Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review
Simple Summary
Abstract
1. Introduction
2. Lipogenic Potential and Fatty Acids Composition in Local and Modern Pig Breeds
2.1. Subcutaneous Adipose Tissue
2.1.1. Fatty Acids Composition of Subcutaneous Adipose Tissue
2.1.2. Lipogenic Enzyme Activities of Subcutaneous Adipose Tissue
2.2. Intramuscular Fat
2.2.1. Fatty Acids Composition of Intramuscular Fat
2.2.2. Lipogenic and Lipolytic Enzyme Activities of Intramuscular Fat
2.3. Summary of Lipogenic Potential and Fatty Acids Composition Differences in Local and Modern Pig Breeds
3. Adipose Tissue Cellularity and Biochemical Processes
4. Transcriptomic Regulation in Fatty and Lean Pig Breeds
4.1. Transcriptional Regulation of Adipogenesis
4.2. Transcriptomic Profile in Fatty and Lean Breeds
4.2.1. Comparison of mRNA Transcriptome of Subcutaneous Adipose Tissue
4.2.2. Comparison of mRNA Transcriptome of Intramuscular Fat
4.3. Involvement of Non-Coding RNAs in Fat Deposition
4.4. Summary of Transcriptomic Regulation Differences in Fatty and Lean Breeds
5. Proteomic profile in local and modern pig breeds
5.1. Proteomic Profile Associated with Fat Metabolism in Subcutaneous Adipose Tissue
5.2. Proteomic Profile Associated with Fat Metabolism in Intramuscular Fat
5.3. Summary of Proteomic Profile Differences in Local and Modern Pig Breeds
6. Adaptation and Selection Induced Specificities of Fatty Pigs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Epstein, H.; Bichard, M. Pig. In Evolution of Domesticated Animals; Mason, I.L., Ed.; Longman: London, UK, 1984; pp. 145–162. [Google Scholar]
- Larson, G.; Albarella, U.; Dobney, K.; Rowley-Conwy, P.; Schibler, J.; Tresset, A.; Vigne, J.D.; Edwards, C.J.; Schlumbaum, A.; Dinu, A.; et al. Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. Proc. Natl. Acad. Sci. USA 2007, 104, 15276–15281. [Google Scholar] [CrossRef]
- Larson, G.; Dobney, K.; Albarella, U.; Fang, M.; Matisoo-Smith, E.; Robins, J.; Lowden, S.; Finlayson, H.; Brand, T.; Willerslev, E.; et al. Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science 2005, 307, 1618–1621. [Google Scholar] [CrossRef]
- Amills, M.; Clop, A.; Ramírez, O.; Pérez-Enciso, M. Origin and genetic diversity of pig breeds. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 1–10. [Google Scholar] [CrossRef]
- Rauw, W.M.; Kanis, E.; Noordhuizen-Stassen, E.N.; Grommers, F.J. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Linan, R.M.N. European Local Pig Breeds—Diversity and Performance. A Study of Project TREASURE; Intech Open: London, UK, 2019; pp. 1–303. [Google Scholar]
- Pugliese, C.; Sirtori, F. Quality of meat and meat products produced from southern European pig breeds. Meat Sci. 2012, 90, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Lebret, B. Production systems and influence on eating quality of pork. Meat Sci. 2010, 84, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Knap, P.W.; Rauw, W.M. Selection for high production in pigs. In Resource Allocation Theory Applied to Farm Animal Production; Rauw, W.M., Ed.; CAB International: Wallingford, UK, 2009; pp. 210–229. [Google Scholar]
- Čandek-Potokar, M.; Škrlep, M. Factors in pig production that impact the quality of dry-cured ham: A review. Animal 2012, 6, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Switonski, M.; Stachowiak, M.; Cieslak, J.; Bartz, M.; Grzes, M. Genetics of fat tissue accumulation in pigs: A comparative approach. J. Appl. Genet. 2010, 51, 153–168. [Google Scholar] [CrossRef]
- Brossard, L.; Nieto, R.; Charneca, R.; Araujo, J.P.; Pugliese, C.; Radović, Č.; Čandek-Potokar, M. Modeling nutritional requirements of growing pigs from local breeds using InraPorc. Animals 2019, 9, 169. [Google Scholar] [CrossRef]
