Effects of Dietary Starch Structure on Growth Performance, Serum Glucose–Insulin Response, and Intestinal Health in Weaned Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Starch
2.2. Animal Management and Housing
2.3. Dietary Treatments
2.4. Sample Collections
2.5. Apparent Digestibility of Nutrients and Starch
2.6. VFA Analysis
2.7. Serum Glucose and Insulin
2.8. Total RNA Extraction and Real-Time Quantitative PCR
2.9. DNA Extraction and Quantification of Intestinal Microflora
2.10. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Glucose and Insulin
3.3. Nutrient Digestibility
3.4. Concentration of Cecal Digesta VFA and pH Value
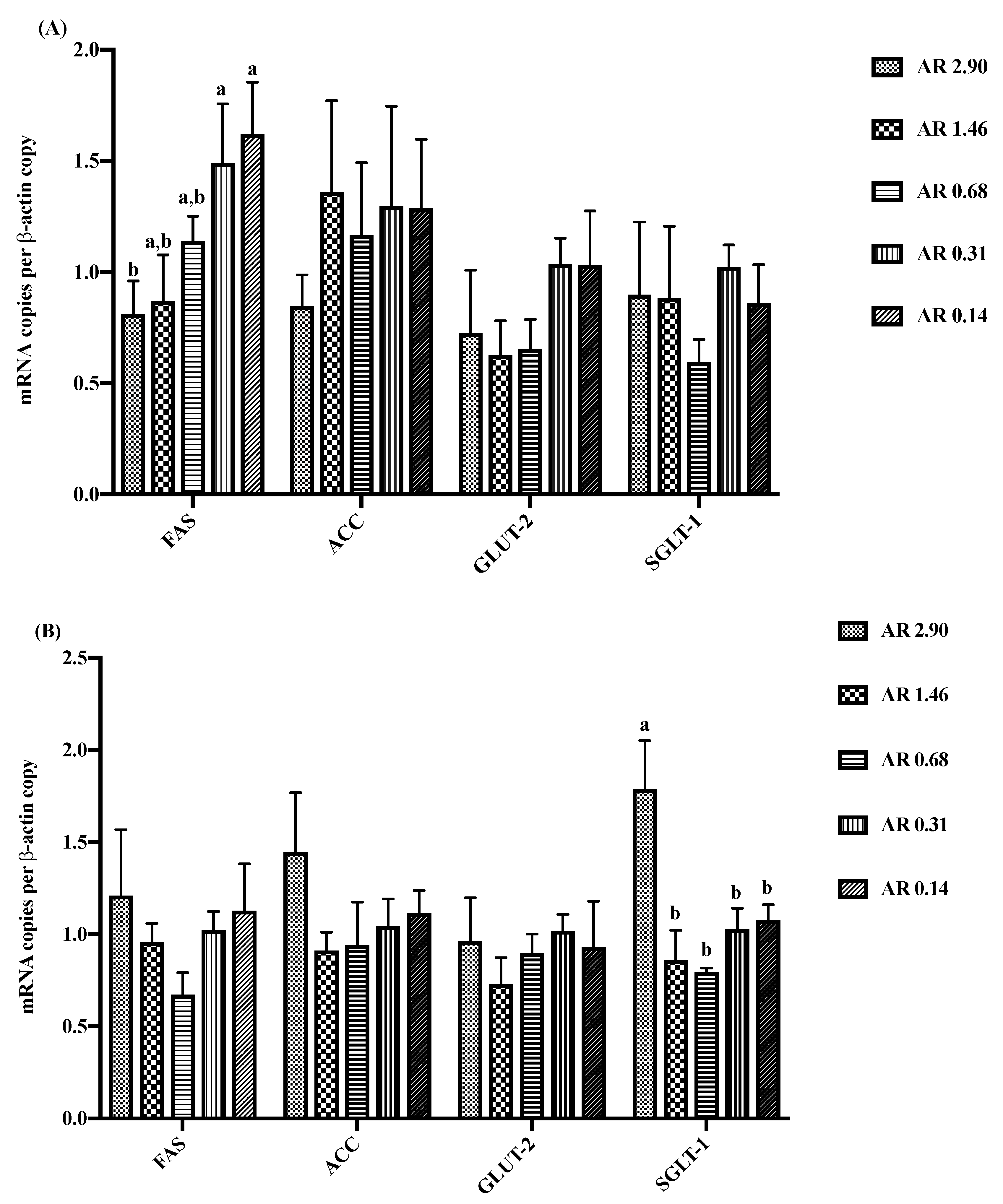
3.5. Intestinal Gene Expression
3.6. Intestinal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knudsen, K.E.B.; Lrke, H.N.; Steenfeldta, S.; Hedemann, M.S.; Jørgensen, H. In vivo methods to study the digestion of starch in pigs and poultry. Anim. Feed Sci. Technol. 2006, 130, 114–135. [Google Scholar] [CrossRef]
- Camp, L.K.; Bidner, T.D.; Southern, L.L. Effect of carbohydrate source on growth performance, carcass traits, and meat quality of growing-finishing pigs1,2,3. J. Anim. Sci. 2003, 81, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Z.; Shu, X.L.; Zhang, L.L.; Wang, X.Y.; Zhao, H.J.; Ma, C.X.; Wu, D.X. Starch properties of mutant rice high in resistant starch. J. Agric. Food Chem. 2006, 54, 523. [Google Scholar] [CrossRef] [PubMed]
- Englyst, K.; Englyst, H.G.J.; Cole, T.; Cummings, J. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Warren, F.J.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.R.; van Kempen, T.A.T.G.; Matte, J.J.; Zijlstra, R.T. Starch with high amylose and low in vitro digestibility increases short-chain fatty acid absorption, reduces peak insulin secretion, and modulates incretin secretion in pigs. J. Nutr. 2011, 141, 398–405. [Google Scholar] [CrossRef]
- He, J.; Chen, D.W.; Zhang, K.Y.; Yu, B. A high-amylopectin diet caused hepatic steatosis associated with more lipogenic enzymes and increased serum insulin concentration. Br. J. Nutr. 2011, 106, 1470–1475. [Google Scholar] [CrossRef]
- Yin, F.G.; Yin, Y.L.; Zhang, Z.Z.; Xie, M.Y.; Huang, J.; Huang, R.L.; Li, T.J. Digestion rate of dietary starch affects the systemic circulation of lipid profiles and lipid metabolism-related gene expression in weaned pigs. Br. J. Nutr. 2011, 106, 369–377. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.T.; Li, S.; Fang, Q.C.; Qian, L.L.; Jia, W.P. Effect of dietary resistant starch on prevention and treatment of obesity-related diseases and its possible mechanisms. Biomed. Environ. Sci. 2015, 28, 291–297. [Google Scholar]
