Study of the AMP-activated Protein Kinase Role in Energy Metabolism Changes during the Postmortem Aging of Yak Longissimus dorsal
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment
2.2. Measurement of the pH
2.3. Lactic Acid Concentration
2.4. ATP, ADP, AMP, and IMP Activity
2.5. AMPK Activity
2.6. Immunoblotting
2.7. Real-time PCR Analysis
2.8. Data Processing and Statistical Analyses
3. Results
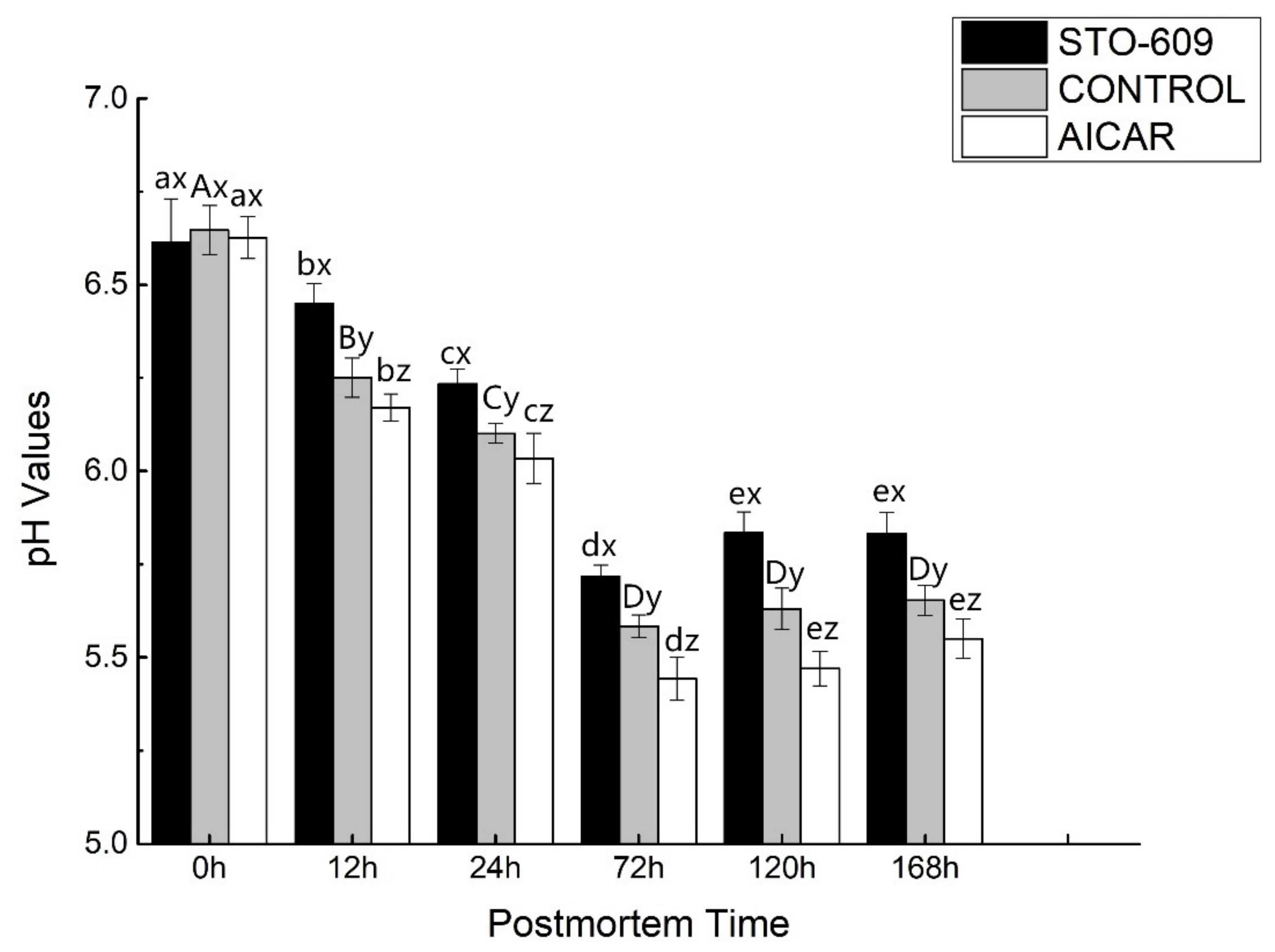
3.1. pH Value Determination
3.2. Lactic Acid Concentration
3.3. ATP, ADP, AMP, and IMP Activities
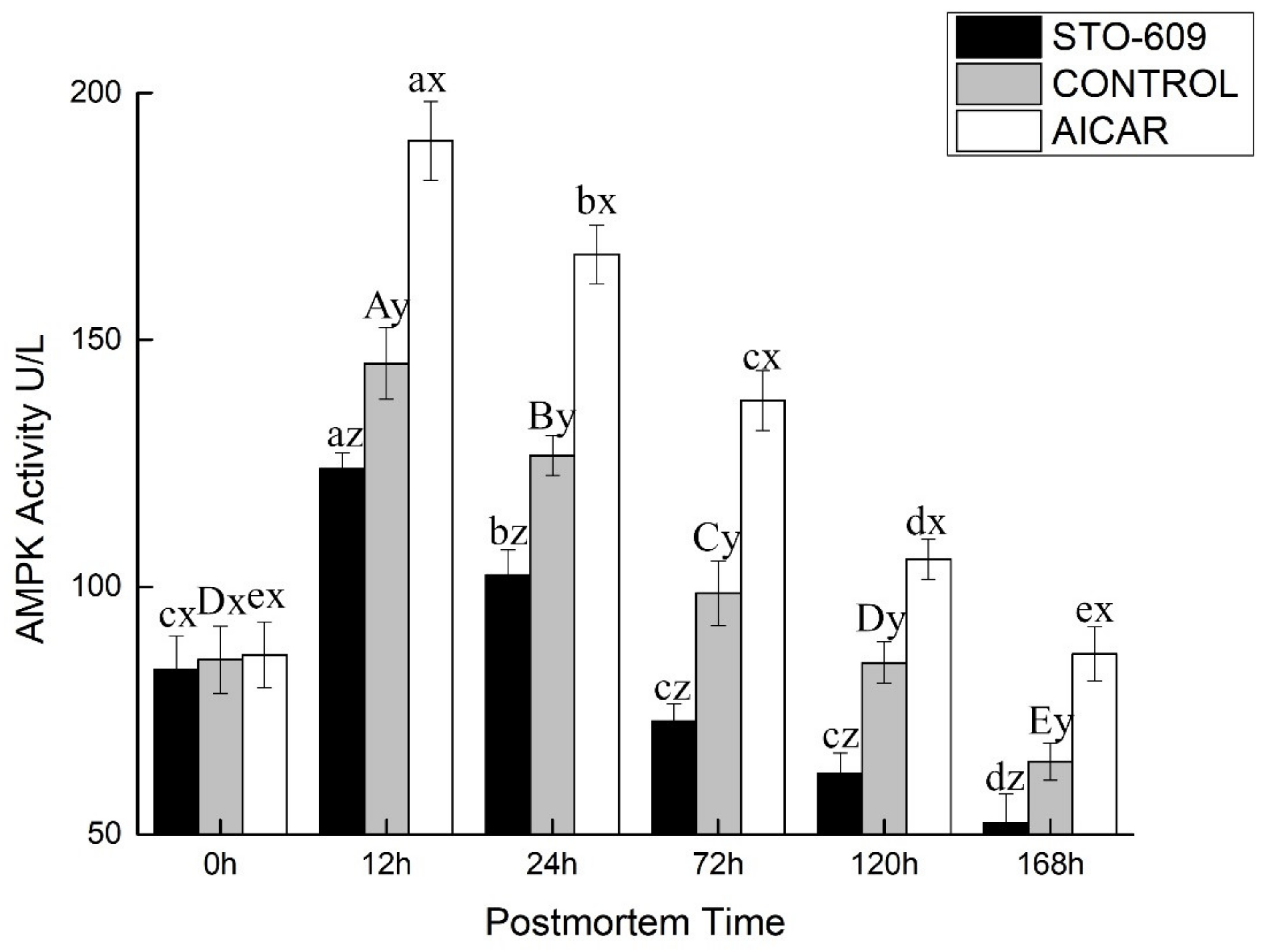
3.4. AMPK Activity
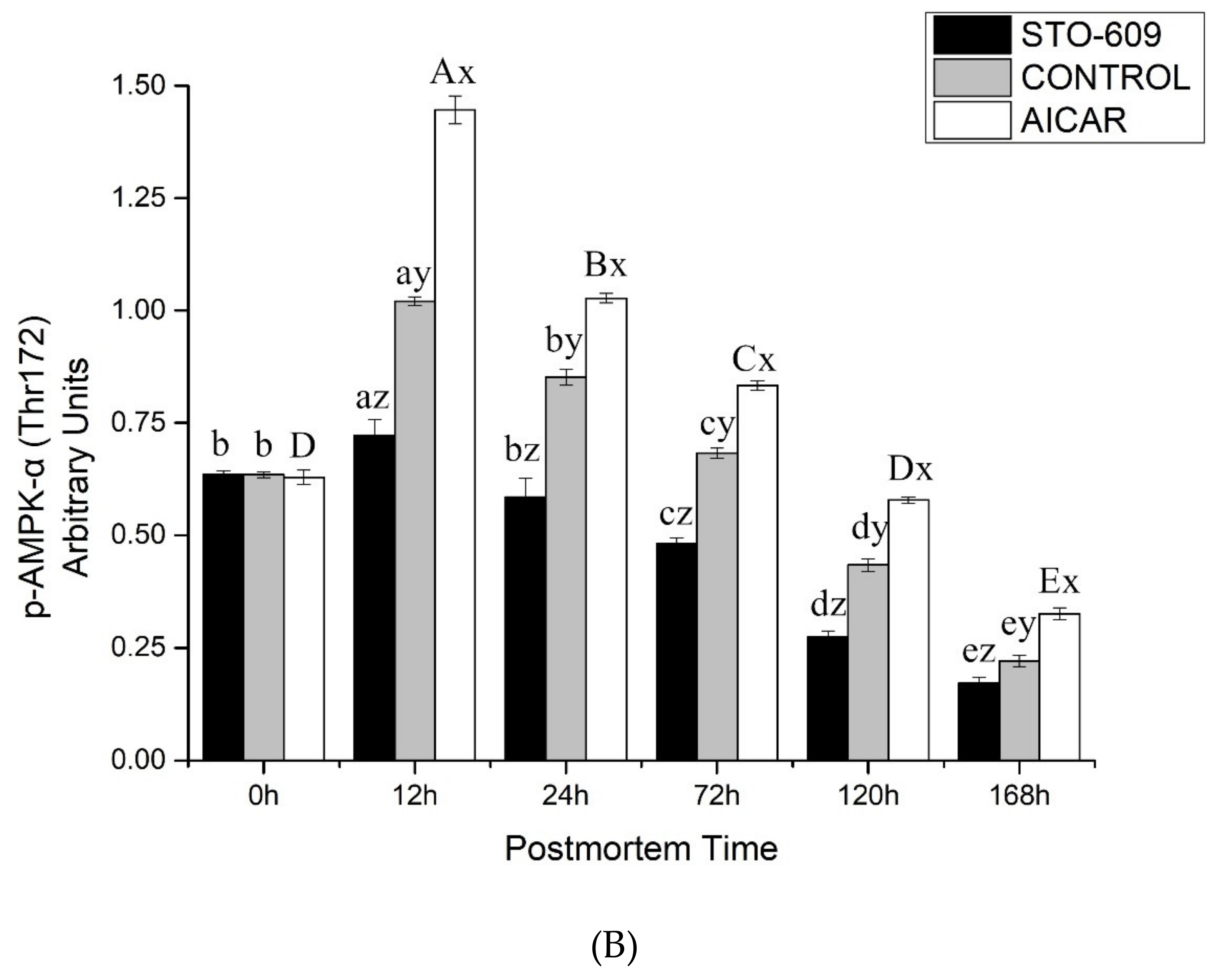
3.5. Immunoprecipitation of AMPK
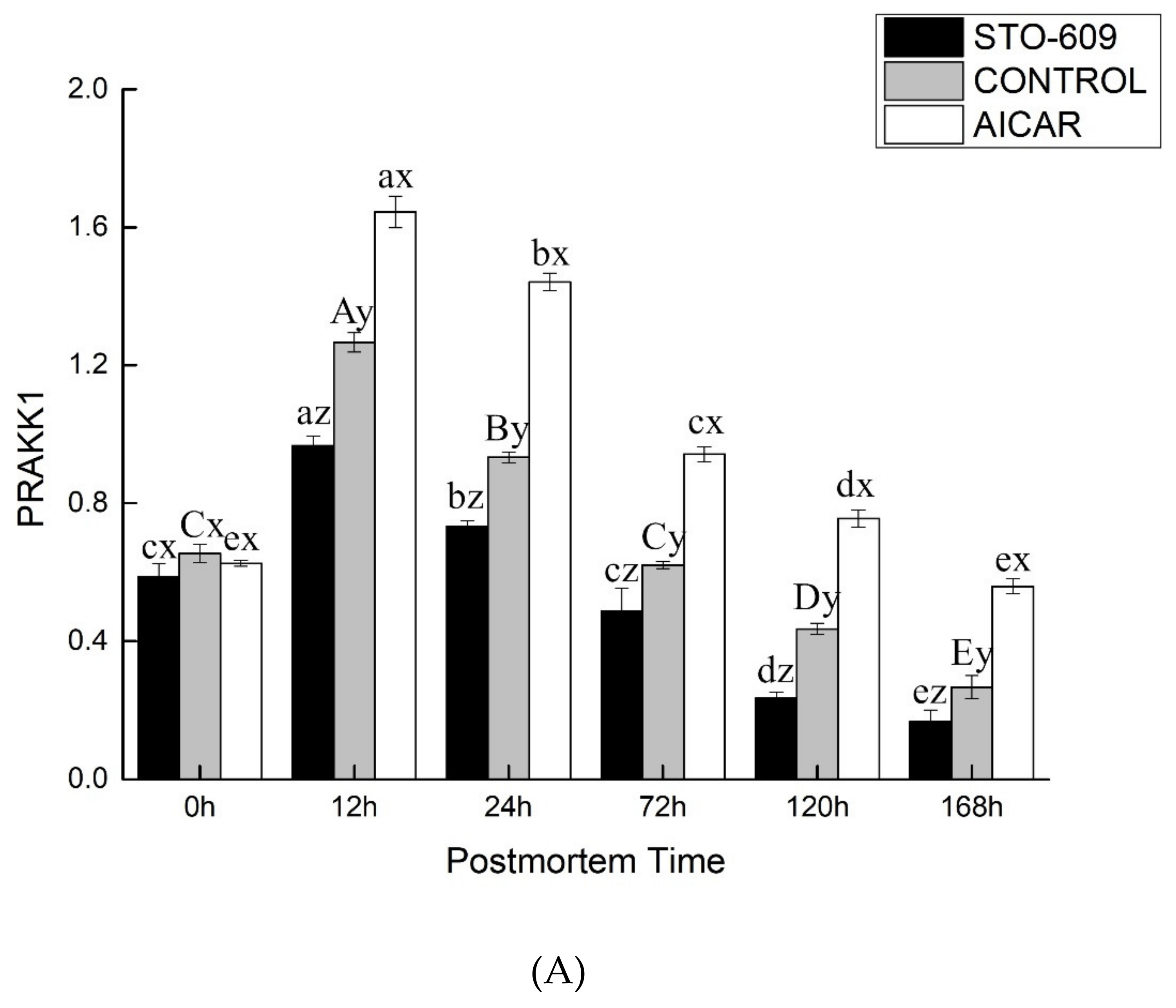
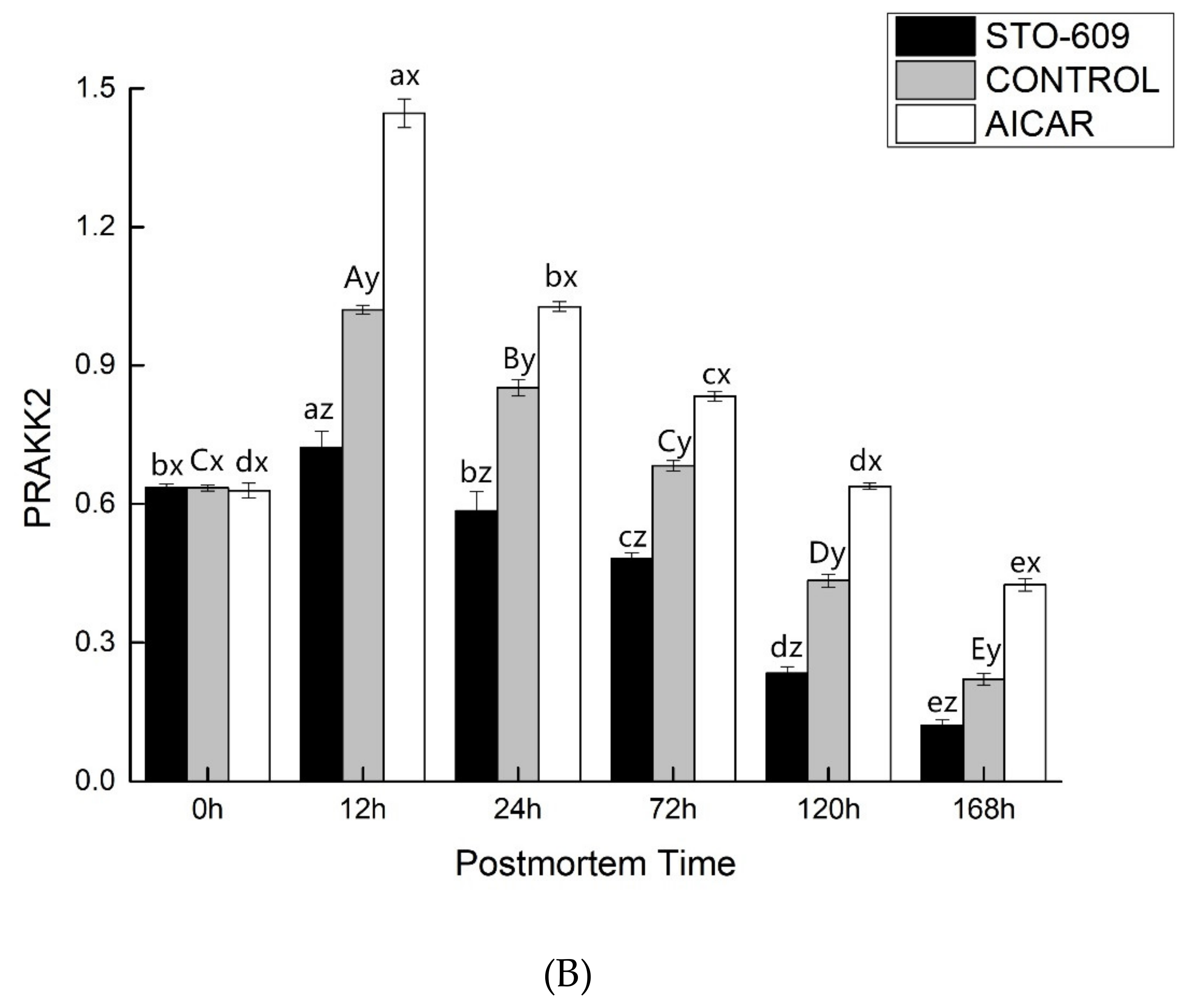
3.6. Gene Expression of AMPK
4. Dicussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zuo, H.X.; Han, L.; Yu, Q. Proteomics and bioinformatics analyses of differentially expressed proteins in yak and beef cattle muscle. Trans. Chin. Soc. Agric. Mach. 2017, 48, 313–320. [Google Scholar]
- Hardie, D.G.; Scott, J.W.; Pan, D.A.; Hudson, E.R. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003, 546, 113–120. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient andenergy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Z.; Liang, C.N.; Guo, X.; Wu, X.Y.; Wang, H.B.; Johnson, K.A.; Yan, P. Physiological insight into the high-altitude adaptations in domesticated yaks (Bos grunniens) along the Qinghai-Tibetan Plateau altitudinal gradient. Livest. Sci. 2014, 162, 233–239. [Google Scholar] [CrossRef]