- Vautier, B.; Quiniou, N.; van Milgen, J.; Brossard, L. Accounting for variability among individual pigs in deterministic growth model. Animal 2013, 7, 1265–1273. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Fontanesi, L.; Lebret, B.; Gil, J.M.; Ovilo, C.; Nieto, R.; Fernandez, A.; Pugliese, C.; Oliver, M.A.; Bozzi, R. Introductory chapter: Concept and ambition of project TREASURE. In European Local Pig Breeds—Diversity and Performance. A study of project Treasure; Čandek-Potokar, M., Nieto, R., Eds.; Intech Open: London, UK, 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Batorek-Lukač, N.; Tomažin, U.; Nieto, R. Performances de croissance des races locales de porcs selon la phase de production: Une étude analytique du projet TREASURE. In Journées Recherche Porcine; INRA, Institut Technique du Porc.: Paris, France, 2019; Volume 51, pp. 205–210. [Google Scholar]
- Henry, Y. Développement morphologique et métabolique du tissu adipeux chez le porc: Influence de la sélection, de l’alimentation et du mode d’élevage. Ann. Biol. Anim. Bioch. Biophys. 1977, 17, 923–952. [Google Scholar] [CrossRef]
- Monziols, M.; Bonneau, M.; Davenel, A.; Kouba, M. Comparison of the lipid content and fatty acid composition of intermuscular and subcutaneous adipose tissues in pig carcasses. Meat Sci. 2007, 76, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Barea, R.; Isabel, B.; Nieto, R.; López-Bote, C.; Aguilera, J.F. Evolution of the fatty acid profile of subcutaneous back-fat adipose tissue in growing Iberian and Landrace × Large White pigs. Animal 2013, 7, 688–698. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Serra, X.; Gil, F.; Pérez-Enciso, M.; Oliver, M.A.; Vázquez, J.M.; Gispert, M.; Díaz, I.; Moreno, F.; Latorre, R.; Noguera, J.L. A comparison of carcass, meat quality and histochemical characteristics of Iberian (Guadyerbas line) and Landrace pigs. Livest. Prod. Sci. 1998, 56, 215–223. [Google Scholar] [CrossRef]
- Lebret, B.; Dourmad, J.Y.; Mourot, J.; Pollet, P.Y.; Gondret, F. Production performance, carcass composition, and adipose tissue traits of heavy pigs: Influence of breed and production system. J. Anim. Sci. 2014, 92, 3543–3556. [Google Scholar] [CrossRef]
- Renaudeau, D.; Mourot, J. A comparison of carcass and meat quality characteristics of Creole and Large White pigs slaughtered at 90kg BW. Meat Sci. 2007, 76, 165–171. [Google Scholar] [CrossRef]
- Franci, O.; Bozzi, R.; Pugliese, C.; Acciaioli, A.; Campodoni, G.; Gandini, G. Performance of Cinta Senese pigs and their crosses with Large White. 1 Muscle and subcutaneous fat characteristics. Meat Sci. 2005, 69, 545–550. [Google Scholar] [CrossRef]
- Madeira, M.S.; Pires, V.M.; Alfaia, C.M.; Costa, A.S.; Luxton, R.; Doran, O.; Bessa, R.J.; Prates, J.A. Differential effects of reduced protein diets on fatty acid composition and gene expression in muscle and subcutaneous adipose tissue of Alentejana purebred and Large White × Landrace × Pietrain crossbred pigs. Br. J. Nutr. 2013, 110, 216–229. [Google Scholar] [CrossRef]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br. J. Nutr. 1997, 78 (Suppl. S1), S49–S60. [Google Scholar] [CrossRef] [PubMed]
- O’Hea, E.K.; Leveille, G.A. Significance of adipose tissue and liver as sites of fatty acid synthesis in the pig and the efficiency of utilization of various substrates for lipogenesis. J. Nutr. 1969, 99, 338–344. [Google Scholar] [CrossRef]
- Hillgartner, F.B.; Salati, L.M.; Goodridge, A.G. Physiological and molecular mechanisms involved in nutritional regulation of fatty acid synthesis. Physiol. Rev. 1995, 75, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Kloareg, M.; Noblet, J.; van Milgen, J. Deposition of dietary fatty acids, de novo synthesis and anatomical partitioning of fatty acids in finishing pigs. Br. J. Nutr. 2007, 97, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Vance, J.E. Biochemistry of Lipids, Lipoproteins and Membranes, 4th ed.; Elsevier Science: Amsterdam, The Netherland, 2002; pp. 1–607. [Google Scholar]
