Effects of Microencapsulated Blends of Organics Acids (OA) and Essential Oils (EO) as a Feed Additive for Broiler Chicken. A Focus on Growth Performance, Gut Morphology and Microbiology
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals and Diets
2.2. Feed Analyses and Chicken Performance
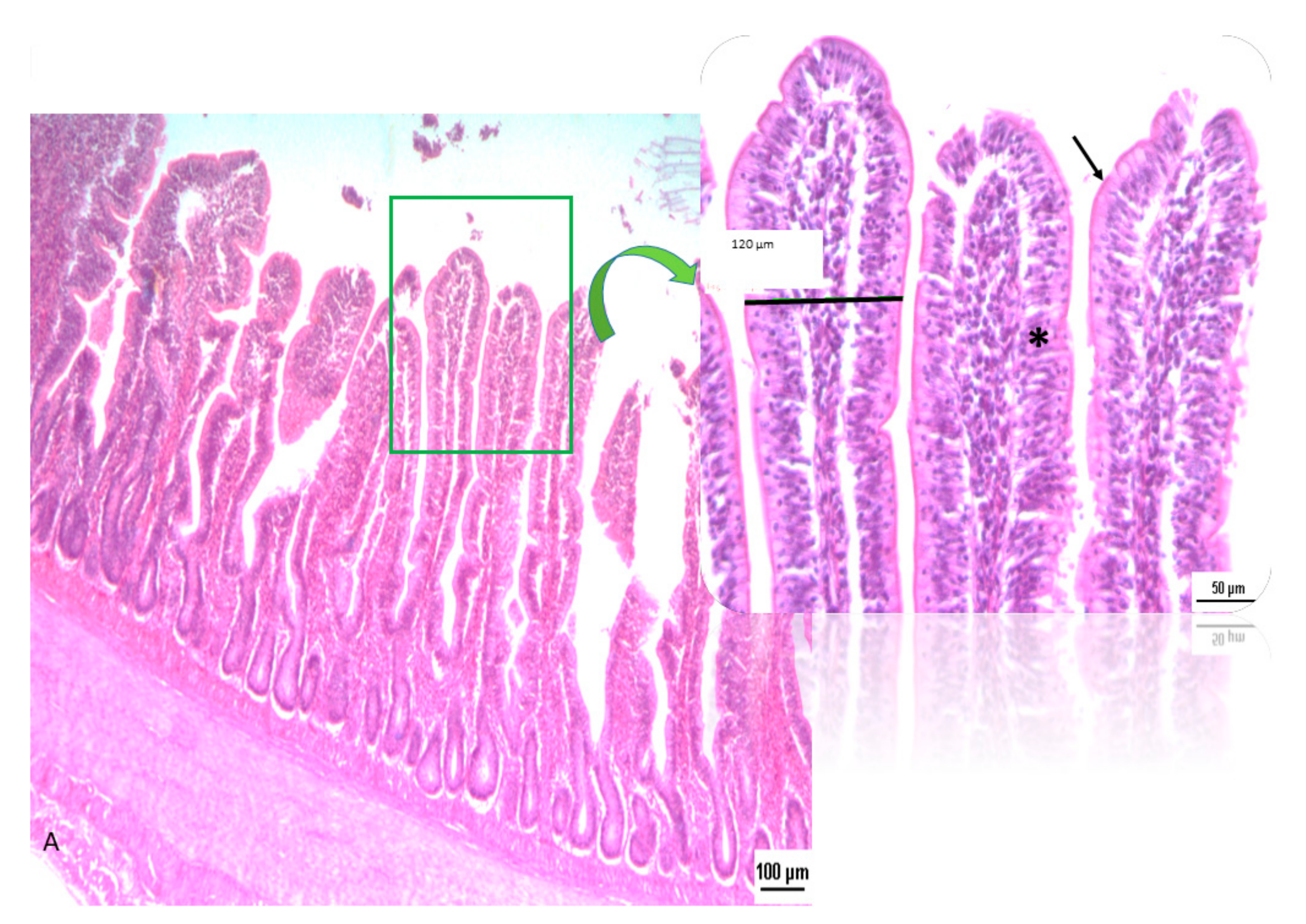
2.3. Intestinal Morphology and Morphometry
2.4. Microbiological Measurements
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Intestinal Morphology and Morphometric Analysis
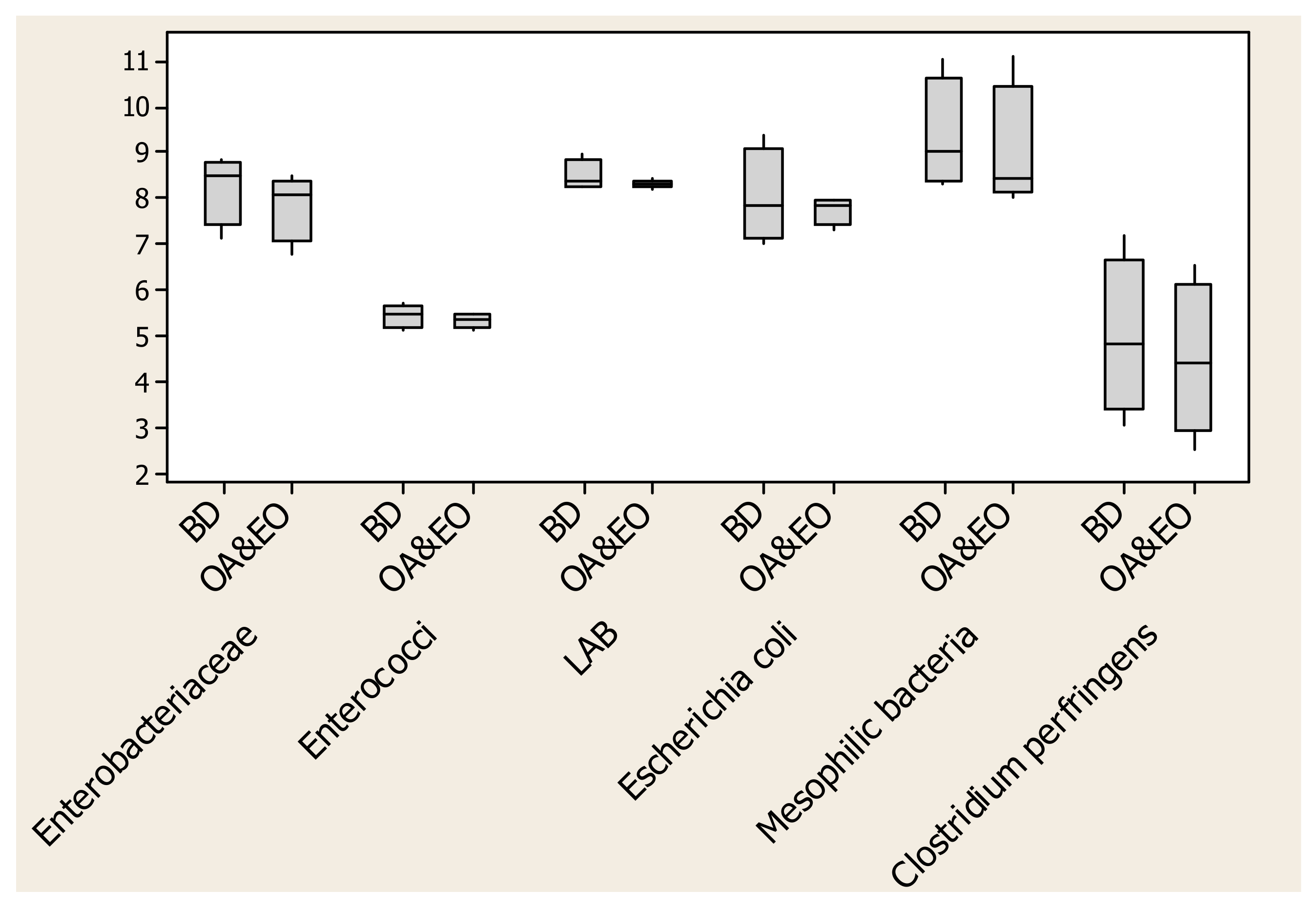
3.3. Intestinal and Litter Microflora Population
4. Discussion
4.1. Growth Performance
4.2. Intestinal Morphology and Morphometric Analysis
4.3. Modification of Ileal, Caecum and Litter Microflora
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 2003, 268, 29–43. [Google Scholar]
- European Commission. Directive 2004/28/EC of the European Parliament and of the Council of 31 March 2004 amending Directive 2001/82/EC on the Community code relating to veterinary medicinal products. 136/58. Off. J. Eur. Union 2004, 136, 58–84. [Google Scholar]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics, and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Tanhaiean, A.; Azghandi, M.; Razmyar, J.; Mohammadi, E.; Sekhavati, M.H. Recombinant production of a chimeric antimicrobial peptide in E. coli and assessment of its activity against some avian clinically isolated pathogens. Microb. Pathog. 2018, 122, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.M.; Reid, K.P.; Donovan, D.M.; Ramsay, T.G. Thermophile lytic enzyme fusion proteins that target Clostridium perfringens. Antibiotics 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Suiryanrayna, M.V.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Polycarpo, G.V.; Andretta, I.; Kipper, M.; Cruz-Polycarpo, V.C.; Dadalt, J.C.; Rodrigues, P.H.M.; Albuquerque, R. Meta-analytic study of organic acids as an alternative performance enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017, 96, 3645–3653. [Google Scholar] [CrossRef]
- Patten, J.D.; Waldroup, P.W. Use of organic acids in broiler diets. Poult. Sci. 1988, 67, 1178–1182. [Google Scholar] [CrossRef]