- Chen, Z.P.; Stephens, T.J.; Murthy, S.; Canny, B.J.; Hargreaves, M.; Witters, L.A.; Kemp, B.E.; McConell, G.K. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 2003, 52, 2205–2212. [Google Scholar] [CrossRef]
- Wojtaszewski, J.F.; Nielsen, P.; Hansen, B.F.; Richter, E.A.; Kiens, B. Isoform specific and exercise intensity- dependent activation of 5′- AMP- activated protein kinase in human skeletal muscle. J. Physiol. 2000, 528, 221–226. [Google Scholar] [CrossRef]
- Musi, N.; Hayashi, T.; Fujii, N.; Hirshman, M.F.; Witters, L.A.; Goodyear, L.J. AMP- activated protein kinase activity and glucose uptake in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E677–E684. [Google Scholar] [CrossRef]
- Park, S.H.; Gammon, S.R.; Knippers, J.D.; Paulsen, S.R.; Rubink, D.S.; Winder, W.W. Phosphorylation activity relationships of AMPK and acetyl- CoA carboxylase in muscle. J. Appl. Physiol. 2002, 92, 2475–2482. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: The guardian of cardiac energy status. J. Clin. Investig. 2004, 114, 465–468. [Google Scholar] [CrossRef]
- Carling, D. The AMP-activated protein kinase cascade—A unifying system for energy control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef]