- Kouba, M.; Sellier, P. A review of the factors influencing the development of intermuscular adipose tissue in the growing pig. Meat Sci. 2011, 88, 213–220. [Google Scholar] [CrossRef]
- Freire, J.P.; Mourot, J.; Cunha, L.F.; Almeida, J.A.; Aumaitre, A. Effect of the source of dietary fat on postweaning lipogenesis in lean and fat pigs. Ann. Nutr. Metab. 1998, 42, 90–95. [Google Scholar] [CrossRef]
- Palma-Granados, P.; Seiquer, I.; Benítez, R.; Óvilo, C.; Nieto, R. Effects of lysine deficiency on carcass composition and activity and gene expression of lipogenic enzymes in muscles and backfat adipose tissue of fatty and lean piglets. Animal 2019, 10, 2406–2418. [Google Scholar] [CrossRef]
- Kouba, M.; Mourot, J.; Peiniau, P. Stearoyl-CoA desaturase activity in adipose tissues and liver of growing Large White and Meishan pigs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 118, 509–514. [Google Scholar] [CrossRef]
- Mauvoisin, D.; Mounier, C. Hormonal and nutritional regulation of SCD1 gene expression. Biochimie 2011, 93, 78–86. [Google Scholar] [CrossRef]
- Benítez, R.; Fernández, A.; Isabel, B.; Núñez, Y.; De Mercado, E.; Gómez-Izquierdo, E.; García-Casco, J.; López-Bote, C.; Óvilo, C. Modulatory effects of breed, feeding status, and diet on adipogenic, lipogenic, and lipolytic gene expression in growing Iberian and Duroc pigs. Int. J. Mol. Sci. 2017, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [PubMed]
- DeVol, D.K.; McKeith, F.K.; Bechtel, P.J.; Novakofski, J.; Shanks, R.D.; Carr, T.R. Variation in composition and palatability traits and relationships between muscle characteristics and palatability in a random sample of pork carcasses. J. Anim. Sci. 1988, 66, 385–395. [Google Scholar] [CrossRef]
- Fortin, A.; Robertson, W.M.; Tong, A.K. The eating quality of Canadian pork and its relationship with intramuscular fat. Meat Sci. 2005, 69, 297–305. [Google Scholar] [CrossRef]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of intramuscular fat content on the quality of pig meat—2. Consumer acceptability of m. longissimus lumborum. Meat Sci. 1999, 53, 67–72. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhu, L.; Teng, X.; Xiao, H.; Shuai, S.; Chen, L.; Li, Q.; Guo, Y. Differential expression analysis and regulatory network reconstruction for genes associated with muscle growth and adipose deposition in obese and lean pigs. Prog. Nat. Sci. 2008, 18, 387–399. [Google Scholar] [CrossRef]
- Ciobanu, D.C.; Lonergan, S.M.; Huff-Lonergan, J. Genetics of meat quality. In The Genetics of the Pig, 2nd ed.; Rothschild, M.F., Ruvinsky, A., Eds.; CAB International: Wallingford, UK, 2011; pp. 355–389. [Google Scholar]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef]
- Klont, R.E.; Brocks, L.M.; Eikelenboom, G. Muscle fibre type and meat quality. Meat Sci. 1998, 49, S219–S229. [Google Scholar] [CrossRef]
- Warris, P.D.; Brown, S.N.; Franklin, J.G.; Kestin, S.C. The thickness and quality of backfat in various pig breeds and their relationship to intramuscular fat and the setting of joints from carcasses. Meat Sci. 1990, 28, 21–29. [Google Scholar] [CrossRef]
- Jacyno, E.; Pietruszka, A.; Kawęcka, M.; Biel, W.; Kołodziej-Skalska, A. Phenotypic correlations of backfat thickness with meatiness traits, intramuscular fat, longissimus muscle cholesterol and fatty acid composition in pigs. S. Afr. J. Anim. Sci. 2015, 45, 122–128. [Google Scholar] [CrossRef]