- Manzanilla, E.G.; Perez, J.F.; Martin, M.; Kamel, C.; Baucells, F.; Gasa, J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J. Anim. Sci. 2004, 82, 3210–3218. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Emami, N.K.; Samie, A.; Rahmani, H.R.; Ruiz-Feria, C.A. The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim. Feed Sci. Technol. 2012, 175, 57–64. [Google Scholar] [CrossRef]
- Placha, I.; Takacova, J.; Ryzner, M.; Cobanova, K.; Laukova, A.; Strompfova, V.; Venglovska, K.; Faix, S. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br. Poult. Sci. 2014, 55, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.K.; Li, Q.Y.; Piao, X.S.; Liu, J.D.; Zhao, P.F.; Xu, X.; Zhang, S.; Niu, S. Forsythia suspensa extract attenuates corticosterone-induced growth inhibition, oxidative injury, and immune depression in broilers. Poult. Sci. 2014, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Piva, A.; Pizzamiglio, V.; Morlacchini, M.; Tedeschi, M.; Piva, G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J. Anim. Sci. 2007, 85, 486–493. [Google Scholar] [CrossRef]
- Piva, A.; Anfossi, P.; Meola, E.; Pietri, A.; Panciroli, A.; Bertuzzi, T.; Formigoni, A. Effect of microencapsulation on absorption processes in the pig. Livest. Prod. Sci. 1997, 51, 53–61. [Google Scholar] [CrossRef]
- Grilli, E.; Messina, M.R.; Tedeschi, M.; Piva, A. Feeding a microencapsulated blend of organic acids and nature identical compounds to weaning pigs improved growth performance and intestinal metabolism. Livest. Sci. 2010, 133, 173–175. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Xin, H.; Chen, S.; Yang, C.; Duan, Y.; Yang, X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 2017, 88, 1414–1424. [Google Scholar] [CrossRef]
- Gauthier, R.; Grilli, E.; Piva, A. A microencapsulated blend of organic acids and natural identical flavours reduces necrotic enteritis-associated damages in broiler chickens. In Proceedings of the 16th European Symposium Poultry Nutrition, Strasbourg, France, 26–30 August 2007; pp. 515–518. [Google Scholar]