- Bird, A.R.; Vuaran, M.; Brown, I.; Topping, D.L. Two high-amylose maize starches with different amounts of resistant starch vary in their effects on fermentation, tissue and digesta mass accretion, and bacterial populations in the large bowel of pigs. Br. J. Nutr. 2007, 97, 134–144. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Yu, B. Metabolic and transcriptomic responses of weaned pigs induced by different dietary amylose and amylopectin ratio. PLoS ONE 2010, 5, e15110. [Google Scholar]
- Yang, C.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Yu, J.; Zheng, P.; He, J. Effect of dietary amylose/amylopectin ratio on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 2015, 108, 55–60. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine: Eleventh Revised Edition; National Academies Press: Washington, DC, USA, 2012; pp. 210–211. [Google Scholar]
- Liu, J.B.; Xue, P.C.; Cao, S.C.; Liu, J.; Chen, L.; Zhang, H.F. Effects of dietary phosphorus concentration and body weight on postileal phosphorus digestion in pigs. Anim. Feed Sci. Technol. 2018, 242, 86–94. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.B.; He, J.; Yu, B.; Huang, Z.Q.; Yu, J.; Zheng, P.; Chen, D.W. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; Aherne, F.; Okai, D. Use of HCl insoluble ash as an index material for determining apparent digestibility with pigs. Can. J. Anim. Sci. 1974, 54, 107–109. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC International: Washington, DC, USA, 1995. [Google Scholar]
- Van Leeuwen, P.; Veldman, A.; Boisen, S.; Deuring, K.; Van Kempen, G.; Derksen, G.; Verstegen, M.; Schaafsma, G. Apparent ileal dry matter and crude protein digestibility of rations fed to pigs and determined with the use of chromic oxide (Cr2O3) and acid-insoluble ash as digestive markers. Br. J. Nutr. 1996, 76, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.W.; Xiang, Z.T.; Han, G.; Yu, B.; Huang, Z.Q.; Chen, D.W. Effects of different dietary protein sources on cecal microflora in rats. Afr. J. Biotechnol. 2011, 10, 3704–3708. [Google Scholar]
- Fierer, N.; Jackson, J.A.; Vilgalys, R.; Jackson, R.B. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 2005, 71, 4117–4120. [Google Scholar] [CrossRef]
- Diao, H.; Zheng, P.; Yu, B.; He, J.; Mao, X.B.; Yu, J.; Chen, D.W. Effects of dietary supplementation with benzoic acid on intestinal morphological structure and microflora in weaned piglets. Livest. Sci. 2014, 167, 249–256. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Regmi, P.R.; Metzler-Zebeli, B.U.; Gänzle, M.G.; van Kempen, T.A.; Zijlstra, R.T. Starch with high amylose content and low in vitro digestibility increases intestinal nutrient flow and microbial fermentation and selectively promotes bifidobacteria in pigs. J. Nutr. 2011, 141, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33. [Google Scholar]