- Wojtysiak, D.; Połtowicz, K. Carcass quality, physico-chemical parameters, muscle fibre traits and myosin heavy chain composition of m. longissimus lumborum from Puławska and Polish Large White pigs. Meat Sci. 2014, 97, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Kim, N.K.; Lee, C.S.; Hwang, I.H. Effect of fiber type on postmortem proteolysis in longissimus muscle of Landrace and Korean native black pigs. Meat Sci. 2007, 77, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Vazquez, J.A.; Lorenzo, J.M. Growth performance, carcass and meat quality of the Celta pig crossbred with Duroc and Landrance genotypes. Meat Sci. 2014, 96, 195–202. [Google Scholar] [CrossRef]
- Ayuso, M.; Fernández, A.; Núñez, Y.; Benítez, R.; Isabel, B.; Fernández, A.I.; Rey, A.I.; González-Bulnes, A.; Medrano, J.F.; Cánovas, Á.; et al. Developmental stage, muscle and genetic type modify muscle transcriptome in pigs: Effects on gene expression and regulatory factors involved in growth and metabolism. PLoS ONE 2016, 11, e0167858. [Google Scholar] [CrossRef] [PubMed]
- Palma-Granados, P.; Haro, A.; Seiquer, I.; Lara, L.; Aguilera, J.F.; Nieto, R. Similar effects of lysine deficiency in muscle biochemical characteristics of fatty and lean piglets. J. Anim. Sci. 2017, 95, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Tomović, V.M.; Šević, R.; Jokanović, M.; Šojić, B.; Škaljac, S.; Tasić, T.; Ikonić, P.; Lušnic Polak, M.; Polak, T.; Demšar, L. Quality traits of longissimus lumborum muscle from White Mangalica, Duroc × White Mangalica and Large White pigs reared under intensive conditions and slaughtered at 150 kg live weight: A comparative study. Arch. Anim. Breed 2016, 59, 401–415. [Google Scholar] [CrossRef]
- Zhao, S.M.; Ren, L.J.; Chen, L.; Zhang, X.; Cheng, M.L.; Li, W.Z.; Zhang, Y.Y.; Gao, S.Z. Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition. Lipids 2009, 44, 1029–1037. [Google Scholar] [CrossRef]
- Parunović, N.; Petrović, M.; Matekalo-Sverak, V.; Radović, Č.; Stanišić, N. Carcass properties, chemical content and fatty acid composition of the musculus longissimus of different pig genotypes. S. Afr. J. Anim. Sci. 2013, 43, 123–136. [Google Scholar] [CrossRef]
- Alfonso, L.; Mourot, J.; Insausti, K.; Mendizabal, J.A.; Arana, A. Comparative description of growth, fat deposition, carcass and meat quality characteristics of Basque and Large White pigs. Anim. Res. 2005, 54, 33–42. [Google Scholar] [CrossRef]
- Mourot, J.; Kouba, M. Lipogenic enzyme activities in muscles of growing Large White and Meishan pigs. Livest. Prod. Sci. 1998, 55, 127–133. [Google Scholar] [CrossRef]
- Zhao, J.; Li, K.; Yang, Q.; Du, M.; Liu, X.; Cao, G. Enhanced adipogenesis in Mashen pigs compared with Large White pigs. Ital. J. Anim. Sci. 2017, 16, 217–225. [Google Scholar] [CrossRef]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The subtle balance between lipolysis and lipogenesis: A critical point in metabolic homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, I.; Oe, M.; Ojima, K.; Muroya, S.; Shibata, M.; Chikuni, K. Cellularity of developing subcutaneous adipose tissue in Landrace and Meishan pigs: Adipocyte size differences between two breeds. Anim. Sci. J. 2011, 82, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Mersmann, H.J.; Smith, S.B. Chapter 11: Development of white adipose tissue metabolism. In Biology of Metabolism in Growing Animals; Burrin, D., Mersmann, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 275–303. [Google Scholar]
- Urrutia, O.; Alfonso, L.; Mendizabal, J.A. Cellularity description of adipose depots in domesticated animals. In Adipose Tissue; Intech Open: London, UK, 2018; pp. 73–90. [Google Scholar] [CrossRef]