- Mitsch, P.; Zitterl-Eglseer, K.; Kohler, B.; Gabler, C.; Losa, R.; Zimpernik, I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult. Sci. 2004, 83, 669–675. [Google Scholar] [CrossRef]
- Desai, D.; Patwardhan, D.; Ranade, A. Acidifiers in Poultry Diets and Poultry Production. Acidifiers in Animal Nutrition–A Guide for Feed Preservation and Acidification to Promote Animal Performance; Luckstdat, C., Ed.; Nottingham University Press: Nottingham, UK, 2007; pp. 63–69. [Google Scholar]
- Mroz, Z. Organic Acids as Potential Alternatives to Antibiotic Growth Promoters for Pigs. Advances in Pork Production; Foxcroft, G., Ed.; University of Alberta Press: Edmonton, Alberta, 2005; pp. 169–182. [Google Scholar]
- Yang, X.; Liu, Y.; Yan, F.; Yang, C.; Yang, X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019, 98, 2858–2865. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, 140–148. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in food-A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Purohit, A.S. Anti-Salmonella activity of pyruvic and succinic acid in combination with oregano essential oil. Food Control 2020, 110, 106960. [Google Scholar] [CrossRef]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. 276/33. Off. J. Eur. Union 2010, 53, 33–79. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) No 849/2012 of 19 September 2012 concerning the authorisation of the preparation of citric acid, sorbic acid, thymol and vanillin as a feed additive for chickens for fattening, chickens reared for laying, all minor avian species for fattening and reared for laying and weaned Suidae other than Sus scrofa domesticus (holder of the authorisation Vetagro SpA). 253/8. Off. J. Eur. Union 2012, 253, 8–10. [Google Scholar]
- Gheisar, M.M.; Hosseindoust, A.; Kim, I.H. Evaluating the effect of microencapsulated blends of organic acids and essential oils in broiler chickens diet. J. Appl. Poult. Res. 2015, 24, 511–519. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, WA, USA, 1994. [Google Scholar]
- Commission Regulation EC no.152 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, 54, 1–130.