- Deng, J.; Wu, X.; Bin, S.; Li, T.-J.; Huang, R.; Liu, Z.; Liu, Y.; Ruan, Z.; Deng, Z.; Hou, Y. Dietary amylose and amylopectin ratio and resistant starch content affects plasma glucose, lactic acid, hormone levels and protein synthesis in splanchnic tissues. J. Anim. Physiol. Anim. Nutr. 2010, 94, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhang, Z.; Huang, J.; Yin, Y. Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. Br. J. Nutr. 2010, 103, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M. Dietary carbohydrates and insulin action in humans. Br. J. Nutr. 2000, 83, S97–S102. [Google Scholar] [CrossRef]
- Armentano, L.; Mills, S.; Young, J. Effects of feeding frequency on glucose concentration, glucose turnover, and insulin concentration in steers. J. Dairy Sci. 1984, 67, 1445–1451. [Google Scholar] [CrossRef]
- Behall, K.M.; Scholfield, D.J. Food amylose content affects postprandial glucose and insulin responses. Cereal Chem. 2005, 82, 654–659. [Google Scholar] [CrossRef]
- Chang, L.; Chiang, S.-H.; Saltiel, A.R. Insulin signaling and the regulation of glucose transport. Mol. Med. 2004, 10, 65. [Google Scholar] [CrossRef]
- Yang, C.; He, J.; Yu, B.; Yu, J.; Mao, X.; Chen, D.; Yin, Y. The effect of dietary amylose/amylopectin ratio on serum and hepatic lipid content and its molecular mechanisms in growing-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1657–1665. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, A.; Jiang, N.; Zhu, S.; Zhao, F.; Wu, Q.; Liu, W.; Wang, L.; Cai, P.; Wang, F. Effects of different amylose to amylopectin ratios on serum indices related to glucose metabolism and glucose transporter expression in fattening lambs. Anim. Feed Sci. Technol. 2015, 202, 106–111. [Google Scholar] [CrossRef]
- Polakof, S.; Díaz-Rubio, M.E.; Dardevet, D.; Martin, J.-F.; Pujos-Guillot, E.; Scalbert, A.; Sebedio, J.-L.; Mazur, A.; Comte, B. Resistant starch intake partly restores metabolic and inflammatory alterations in the liver of high-fat-diet-fed rats. J. Nutr. Biochem. 2013, 24, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Sa’ad, H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2010, 1801, 1175–1183. [Google Scholar]
- Arora, T.; Sharma, R.; Frost, G. Propionate. Anti-obesity and satiety enhancing factor? Appetite 2011, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Krog, I.A.; Kappel, T.P.; Skou, H.M.; Nygaard, L.H.; Erik, B.K.K. Resistant starch and arabinoxylan augment SCFA absorption, but affect postprandial glucose and insulin responses differently. Br. J. Nutr. 2014, 111, 1564–1576. [Google Scholar]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, C.; Wright, A.D.G.; He, J.; Chen, D.-W. Responses in ileal and cecal bacteria to low and high amylose/amylopectin ratio diets in growing pigs. Appl. Microbiol. Biotechnol. 2015, 99, 10627–10638. [Google Scholar] [CrossRef]