- Salans, L.B.; Dougherty, J.W. The effect of insulin upon glucose metabolism by adipose cells of different size. Influence of cell lipid and protein content, age, and nutritional state. J. Clin. Investig. 1971, 50, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Cignarelli, A.; Genchi, V.A.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and Insulin Receptors in Adipose Tissue Development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef] [PubMed]
- Barb, C.R.; Hausman, G.J.; Houseknecht, K.L. Biology of leptin in the pig. Domest. Anim. Endocrinol. 2001, 21, 297–317. [Google Scholar] [CrossRef]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 2014, 1842, 414–423. [Google Scholar] [CrossRef]
- Myers, M.G.; Cowley, M.A.; Münzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef]
- Spurlock, M.E.; Gabler, N.K. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [CrossRef]
- Torres-Rovira, L.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Ovilo, C.; Pallares, P.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Diet-induced swine model with obesity/leptin resistance for the study of metabolic syndrome and type 2 diabetes. Sci. World J. 2012, 2012, 510149. [Google Scholar] [CrossRef]
- Fernández-Fígares, I.; Lachica, M.; Nieto, R.; Rivera-Ferre, M.G.; Aguilera, J.F. Serum profile of metabolites and hormones in obese (Iberian) and lean (Landrace) growing gilts fed balanced or lysine deficient diets. Livest. Sci. 2007, 110, 73–81. [Google Scholar] [CrossRef]
- Vincent, A.; Louveau, I.; Gondret, F.; Lebret, B.; Damon, M. Mitochondrial function, fatty acid metabolism, and immune system are relevant features of pig adipose tissue development. Physiol. Genom. 2012, 44, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Mourot, J.; Kouba, M.; Bonneau, M. Comparative study of in vitro lipogenesis in various adipose tissues in the growing Meishan pig: Comparison with the Large White pig (Sus domesticus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 115, 383–388. [Google Scholar] [CrossRef]
- Hausman, G.J.; Dodson, M.V.; Ajuwon, K.; Azain, M.; Barnes, K.M.; Guan, L.L.; Jiang, Z.; Poulos, S.P.; Sainz, R.D.; Smith, S.; et al. Board-invited review: The biology and regulation of preadipocytes and adipocytes in meat animals. J. Anim. Sci. 2009, 87, 1218–1246. [Google Scholar] [CrossRef] [PubMed]
- Louveau, I.; Perruchot, M.H.; Bonnet, M.; Gondret, F. Invited review: Pre- and postnatal adipose tissue development in farm animals: From stem cells to adipocyte physiology. Animal 2016, 10, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Hausman, G.J.; Sanz Fernandez, M.V. Insulin: Pancreatic secretion and adipocyte regulation. Domest. Anim. Endocrinol. 2016, 54, 76–84. [Google Scholar] [CrossRef] [PubMed]
- McNeel, R.L.; Ding, S.T.; Smith, E.O.; Mersmann, H.J. Expression of porcine adipocyte transcripts during differentiation in vitro and in vivo. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, 291–302. [Google Scholar] [CrossRef]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef]
- Ding, S.; Mersmann, H.J. Fatty acids modulate porcine adipocyte differentiation and transcripts for transcription factors and adipocyte-characteristic proteins. J. Nutr. Biochem. 2001, 12, 101–108. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Damon, M.; Wyszynska-Koko, J.; Vincent, A.; Hérault, F.; Lebret, B. Comparison of muscle transcriptome between pigs with divergent meat quality phenotypes identifies genes related to muscle metabolism and structure. PLoS ONE 2012, 7, e33763. [Google Scholar] [CrossRef] [PubMed]