- European Commission. Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Union 2009, 303, 1–30. [Google Scholar]
- Rubio, L.A.; Ruiz, R.; Peinado, M.J.; Echavarri, A. Morphology and enzymatic activity of the small intestinal mucosa of Iberian pigs as compared with a lean pig strain. J. Anim. Sci. 2010, 88, 3590–3597. [Google Scholar] [CrossRef]
- Gheisari, A.A.; Heidari, M.; Kermanshahi, R.K.; Togiani, M.; Saraeian, S. Effects of dietary supplementation of protected organic acids on ileal microflora and protein digestibility in broiler chickens. In Proceedings of the 16th European Symposium Poultry Nutrition, Strasbourg, France, 26–30 August 2007; pp. 519–522. [Google Scholar]
- ISO 10272-1. Microbiology of the Food Chain–Horizontal Method for the Detection and Enumeration of Campylobacter Spp. Available online: https://www.iso.org/standard/63225.html (accessed on 22 May 2019).
- ISO 11290-1. Microbiology of the food Chain–Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and Listeria Spp. Available online: https://www.iso.org/standard/60313.html (accessed on 22 May 2019).
- ISO 6579-1. Microbiology of the Food Chain–Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Available online: https://www.iso.org/standard/56712.html (accessed on 22 May 2019).
- McNight, L.L.; Pepper, W.; Wright, D.C.; Page, G.; Han, Y. A blend of fatty acids, organic acids, and phytochemicals induced changes in intestinal morphology and inflammatory gene expression in coccidiosis-vaccinated broiler chickens. Poult. Sci. 2019, 98, 4901–4908. [Google Scholar] [CrossRef]
- Grilli, E.; Vitari, F.; Domeneghini, C.; Palmonari, A.; Tosi, G.; Fantinati, P.; Massi, P.; Piva, A. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: From in vitro to in vivo, a proof of concept. J. Appl. Microbiol. 2013, 114, 308–317. [Google Scholar] [CrossRef]
- Pourabedin, M.; Zhao, X. Prebiotics and gut microbiota in chickens. FEMS Microbiol Lett. 2015, 362, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Verstegen, M.W.A.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial community in broiler chickens. World’s Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef]
- Rebolé, A.; Ortiz, L.T.; Rodríguez, M.L.; Alzueta, C.; Treviño, J.; Velasco, S. Effects of inulin and enzyme complex, individually or in combination, on growth performance, intestinal microflora, cecal fermentation characteristics, and jejunal histomorphology in broiler chickens fed a wheat- and barley-based diet. Poult. Sci. 2010, 89, 276–286. [Google Scholar]
- Xia, M.S.; Hu, C.H.; Xu, Z.R. Effects of copper-bearing montmorillonite on growth performance, digestive enzyme activities, and intestinal microflora and morphology of male broilers. Poult. Sci. 2004, 83, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Sethiya, N.K. Review on natural growth promoters available for improving gut health of poultry: An alternative to antibiotic growth promoters. Asian J. Poult. Sci. 2016, 10, 1–29. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Böhm, J. Intestinal structure and function of broiler chickens on diets supplemented with a synbiotic containing Enterococcus faecium and oligosaccharides. Int. J. Mol. Sci. 2008, 9, 2205–2216. [Google Scholar] [CrossRef]