- Bhandari, S.; Nyachoti, C.; Krause, D. Raw potato starch in weaned pig diets and its influence on postweaning scours and the molecular microbial ecology of the digestive tract. J. Anim. Sci. 2009, 87, 984–993. [Google Scholar] [CrossRef]
| Item | Content |
|---|---|
| Ingredients (%) | |
| Extruded soya bean | 6.50 |
| Soybean meal, dehulled | 14.00 |
| Fish meal (62.5%) | 5.00 |
| Whey powder | 8.50 |
| Blood plasma meal | 3.25 |
| Soy protein concentrate | 7.50 |
| Cellulose | 1.20 |
| Limestone | 1.04 |
| Dicalcium phosphate | 0.13 |
| Glucose | 2.00 |
| Salt | 0.35 |
| L-Lys·HCl (78%) | 0.04 |
| DL- Methionine | 0.09 |
| Chloride choline | 0.15 |
| Vitamin and minerals premix 1 | 0.25 |
| Total | 100.00 |
| Calculated content (as DM) | |
| Dietary energy (Mcal/kg) | 3.68 |
| Crude protein (%) | 19.37 |
| Ca (%) | 0.80 |
| Available P (%) | 0.36 |
| Methionine+ cystiene (%) | 0.72 |
| Threonine (%) | 0.82 |
| Tryptophan (%) | 2.05 |
| Crude fiber (%) | 2.05 |
| Gene | Primer Sequence (5’-3’) | Accession No. | Temp (°C) |
|---|---|---|---|
| FAS | Forward: GGACCTGGTGATGAACGTCT | EF589048 | 65.0 |
| Reverse: CGGAAGTTGAGGGAGGTGTA | |||
| ACC | Forward: ATGTTTCGGCAGTCCCTGAT | EF618729 | 59.7 |
| Reverse: TGTGGACCAGCTGACCTTGA | |||
| GLUT2 | Forward: TGGAATCAGCCAACCTGTTT | NM_001097417.1 | 59.7 |
| Reverse: ACAAGTCCCACCGACATGA | |||
| SGLT1 | Forward: AGAAGGGCCCCAAAATGACC | NM_001164021.1 | 65.0 |
| Reverse: TGTTCACTACTGTCCGCCAC | |||
| β-Actin | Forward: TCTGGCACCACACCTTCT | XM_003124280.3 | 60.0 |
| Reverse: TGATCTGGGTCATCTTCTCAC |
| Item | Primer/Probe Name and Sequence (5’-3’) | Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| Bifidobacterium | SQ-F, CGCGTCCGGTGTGAAAG | 121 | 55.0 |
| SQ-R, CTTCCCGATATCTACACATTCCA | |||
| SQ-P, (FMA) ATTCCACCGTTACACCGGGAA(BHQ-1) | |||
| Lactobacillus | RS-F, GAGGCAGCAGTAGGGAATCTTC | 126 | 53.0 |
| RS-R, CAACAGTTACTCTGACACCCGTTCTTC | |||
| RS-P, (FMA)AAGAAGGGTTTCGGCTCGTAAAACTCTGTT(BHQ-1) | |||
| Bacillus | YB-F, GCAACGAGCGCAACCCTTGA | 92 | 53.0 |
| YB-R, TCATCCCCACCTTCCTCCGGT | |||
| YB-P, (FMA)CGGTTTGTCACCGGCAGTCACCT(BHQ-1) | |||
| Escherichia coli | DC-F, CATGCCGCGTGTATGAAGAA | 96 | 55.0 |
| DC-R, CGGGTAACGTCAATGAGCAAA | |||
| DC-P, (FMA)AGGTATTAACTTTACTCCCTTCCTC(BHQ-1) | |||
| Total bacteria | Eub338F, ACTCCTACGGGAGGCAGCAG | ||
| Eub518R, ATTACCGCGGCTGCTGG | 200 | 61.5 |
| Item | Treatments | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| AR 2.90 1 | AR 1.46 | AR 0.68 | AR 0.31 | AR 0.14 | ANOVA | Linear | Quadratic | |
| ADFI, g/d 2 | 522.46 ± 15.86 | 541.78 ± 22.71 | 539.11 ± 7.18 | 531.90 ± 16.99 | 533.96 ± 14.04 | 0.949 | 0.810 | 0.812 |