- Ovilo, C.; Benítez, R.; Fernández, A.; Núñez, Y.; Ayuso, M.; Fernández, A.I.; Rodríguez, C.; Isabel, B.; Rey, A.I.; López-Bote, C.; et al. Longissimus dorsi transcriptome analysis of purebred and crossbred Iberian pigs differing in muscle characteristics. BMC Genom. 2014, 15, 413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Lu, J.X.; Chen, Y.; Zhao, Y.Q.; Guo, P.H.; Yang, J.T.; Zang, R.X. Comparison of the adipogenesis in intramuscular and subcutaneous adipocytes from Bamei and Landrace pigs. Biochem. Cell Biol. Biochim. Biol. Cell 2014, 92, 259–267. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Benítez, R.; Trakooljul, N.; Núñez, Y.; Isabel, B.; Murani, E.; De Mercado, E.; Gómez-Izquierdo, E.; García-Casco, J.; López-Bote, C.; Wimmers, K.; et al. Breed, diet, and interaction efects on adipose tissue transcriptome in Iberian and Duroc pigs fed different energy sources. Genes 2019, 10, 589. [Google Scholar] [CrossRef]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef]
- Xing, K.; Wang, K.; Ao, H.; Chen, S.; Tan, Z.; Wang, Y.; Xitong, Z.; Yang, T.; Zhang, F.; Iiu, Y.; et al. Comparative adipose tissue transcriptome analysis digs out genes related to fat deposition in two pig breeds. Sci. Rep. 2019, 9, 12925. [Google Scholar] [CrossRef]
- Tao, X.; Liang, Y.; Yang, X.; Pang, J.; Zhong, Z.; Chen, X.; Yang, Y.; Zeng, K.; Kang, R.; Lei, Y.; et al. Transcriptomic profiling in muscle and adipose tissue identifies genes related to growth and lipid deposition. PLoS ONE 2017, 12, e0184120. [Google Scholar] [CrossRef]
- Song, B.; Di, S.; Cui, S.; Chen, N.; Wang, H.; Wang, X.; Gao, Q.; Tong, G.; Wang, H.; Huang, X.; et al. Distinct patterns of PPARγ promoter usage, lipid degradation activity, and gene expression in subcutaneous adipose tissue of lean and obese swine. Int. J. Mol. Sci. 2018, 19, 3892. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Chamba, Y.; Zhang, B.; Shang, P.; Zhang, H.; Wu, C. Identification of genes related to growth and lipid deposition from transcriptome profiles of pig muscle tissue. PLoS ONE 2015, 10, e0141138. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, Z.; Yuan, Z.; Lo, J.L.; Chen, J.; Wang, Y.; Peng, J. Distinctive genes determine different intramuscular fat and muscle fiber ratios of the longissimus dorsi muscles in Jinhua and Landrace pigs. PLoS ONE 2013, 8, e53181. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Zhao, S.M.; Huang, Y.; Yang, M.H.; Pan, H.B.; Zhang, X.; Ge, C.R.; Gao, S.Z. Expression of lipogenic genes during porcine intramuscular preadipocyte differentiation. Res. Vet. Sci. 2012, 93, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Van Solingen, C.; Scacalossi, K.R.; Moore, K.J. Long noncoding RNAs in lipid metabolism. Curr. Opin. Lipidol. 2018, 29, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cooper, D.K.C.; Cai, Z.; Mou, L. Expression and regulation profile of mature microRNA in the pig: Relevance to xenotransplantation. Biomed. Res. Int. 2018, 2018, 21. [Google Scholar] [CrossRef]
- Chen, C.; Deng, B.; Qiao, M.; Zheng, R.; Chai, J.; Ding, Y.; Peng, J.; Jiang, S. Solexa sequencing identification of conserved and novel microRNAs in backfat of Large White and Chinese Meishan pigs. PLoS ONE 2012, 7, e31426. [Google Scholar] [CrossRef]
- Xie, S.; Chen, L.; Zhang, X.; Liu, X.; Chen, Y.; Mo, D. An integrated analysis revealed different microRNA-mRNA profiles during skeletal muscle development between Landrace and Lantang pigs. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Hou, X.; Yang, Y.; Zhu, S.; Hua, C.; Zhou, R.; Mu, Y.; Tang, Z.; Li, K. Comparison of skeletal muscle miRNA and mRNA profiles among three pig breeds. Mol. Genet. Genom. 2016, 291, 559–573. [Google Scholar] [CrossRef]
- Li, H.Y.; Xi, Q.Y.; Xiong, Y.Y.; Liu, X.L.; Cheng, X.; Shu, G.; Wang, S.B.; Wang, L.N.; Gao, P.; Zhu, X.T.; et al. Identification and comparison of microRNAs from skeletal muscle and adipose tissues from two porcine breeds. Anim. Genet. 2012, 43, 704–713. [Google Scholar] [CrossRef]