- Bogucka, J.; Ribeiro, D.M.; Boguslawska-Tryk, M.; Dankowiakowska, A.; da Costa, R.P.R.; Bednarczyk, M. Microstructure of the small intestine in broiler chickens fed a diet with probiotic or symbiotic supplementation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1785–1791. [Google Scholar] [CrossRef]
- Grilli, E.; Tugnoli, B.; Passey, J.L.; Stahl, C.H.; Piva, A.; Moeser, A.J. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet. Res. 2015, 11, 96. [Google Scholar] [CrossRef]
- Stanley, D.; Keyburn, A.L.; Denman, S.E.; Moore, R.J. Changes in caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012, 159, 155–162. [Google Scholar] [CrossRef]
- Briozzo, J.; Nunez, L.; Chirife, J.; Herszage, L.; D’Aquino, M. Antimicrobial activity of clove oil dispersed in a concentrated sugar solution. J. Appl. Bacteriol. 1988, 66, 69–75. [Google Scholar] [CrossRef]
- Dibner, J.J.; Buttin, P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Smulikowska, S.; Czerwiński, J.; Mieczkowska, A. Effect of an organic acid blend and phytase added to a rapeseed cake-containing diet on performance, intestinal morphology, caecal microflora activity and thyroid status of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2010, 94, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Tiwari, R.; Khan, R.U.; Chakroborty, S.; Gopi, M.; Karthik, K.; Saminathan, M. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: The trend an advances–a review. Int. J. Pharmacol. 2014, 10, 129–159. [Google Scholar] [CrossRef]
- Sunkara, L.B.; Achanta, M.; Schreiber, N.B.; Bommineni, Y.R.; Dai, G. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defence peptide gene expression. PLoS ONE 2011, 6, e27225. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, L.B.; Jiang, W.; Zhang, G. Modulations of antimicrobial host defence peptide gene expression by free fatty acids. PLoS ONE 2012, 7, e49558. [Google Scholar] [CrossRef] [PubMed]

| Ingredients, g/100 Gas-Fed | Diet | |||
|---|---|---|---|---|
| Starter (0–12 d) | Grower 1 (12–26d) | Grower 2 (26–35 d) | Finisher (35–47d) | |
| Corn | 35 | 50 | 51 | 50 |
| Soybean meal 48% | 27.15 | 28.9 | 26 | 23.5 |
| Soybean | 10 | 3 | 2 | 2 |
| Wheat | 10 | 0 | 0 | 0 |
| Wheat pollard | 9 | 9 | 10 | 15 |
| Animal Fat | 3.9 | 4.5 | 6.4 | 5.3 |
| Dicalcium Phosphate | 1.75 | 1.5 | 1.5 | 1.2 |
| Mineral-vitamin premix 1 | 2.5 | 2.5 | 2.5 | 2.5 |
| Calcium carbonate | 0.7 | 0.6 | 0.6 | 0.5 |
| Chemical composition | ||||
| Dry matter (DM), g/100g as fed | 88.89 | 88.66 | 89.26 | 90.08 |
| Protein, g/100g DM | 21.45 | 19.67 | 18.76 | 18.46 |