- Winder, W.W. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J. Appl. Physiol. 2001, 91, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D. The AMP-activated protein kinase– fuel gauge of the mammalian cell? Eur. J. Biochem. 1997, 246, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferré, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Salt, I.P.; Hawley, S.A.; Davies, S.P. AMP activated protein kinase: An ultrasensitive system for monitoring cellular energy charge. Biochem. J. 1999, 338, 717–722. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hardie, D.G. Role of the AMPactivated protein kinase in the cellular stress response. Curr. Biol. 1994, 4, 315–324. [Google Scholar] [CrossRef]
- Shen, Q.W.; Means, W.J.; Underwood, K.R.; Thompson, S.A.; Zhu, M.J.; McCormick, R.J.; Ford, S.P.; Ellis, M.; Du, M. Early post-mortem AMPactivated protein kinase (AMPK) activation leads to phosphofructokinase-2 and-1 (PFK-2 and PFK-1) phosphorylation and the development of pale, soft, and exudative (PSE) conditions in porcine longissimus muscle. J. Agric. Food Chem. 2006, 54, 5583–5589. [Google Scholar] [CrossRef]
- Fan, X.; Ding, Y.; Brown, S.; Zhou, L.; Shaw, M.; Vella, M.C.; Cheng, H.; McNay, E.C.; Sherwin, R.S.; McCrimmon, R.J. Hypothalamic AMP-activated protein kinase activation with AICAR amplifies counterregulatory responses to hypoglycemia in a rodent model of type 1 diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1702–R1708. [Google Scholar] [CrossRef]
