. Effects of Chinese herbal ultra-fine powder as a dietary additive on growth performance, serum metabolites and intestinal health in early-weaned piglets. Livest. Sci. 2007, 108, 272–275. [Google Scholar] [CrossRef]
- Bavarsadi, M.; Mahdavi, A.H.; Ansari-Mahyari, S.; Jahanian, E. Effects of different levels of sanguinarine on antioxidant indices, immunological responses, ileal microbial counts and jejunal morphology of laying hens fed diets with different levels of crude protein. J. Anim. Physiol. Anim. Nutr. 2017, 101, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.F.; Boris, S.; Barbes, C. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 2003, 94, 449–455. [Google Scholar] [CrossRef]
- Zuo, Z.H.; Shang, B.J.; Shao, Y.C.; Li, W.Y.; Sun, J.S. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immun. 2019, 86, 160–168. [Google Scholar] [CrossRef]
- Whitley, N.C.; Cazac, D.; Rude, B.J.; Jackson-O’Brien, D.; Parveen, S. Use of a commercial probiotic supplement in meat goats. J. Anim. Sci. 2009, 87, 723–728. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.N.; Kumar, S.; Tyagi, A.K. Effects of mannan-oligosaccharides and Lactobacillus acidophilus supplementation on growth performance, nutrient utilization and faecal characteristics in Murrah buffalo calves. J. Anim. Physiol. Anim. Nutr. 2018, 102, 679–689. [Google Scholar] [CrossRef]
| Item 1 | Age | SEM | Gut | SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preweaned | Postweaned | Jejunum | Ileum | Colon | Gut | Age | Interaction | |||
| SOD (U/mg·protein) | 98.8 a | 56.3 b | 8.0 | 85.1 | 73.1 | 74.35 | 11.9 | 0.829 | 0.012 | 0.701 |
| GSH-Px (U/mg·protein) | 62.0 | 64.4 | 10.7 | 88.8 a | 53.0 b | 47.87 b | 12.5 | 0.037 | 0.846 | 0.349 |
| IL-2 (pg/mg·protein) | 319.5 | 327.3 | 23.9 | 329.8 | 267.3 | 373.2 | 34.2 | 0.147 | 0.855 | 0.427 |
| IL-6 (pg/mg·protein) | 28.9 | 33.2 | 2.2 | 28.3 | 26.6 | 38.2 | 3.2 | 0.092 | 0.307 | 0.683 |
| Item 1 | Additive 2 | SEM | Gut | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | LAC | MAC | Jejunum | Ileum | Colon | Gut | Additive | Interaction | |||
| IL2 | 327.3 | 294.9 | 278.0 | 24.0 | 381.46 a | 254.5 b | 266.0 b | 26.8 | 0.020 | 0.536 | 0.304 |
| IL6 | 33.2 | 26.9 | 27.1 | 2.2 | 38.9 a | 23.4 b | 25.0 b | 2.3 | <0.001 | 0.185 | 0.397 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Liu, Y.; Cheng, Y.; Yan, Q.; Zhou, C.; He, Z.; Zeng, J.; He, J.; Tan, Z. Supplementation of Lactobacillus plantarum or Macleaya cordata Extract Alleviates Oxidative Damage Induced by Weaning in the Lower Gut of Young Goats. Animals 2020, 10, 548. https://doi.org/10.3390/ani10040548
Chen K, Liu Y, Cheng Y, Yan Q, Zhou C, He Z, Zeng J, He J, Tan Z. Supplementation of Lactobacillus plantarum or Macleaya cordata Extract Alleviates Oxidative Damage Induced by Weaning in the Lower Gut of Young Goats. Animals. 2020; 10(4):548. https://doi.org/10.3390/ani10040548
Chicago/Turabian StyleChen, Kai, Yong Liu, Yan Cheng, Qiongxian Yan, Chuanshe Zhou, Zhixiong He, Jianguo Zeng, Jianhua He, and Zhiliang Tan. 2020. "Supplementation of Lactobacillus plantarum or Macleaya cordata Extract Alleviates Oxidative Damage Induced by Weaning in the Lower Gut of Young Goats" Animals 10, no. 4: 548. https://doi.org/10.3390/ani10040548