| ADG, g/d 3 | 302.92 ± 15.86 | 314.76 ± 14.07 | 304.97 ± 9.69 | 296.85 ± 8.51 | 300.22 ± 12.26 | 0.876 | 0.540 | 0.786 |
| F/G, g/d 4 | 1.73 ± 0.04 | 1.73 ± 0.06 | 1.78 ± 0.07 | 1.79 ± 0.04 | 1.79 ± 0.06 | 0.851 | 0.288 | 0.564 |
| Item | Treatments | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| AR 2.90 2 | AR 1.46 | AR 0.68 | AR 0.31 | AR 0.14 | ANOVA | Linear | Quadratic | |
| GLU F 3 (mmol/L) | 5.95 ± 0.52 | 6.11 ± 0.35 | 6.33 ± 0.33 | 5.70 ± 0.49 | 5.76 ± 0.64 | 0.882 | 0.589 | 0.727 |
| GLU 1h 4 (mmol/L) | 8.66 ± 0.57 | 8.42 ± 1.07 | 8.57 ± 0.73 | 8.32 ± 0.67 | 8.70 ± 1.20 | 0.998 | 0.332 | 0.629 |
| GLU 2h (mmol/L) | 6.84 ± 0.45 | 7.48 ± 0.52 | 7.45 ± 0.65 | 7.63 ± 0.30 | 7.82 ± 0.72 | 0.769 | 0.208 | 0.432 |
| GLU 3h (mmol/L) | 6.89 ± 0.35 b | 6.59 ± 0.26 b | 8.76 ± 0.51 a | 8.88 ± 0.45 a | 8.34 ± 0.37 a | 0.001 | 0.002 | 0.004 |
| GLU 4h (mmol/L) | 6.05 ± 0.21 | 5.92 ± 0.16 | 6.90 ± 0.37 | 6.55 ± 0.36 | 7.02 ± 0.54 | 0.103 | 0.022 | 0.077 |
| GLU 5h (mmol/L) | 5.60 ± 0.11 b | 6.06 ± 0.18 b | 6.97 ± 0.43 a | 5.70 ± 0.25 b | 5.59 ± 0.31 b | 0.008 | 0.723 | 0.024 |
| Item | Treatments | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| AR 2.902 | AR 1.46 | AR 0.68 | AR 0.31 | AR 0.14 | ANOVA | Linear | Quadratic | |
| INS F 3 (mIU/L) | 57.20 ± 4.96 a | 61.72 ± 3.98 a | 65.00 ± 1.95 a | 54.41 ± 3.07 a | 37.69 ± 3.61 b | <0.001 | 0.003 | <0.001 |
| INS 1h 4 (mIU/L) | 77.73 ± 4.01 b | 84.15 ± 1.43 ab | 89.82 ± 2.29 a | 66.25 ± 2.97 c | 69.27 ± 1.23 c | <0.001 | 0.009 | 0.001 |
| INS 2h (mIU/L) | 91.40 ± 6.19 b | 91.57 ± 2.12 b | 90.96 ± 2.32 b | 112.26 ± 1.88 a | 108.45 ± 3.53 a | <0.001 | <0.001 | 0.001 |
| INS 3h (mIU/L) | 54.62 ± 2.42 b | 64.72 ± 5.93 b | 66.49 ± 1.73 b | 100.59 ± 6.08 a | 104.73 ± 4.06 a | <0.001 | <0.001 | <0.001 |
| INS 4h (mIU/L) | 90.98 ± 3.12 ab | 83.26 ± 4.09 b | 85.29 ± 2.91 b | 80.31 ± 3.27 b | 100.33 ± 6.64 a | 0.020 | 0.385 | 0.015 |
| INS 5h (mIU/L) | 53.55 ± 3.83 b | 47.21 ± 0.46 b | 47.70 ± 5.59 b | 59.01 ± 3.03 ab | 69.83 ± 8.27 a | 0.026 | 0.092 | 0.022 |
| Items | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| AR 2.90 2 | AR 1.46 | AR 0.68 | AR 0.31 | AR 0.14 | ||
| Dry matter | 85.39 ± 0.58 c | 83.98 ± 0.41c | 70.36 ± 3.56 d | 87.15 ± 0.44 bc | 93.14 ± 0.93 a | <0.001 |
| Ether extract | 90.84 ± 0.17 a | 78.11 ± 1.20 b | 64.02 ± 7.57 c | 80.31 ± 0.64 b | 92.56 ± 0.24 a | <0.001 |
| Gross energy | 83.26 ± 0.67 b | 81.06 ± 0.56 b | 64.99 ± 4.95 c | 85.52 ± 0.40 b | 92.71 ± 0.34 a | <0.001 |
| Crude protein | 73.43 ± 0.88 c | 64.19 ± 0.79 d | 37.47 ± 3.02 e | 78.89 ± 0.47 b | 88.19 ± 0.45 a | <0.001 |
| Crude Ash | 60.77 ± 1.51 bc | 49.31 ± 1.94 cd | 38.36 ± 6.09 d | 64.26 ± 5.24 ab | 77.09 ± 0.81 a | <0.001 |