- Vincent, M.F.; Erion, M.D.; Gruber, H.E.; Van den Berghe, G. Hypoglycaemic effect of AICA riboside in mice. Diabetologia 1996, 39, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Miller, I.; Aja, S.; Landree, L.E.; Pinn, M.; McFadden, J.; Kuhajda, F.P.; Moran, T.H.; Ronnett, G.V. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J. Biol. Chem. 2004, 279, 19970–19976. [Google Scholar] [CrossRef]
- Fernandez, X.; Neyraud, E.; Astruc, T.; Sante, V. Effects of halothane genotype and pre-slaughter treatment on pig meat quality. Part 1. Post mortem metabolism, meat quality indicators and sensory traits of m. Longissimus Lumborum. Meat Sci. 2002, 62, 429–437. [Google Scholar] [CrossRef]
- Shen, Q.W.; Means, W.J.; Thompson, S.A.; Underwood, K.R.; Zhu, M.J.; McCormick, R.J.; Ford, S.P.; Du, M. Pre-slaughter transport, AMP-activated protein kinase, glycolysis, and quality of pork loin. Meat Sci. 2006, 74, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yao, K.; Wang, L.; Ding, B.; Fu, D.; Wu, G. Effects of alpha-ketoglutarate on energy status in the intestinal mucosa of weaned piglets chronically challenged with lipopolysaccharide. Br. J. Nutr. 2011, 106, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.W.; Jones, C.S.; Kalchayanand, N.; Zhu, M.J.; Du, M. Effect of dietary alpha-lipoic acid on growth, body composition, muscle pH, and AMP-activated protein kinase phosphorylation in mice. J. Anim. Sci. 2005, 83, 2611–2617. [Google Scholar] [CrossRef]