| Lipid, g/100g DM | 8.99 | 7.23 | 7.75 | 7.86 |
| Crude fiber, g/100g DM | 3.65 | 3.07 | 3.38 | 3.35 |
| Ash, g/100g DM | 5.59 | 5.61 | 5.82 | 5.34 |
| Calcium, g/100g DM | 0.87 | 0.84 | 0.78 | 0.63 |
| Sodium, g/100g DM | 0.18 | 0.16 | 0.17 | 0.17 |
| Phosphorus, g/100g DM | 0.61 | 0.61 | 0.59 | 0.55 |
| Lysine, Lys | 1.37 | 1.44 | 1.42 | 1.29 |
| Methionine, Met | 0.75 | 0.76 | 0.70 | 0.58 |
| Metabolizable Energy (kcal/kg) | 3200 | 3060 | 3062 | 3060 |
| Treatment | 0–12 Days | 12–25 Days | 25–35 Days | 35–47 Days | 0–47 Days | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADG a | FI b | FCR c | ADG a | FI b | FCR c | ADG a | FI b | FCR c | ADG a | FI b | FCR c | ADG a | FI b | FCR c | |
| BD d | 29.79 | 32.37 | 1.09 | 92.39 | 119.25 | 1.29 | 89.82 | 113.4 | 1.27 | 78.151 | 185.81 | 2.38 | 70.66 | 88.34 | 1.21 |
| OA&EO e | 30.14 | 31.5 | 1.05 | 87.61 | 117.65 | 1.34 | 83.59 | 111.7 | 1.34 | 104.55 | 195.66 | 1.88 | 67.11 | 86.95 | 1.24 |
| SEM f | 0.318 | 0.262 | 0.019 | 1.61 | 0.878 | 0.016 | 3.05 | 1.29 | 0.031 | 6.45 | 3.84 | 0.121 | 6.77 | 9.58 | 0.031 |
| p-values | 0.635 | 0.091 | 0.298 | 0.152 | 0.422 | 0.100 | 0.363 | 0.571 | 0.318 | 0.01 | 0.233 | 0.006 | 0.802 | 0.945 | 0.683 |
| Treatment 1 | Live Weight, g | |||
|---|---|---|---|---|
| 12 Days | 25 Days | 35 Days | 47 Days | |
| BD | 401.13 | 1602.18 | 2500.37 | 3438.18 |
| OA&EO | 405.07 | 1543.99 | 2379.88 | 3634.42 |
| SEM 2 | 3.21 | 11.8 | 19.3 | 29.2 |
| p-Values | 0.541 | 0.013 | 0.002 | 0.001 |
| Items | Day 11 | Day 25 | Day 34 | Day 46 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment, T 1 | SEM 2 | p-Value 3 | Treatment, T 1 | SEM 2 | p-Value 3 | Treatment, T 1 | SEM 2 | p-Value 3 | Treatment, T 1 | SEM 2 | p-Value 3 | |||||
| BD | OA&EO | BD | OA&EO | BD | OA&EO | BD | OA&EO | |||||||||
| Villi, n. | ||||||||||||||||
| Duodenum | 11.1 | 10.3 | 0.889 | NS | 11.3 | 9.1 | 1.1 | NS | 6.0 | 9.4 | 0.871 | 0.049 | 5.7 | 6.2 | 0.468 | NS |
| Jejunum | 14.6 | 15.4 | 1.62 | NS | 13.3 | 10.8 | 1.64 | NS | 10.7 | 14.0 | 1.38 | NS | 8.3 | 8.6 | 0.883 | NS |
| Ileum | 14.9 | 16.3 | 2 | NS | 17 | 16.9 | 2 | NS | 18.1 | 18.0 | 1 | NS | 10.7 | 13.6 | 12.5 | NS |
| Villi height, VH, μm | ||||||||||||||||
| Duodenum | 182,4775 | 170,7206 | 6,8104 | NS | 1,627,299 | 1,651,397 | 9,3101 | NS | 179,1973 | 194,9865 | 11,6612 | NS | 166,2088 | 181,2208 | 7,0772 | NS |
| Jejunum | 79,1347 | 76,0604 | 3,6103 | NS | 821,632 | 960,890 | 6,7449 | NS | 91,7661 | 101,1476 | 6,3316 | NS | 90,5051 | 107,8127 | 5,0823 | 0.089 |
| Ileum | 54,9170 | 51,9617 | 1,3956 | NS | 646,283 | 767,625 | 3,5861 | 0.091 | 58,6361 | 76,8206 | 2,9264 | 0.001 | 81,1187 | 86,2729 | 6,4917 | NS |
| Villi width, VW, μm | ||||||||||||||||
| Duodenum | 16,6146 | 16,7485 | 9500 | NS | 17,4806 | 15,3437 | 9560 | NS | 19,4824 | 18,7712 | 1,8789 | NS | 22,7073 | 21,8347 | 1,5191 | NS |
| Jejunum | 14,8624 | 14,9632 | 1,0872 | NS | 13,8523 | 14,7230 | 9648 | NS | 16,8853 | 18,3951 | 9788 | NS | 20,4966 | 19,2229 | 1,2640 | NS |