| Item | Treatments | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| AR 2.902 | AR 1.46 | AR 0.68 | AR 0.31 | AR 0.14 | ANOVA | Linear | Quadratic | |
| Acetic acid | 58.26 ± 3.11 ab | 51.34 ± 6.20 b | 50.89 ± 3.65 b | 66.70 ± 4.12 a | 61.11 ± 3.51 ab | 0.070 | 0.158 | 0.206 |
| Propionic acid | 31.05 ± 3.98 | 25.07 ± 2.93 | 24.20 ± 4.30 | 22.55 ± 1.06 | 23.65 ± 2.48 | 0.375 | 0.087 | 0.122 |
| Butyric acid | 22.36 ± 3.48 a | 20.62 ± 3.92 a | 20.05 ± 2.20 a | 16.60 ± 1.44 ab | 9.39 ± 1.78 b | 0.020 | 0.002 | 0.003 |
| Total volatile fatty acid | 111.67 ± 2.56 | 97.03 ± 9.02 | 95.13 ± 8.01 | 105.85 ± 5.91 | 94.15 ± 7.52 | 0.335 | 0.248 | 0.409 |
| pH value | 5.39 ± 0.09 b | 5.58 ± 0.10 ab | 5.46 ± 0.08 ab | 5.63 ± 0.14 ab | 5.79 ± 0.17 a | 0.203 | 0.033 | 0.093 |
| Item | Treatments | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| AR 2.90 2 | AR 1.46 | AR 0.68 | AR 0.31 | AR 0.14 | ANOVA | Linear | Quadratic | |
| Total bacteria | 12.16 ± 1.14 c | 12.22 ± 0.85 c | 12.20 ± 0.77 c | 15.45 ± 0.33 b | 17.98 ± 0.66 a | <0.001 | <0.001 | <0.001 |
| Bacillus | 8.75 ± 0.15 | 9.06 ± 0.25 | 8.93 ± 0.14 | 8.69 ± 0.27 | 8.85 ± 0.21 | 0.740 | 0.794 | 0.843 |
| Lactobacillus | 7.49 ± 0.20 | 7.56 ± 0.25 | 7.78 ± 0.26 | 7.25 ± 0.17 | 7.57 ± 0.22 | 0.583 | 0.828 | 0.936 |
| Bifidobacterium | 5.13 ± 0.14 | 5.16 ± 0.20 | 5.03 ± 0.14 | 4.99 ± 0.22 | 5.25 ± 0.27 | 0.891 | 0.909 | 0.748 |
| Escherichia coli | 8.14 ± 0.17 | 7.85 ± 0.40 | 8.18 ± 0.42 | 8.37 ± 0.40 | 8.82 ± 0.20 | 0.344 | 0.075 | 0.113 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Yu, B.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Zheng, P.; He, J.; Chen, D. Effects of Dietary Starch Structure on Growth Performance, Serum Glucose–Insulin Response, and Intestinal Health in Weaned Piglets. Animals 2020, 10, 543. https://doi.org/10.3390/ani10030543
Gao X, Yu B, Yu J, Mao X, Huang Z, Luo Y, Luo J, Zheng P, He J, Chen D. Effects of Dietary Starch Structure on Growth Performance, Serum Glucose–Insulin Response, and Intestinal Health in Weaned Piglets. Animals. 2020; 10(3):543. https://doi.org/10.3390/ani10030543
Chicago/Turabian StyleGao, Xiaoqian, Bing Yu, Jie Yu, Xiangbing Mao, Zhiqing Huang, Yuheng Luo, Junqiu Luo, Ping Zheng, Jun He, and Daiwen Chen. 2020. "Effects of Dietary Starch Structure on Growth Performance, Serum Glucose–Insulin Response, and Intestinal Health in Weaned Piglets" Animals 10, no. 3: 543. https://doi.org/10.3390/ani10030543
APA StyleGao, X., Yu, B., Yu, J., Mao, X., Huang, Z., Luo, Y., Luo, J., Zheng, P., He, J., & Chen, D. (2020). Effects of Dietary Starch Structure on Growth Performance, Serum Glucose–Insulin Response, and Intestinal Health in Weaned Piglets. Animals, 10(3), 543. https://doi.org/10.3390/ani10030543