- Veiseth, E.; Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Technical note: Comparison of myofibril fragmentation index from fresh and frozen pork and lamb longissimus. J. Anim. Sci. 2001, 79, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Raser, K.J.; Posner, A.; Wang, K.K.W. Casein zymography: A method to study μ-calpain, m-calpain, and their inhibitory agents. Arch. Biochem. Biophys. 1995, 319, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.W.; Du, M. Effects of dietary alpha-lipoic acid on glycolysis of postmortem muscle. Meat Sci. 2005, 71, 306–311. [Google Scholar] [CrossRef]
- Brüggemann, M.; Raff, T.; Flohr, T.; Gökbuget, N.; Nakao, M.; Droese, J.; Lüschen, S.; Pott, C.; Ritgen, M.; Scheuring, U.; et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 2006, 107, 1116–1123. [Google Scholar] [CrossRef]
- Kim, E.T.; Madhukar, A.; Ye, Z.; Campbell, J.C. High detectivity inas quantum dot infrared photodetectors. Appl. Phys. Lett. 2004, 84, 3277. [Google Scholar] [CrossRef]
- Proszkowiec-Weglarz, M.; Richards, M.P.; Ramachandran, R.; McMurtry, J.P. Characterization of the AMP-activated protein kinase pathway in chickens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 143, 92–106. [Google Scholar]
- Blattler, S.M.; Rencurel, F.; Kaufmann, M.R.; Meyer, U.A. In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 1045–1050. [Google Scholar] [CrossRef]
- Hamilton, S.R.; O’Donnell, J.B., Jr.; Hammet, A.; Stapleton, D.; Habinowski, S.A.; Means, A.R.; Kemp, B.E.; Witters, L.A. Amp-activated protein kinase kinase: Detection with recombinant AMPK alpha1 subunit. Biochem. Biophys. Res. Commun. 2002, 293, 892–898. [Google Scholar] [CrossRef]