| Ileum | 14,0674 | 12,7699 | 7,269 | NS | 14,7288 | 14,3584 | 6571 | NS | 13,6420 | 178,355 | 1,2961 | 0.1 | 16,4235 | 20,2750 | 1,2136 | NS |
| Crypt, depth CD, μm | ||||||||||||||||
| Duodenum | 19,4587 | 19,0393 | 1,1623 | NS | 23,6624 | 26,7845 | 1,9466 | NS | 28,7915 | 32,8091 | 1,5754 | NS | 22,6123 | 19,7036 | 2,1050 | NS |
| Jejunum | 14,0735 | 15,7599 | 1,0492 | NS | 14,2846 | 18,3143 | 1,3830 | NS | 20,3398 | 17,8364 | 1,6135 | NS | 14,9159 | 16,8538 | 1,5840 | NS |
| Ileum | 13,2409 | 12,9022 | 7489 | NS | 13,4701 | 17,6356 | 1,1009 | 0.055 | 13,6884 | 16,1609 | 9907 | NS | 12,2340 | 12,3774 | 1,2795 | NS |
| Crypt, width CW, μm | ||||||||||||||||
| Duodenum | 1,5268 | 1,7226 | 944 | NS | 1,8779 | 1,8635 | 1014 | NS | 2,3641 | 2,4140 | 1552 | NS | 1,7381 | 1,6459 | 1682 | NS |
| Jejunum | 1,7878 | 1,5713 | 1144 | NS | 1,8334 | 2,0402 | 918 | NS | 2,3566 | 2,2134 | 1495 | NS | 1,8076 | 2,0179 | 1863 | NS |
| Ileum | 1,4568 | 1,3884 | 835 | NS | 2,0121 | 2,0456 | 941 | NS | 2,2037 | 2,0804 | 1144 | NS | 1,8985 | 1,5580 | 2373 | NS |
| IM 4 thickness, μm | ||||||||||||||||
| Duodenum | 11,8121 | 11,2260 | 7393 | NS | 16,3518 | 24,8576 | 1,5416 | 0.002 | 15,1654 | 18,6055 | 1,1616 | NS | 11,3496 | 10,2091 | 1,2472 | NS |
| Jejunum | 10,0921 | 8,5737 | 9282 | NS | 19,5494 | 15,7568 | 1,9865 | NS | 12,5443 | 15,0728 | 1,2978 | NS | 8,5002 | 7,8229 | 9276 | NS |
| Ileum | 12,3312 | 11,8648 | 8753 | NS | 21,7409 | 23,8342 | 1,4278 | NS | 15,7826 | 20,6350 | 1,2914 | 0.057 | 13,2033 | 12,2037 | 1,1551 | NS |
| Villi area, Log10μm 2 | ||||||||||||||||
| Duodenum | 1.20 × 10 | 1.19× 10 | 2.90 × 10−2 | NS | 1.19 × 10 | 1.19 × 10 | 3.40 × 10−2 | NS | 1.20 × 10 | 1.20 × 10 | 4.60 × 10−2 | NS | 1.21 × 10 | 1.21E+01 | 3.10 × 10−2 | NS |
| Jejunum | 1.15 × 10 | 1.15 × 10 | 4.20 × 10−2 | NS | 1.15 × 10 | 1.16 × 10 | 4.40 × 10−2 | NS | 1.17 × 10 | 1.17 × 10 | 4.50 × 10−2 | NS | 1.17 × 10 | 1.18E+01 | 3.80 × 10−2 | NS |
| Ileum | 1.14× 10 | 1.13 × 10 | 2.50 × 10−2 | NS | 1.15 × 10 | 1.15 × 10 | 2.80 × 10−2 | NS | 1.14 × 10 | 1.16 × 10 | 4.20 × 10−2 | 0.005 | 1.16 × 10 | 1.17E+01 | 5.30 × 10−2 | NS |
| Bacterial Group 2 | Treatment | SEM 1 | p-Value | ||
|---|---|---|---|---|---|
| Day | OA&EO | BD | |||
| Enterobatteriaceae | 11 | 6.959 | 6.959 | 0.145 | 0.641 |
| (Log10 CFU/g) | 25 | 6.707 | 6.159 | 0.202 | 0.202 |
| 34 | 8.694 | 8.790 | 0.139 | 0.769 | |
| 46 | 8.144 | 8.415 | 0.273 | 0.673 | |
| Enterococci | 11 | 5.651 | 6.138 | 0.260 | 0.409 |
| (Log10 CFU/g) | 25 | 5.924 | 5.726 | 0.096 | 0.360 |
| 34 | 5.827 | 6.041 | 0.230 | 0.691 | |
| 46 | 5.996 | 5.612 | 0.147 | 0.222 | |
| LAB 3 | 11 | 7.623 | 7.301 | 0.180 | 0.433 |
| (Log10 CFU/g) | 25 | 8.559 | 8.417 | 0.058 | 0.259 |
| 34 | 8.573 | 8.334 | 0.110 | 0.327 | |
| 46 | 9.403 | 9.085 | 0.131 | 0.268 | |
| Escherichia coli | 11 | 5.398 | 5.401 | 0.169 | 0.992 |