- Copenhafer, T.L.; Richert, B.T.; Schinckel, A.P.; Grant, A.L.; Gerrard, D.E. Augmented postmortem glycolysis does not occur early postmortem in AMPK73-mutated porcine muscle of halothane positive pigs. Meat Sci. 2006, 73, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.W.; Du, M. Role of AMP-activated protein kinase in the glycolysis of postmortem muscle. J. Sci. Food Agric. 2005, 85, 2401–2406. [Google Scholar] [CrossRef]
- Shen, Q.W.; Underwood, K.R.; Means, W.J.; McCormick, R.J.; Du, M. The halothane gene, energy metabolism, adenosine monophosphate-activated protein kinase, and glycolysis in postmortem pig longissimus dorsi muscle. J. Anim. Sci. 2007, 85, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, M.J. Activating AMP-activated protein kinase without AMP. Mol. Cells 2005, 19, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef]
- Carling, D.; Zammit, V.A.; Hardie, D.G. A common bicyclic protein-kinase cascade inactivates the regulatory enzymes of fatty-acid and cholesterol-biosynthesis. FEBS Lett. 1987, 223, 217–222. [Google Scholar] [CrossRef]
- Mcfadden, J.W.; Corl, B.A. Activation of amp-activated protein kinase (ampk) inhibits fatty acid synthesis in bovine mammary epithelial cells. Biochem. Biophys. Res. Commun. 2009, 390, 388–393. [Google Scholar] [CrossRef]


| Gene Symbol | No. | Gene Bank | Primers 5′-3′ | GC% | Tm | Amplification Length/bp |
|---|---|---|---|---|---|---|
| PRKAA1 | BA040395 | NM0011098022 | F-CACACATGAATGCAAAGATAGCTGA | 40.0 | 63.5 | 109 |
| R-ATTACTTCTGGTGCAGCATAGTTGG | 44.0 | 62.8 | ||||
| PRKAA2 | BA073991 | NM0012056051 | F-GAAGATCGGCCACTACGTGCT | 57.1 | 63.8 | 93 |
| R-ACTTTATGGCCTGTCAATTGATGCT | 40.0 | 64.1 |
| Time | 0 h | 12 h | 24 h | 72 h | 120 h | 168 h |
|---|---|---|---|---|---|---|
| ATP contents (lmol/g muscle) | ||||||
| STO-609 | 3.00 ± 0.083 ax | 1.69 ± 0.032 bx | 1.58 ± 0.032 cx | 0.11 ± 0.033 ex | 0.32 ± 0.087 dx | 0.45 ± 0.034 dx |
| Control | 3.01 ± 0.093 Ax | 1.95 ± 0.062 By | 1.84 ± 0.053 Cy | 0.22 ± 0.056 Ey | 0.51 ± 0.067 Dy | 0.61 ± 0.055 dy |
| AICAR | 3.01 ± 0.041 ax | 2.26 ± 0.055 bz | 2.18 ± 0.051 cz | 0.57 ± 0.036 ez | 0.73 ± 0.025 dz | 0.81 ± 0.020 dz |
| ADP contents (lmol/g muscle) | ||||||
| STO-609 | 3.91 ± 0.031 ax | 1.72 ± 0.046 bx | 0.035 ± 0.043 ex | 0.041 ± 0.072 dx | 0.57 ± 0.042 cx | 0.58 ± 0.021 cx |
| Control | 3.99 ± 0.026 Ax | 1.91 ± 0.073 By | 0.54 ± 0.070 Dy | 0.77 ± 0.112 Cy | 0.72 ± 0.087 Cy | 0.80 ± 0.025 Cy |
| AICAR | 4.03 ± 0.042 az | 2.11 ± 0.045 bz | 0.89 ± 0.036 dz | 1.02 ± 0.078 cz | 1.05 ± 0.031 cz | 1.06 ± 0.055 cz |
| AMP contents (lmol/g muscle) | ||||||
| STO-609 | 0.24 ± 0.011 ax | 0.09 ± 0.032 cx | 0.12 ± 0.028 bx | 0.065 ± 0.002 dx | 0.058 ± 0.005 ex | 0.031 ± 0.015 fx |
| Control | 0.25 ± 0.015 Ax | 0.14 ± 0.011 Cy | 0.18 ± 0.009 By | 0.11 ± 0.006 Dy | 0.087 ± 0.005 Ey | 0.077 ± 0.007 Fy |
| AICAR | 0.25 ± 0.004 ax | 0.20 ± 0.010 cz | 0.25 ± 0.008 bz | 0.17 ± 0.003 dz | 0.104 ± 0.007 ez | 0.100 ± 0.003 fz |