| (Log10 CFU/g) | 25 | 7.675 | 6.729 | 0.283 | 0.087 |
| 34 | 6.526 | 6.490 | 0.141 | 0.914 | |
| 46 | 7.476 | 7.655 | 0.173 | 0.658 | |
| Mesophilic bacteria | 11 | 7.317 | 7.145 | 0.197 | 0.710 |
| (Log10 CFU/g) | 25 | 7.713 | 7.693 | 0.426 | 0.984 |
| 34 | 8.924 | 9.196 | 0.223 | 0.601 | |
| 46 | 8.306 | 8.578 | 0.152 | 0.432 | |
| Clostridium perfringens | 11 | 5.908 | 5.735 | 0.057 | 0.140 |
| (Log10 CFU/g) | 25 | 1.985 | 1.667 | 0.537 | 0.802 |
| 34 | 1.360 | 5.066 | 1.030 | 0.053 | |
| 46 | 1.230 | 4.154 | 0.879 | 0.090 | |
| Bacterial Group | Treatment | SEM 1 | p-Value | ||
|---|---|---|---|---|---|
| Day | OA&EO | BD | |||
| Mesophilic bacteria | 0 | 5.681 | 5.681 | 0 | |
| (Log10 CFU/g) | 20 | 9.449 | 9.501 | 9.475 | 0.367 |
| 41 | 9.392 | 10.328 | 0.239 | 0.022 | |
| Enterococci | 0 | 3.041 | 3.041 | 0 | |
| (Log10 CFU/g) | 20 | 8.413 | 8.317 | 8.3651 | 0.502 |
| 41 | 7.804 | 8.242 | 0.108 | 0.013 | |
| Enterobatteriaceae | 0 | 3.079 | 3.079 | 0 | |
| (Log10 CFU/g) | 20 | 8.324 | 9.067 | 8.695 | 0.096 |
| 41 | 8.338 | 7.549 | 0.24 | 0.096 | |
| Clostridium perfringens | 0 | 0 | 0 | 0 | |
| (Log10 CFU/g) | 20 | 0.534 | 0 | 0.267 | 0.374 |
| 41 | 3.303 | 4.836 | 0.460 | 0.089 | |
| Escherichia coli | 0 | 0 | 0 | 0 | |
| (Log10 CFU/g) | 20 | 7.766 | 6.580 | 7.173 | 0.013 |
| 41 | 7.454 | 7.031 | 0.172 | 0.257 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamilla, A.; Messina, A.; Sallemi, S.; Condorelli, L.; Antoci, F.; Puleio, R.; Loria, G.R.; Cascone, G.; Lanza, M. Effects of Microencapsulated Blends of Organics Acids (OA) and Essential Oils (EO) as a Feed Additive for Broiler Chicken. A Focus on Growth Performance, Gut Morphology and Microbiology. Animals 2020, 10, 442. https://doi.org/10.3390/ani10030442
Stamilla A, Messina A, Sallemi S, Condorelli L, Antoci F, Puleio R, Loria GR, Cascone G, Lanza M. Effects of Microencapsulated Blends of Organics Acids (OA) and Essential Oils (EO) as a Feed Additive for Broiler Chicken. A Focus on Growth Performance, Gut Morphology and Microbiology. Animals. 2020; 10(3):442. https://doi.org/10.3390/ani10030442
Chicago/Turabian StyleStamilla, Alessandro, Antonino Messina, Sabrina Sallemi, Lucia Condorelli, Francesco Antoci, Roberto Puleio, Guido Ruggero Loria, Giuseppe Cascone, and Massimiliano Lanza. 2020. "Effects of Microencapsulated Blends of Organics Acids (OA) and Essential Oils (EO) as a Feed Additive for Broiler Chicken. A Focus on Growth Performance, Gut Morphology and Microbiology" Animals 10, no. 3: 442. https://doi.org/10.3390/ani10030442
APA StyleStamilla, A., Messina, A., Sallemi, S., Condorelli, L., Antoci, F., Puleio, R., Loria, G. R., Cascone, G., & Lanza, M. (2020). Effects of Microencapsulated Blends of Organics Acids (OA) and Essential Oils (EO) as a Feed Additive for Broiler Chicken. A Focus on Growth Performance, Gut Morphology and Microbiology. Animals, 10(3), 442. https://doi.org/10.3390/ani10030442