| IMP contents (lmol/g muscle) | ||||||
| STO-609 | 1.32 ± 0.021 fx | 1.96 ± 0.098 ex | 2.43 ± 0.121 dx | 5.42 ± 0.024 ax | 3.72 ± 0.021 cx | 4.15 ± 0.045 bx |
| Control | 1.34 ± 0.018 Fy | 2.28 ± 0.110 Ey | 2.67 ± 0.180 Dy | 5.74 ± 0.010 Ay | 4.05 ± 0.015 Cy | 4.38 ± 0.036 By |
| AICAR | 1.34 ± 0.122 fz | 2.57 ± 0.105 ez | 2.84 ± 0.115 dz | 5.96 ± 0.012 az | 4.31 ± 0.002 cz | 4.56 ± 0.101 bz |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Han, L.; Yu, Q.; Gao, Y.; Song, R. Study of the AMP-activated Protein Kinase Role in Energy Metabolism Changes during the Postmortem Aging of Yak Longissimus dorsal. Animals 2020, 10, 427. https://doi.org/10.3390/ani10030427
Yang Y, Han L, Yu Q, Gao Y, Song R. Study of the AMP-activated Protein Kinase Role in Energy Metabolism Changes during the Postmortem Aging of Yak Longissimus dorsal. Animals. 2020; 10(3):427. https://doi.org/10.3390/ani10030427
Chicago/Turabian StyleYang, Yayuan, Ling Han, Qunli Yu, Yongfang Gao, and Rende Song. 2020. "Study of the AMP-activated Protein Kinase Role in Energy Metabolism Changes during the Postmortem Aging of Yak Longissimus dorsal" Animals 10, no. 3: 427. https://doi.org/10.3390/ani10030427
APA StyleYang, Y., Han, L., Yu, Q., Gao, Y., & Song, R. (2020). Study of the AMP-activated Protein Kinase Role in Energy Metabolism Changes during the Postmortem Aging of Yak Longissimus dorsal. Animals, 10(3), 427. https://doi.org/10.3390/ani10030427