- Chen, Z. Progress and prospects of long non-coding RNAs in lipid homeostasis. Mol. Metab. 2016, 5, 164–170. [Google Scholar] [CrossRef]
- Muret, K.; Désert, C.; Lagoutte, L.; Boutin, M.; Gondret, F.; Zerjal, T.; Lagarrigue, S. Long noncoding RNAs in lipid metabolism: Literature review and conservation analysis across species. BMC Genom. 2019, 20, 882. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, X.; Li, A.; Xie, L.; Miao, X. Differential regulation of mRNAs and lncRNAs related to lipid metabolism in two pig breeds. Oncotarget 2017, 8, 87539–87553. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wang, S.; Zhang, J.; Wei, P.; Guo, L.; Liu, D.; Wang, Y.; Shi, M. Identification and comparison of long non-conding RNA in Jinhua and Landrace pigs. Biochem. Biophys. Res. Commun. 2018, 506, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Gondret, F.; Guével, B.; Com, E.; Vincent, A.; Lebret, B. A comparison of subcutaneous adipose tissue proteomes in juvenile piglets with a contrasted adiposity underscored similarity with human obesity. J. Proteom. 2012, 75, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Mo, D.; Zhao, X.; Jiang, W.; Cong, P.; He, Z.; Xiao, S.; Liu, X.; Chen, Y. Comparison of the longissimus muscle proteome between obese and lean pigs at 180 days. Mamm. Genome 2013, 24, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Murgiano, L.; D’Alessandro, A.; Egidi, M.G.; Crisà, A.; Prosperini, G.; Timperio, A.M.; Valentini, A.; Zolla, L. Proteomics and transcriptomics investigation on longissimus muscles in Large White and Casertana pig breeds. J. Proteome Res. 2010, 9, 6450–6466. [Google Scholar] [CrossRef]
- Wilkinson, S.; Lu, Z.H.; Megens, H.J.; Archibald, A.L.; Haley, C.; Jackson, I.J.; Groenen, M.A.; Crooijmans, R.P.; Ogden, R.; Wiener, P. Signatures of Diversifying Selection in European Pig Breeds. PLoS Genet. 2013, 9, e1003453. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Batorek Lukač, N.; Tomažin, U.; Škrlep, M.; Nieto, R. Analytical Review of Productive Performance of Local Pig Breeds. In European Local Pig Breeds—Diversity and Performance. A Study of Project TREASURE; Čandek-Potokar, M., Nieto, R., Eds.; Intech Open: London, UK, 2019; pp. 281–300. [Google Scholar] [CrossRef]
- Wiener, P.; Wilkinson, S. Deciphering the genetic basis of animal domestication. Proc. Biol. Sci. 2011, 278, 3161–3170. [Google Scholar] [CrossRef]
- Herrero-Medrano, J.M.; Megens, H.J.; Groenen, M.A.; Bosse, M.; Pérez-Enciso, M.; Crooijmans, R.P. Whole-genome sequence analysis reveals differences in population management and selection of European low-input pig breeds. BMC Genom. 2014, 15, 601. [Google Scholar] [CrossRef]
- Choi, J.W.; Chung, W.H.; Lee, K.T.; Cho, E.S.; Lee, S.W.; Choi, B.H.; Lee, S.H.; Lim, W.; Lim, D.; Lee, Y.G.; et al. Whole-genome resequencing analyses of five pig breeds, including Korean wild and native, and three European origin breeds. DNA Res. 2015, 22, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Li, K.; Fan, B.; Tang, Z. A genome-wide scan for signatures of selection in Chinese indigenous and commercial pig breeds. BMC Genet. 2014, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Bozzi, R.; García, F.; Núñez, Y.; Geraci, C.; Crovetti, A.; García-Casco, J.; Alves, E.; Škrlep, M.; Charneca, R.; et al. Diversity across major and candidate genes in European local pig breeds. PLoS ONE 2018, 13, e0207475. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Bozzi, R.; García-Casco, J.; Núñez, Y.; Ribani, A.; Franci, O.; García, F.; Škrlep, M.; Schiavo, G.; Bovo, S.; et al. Genomic diversity, linkage disequilibrium and selection signatures in European local pig breeds assessed with a high density SNP chip. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
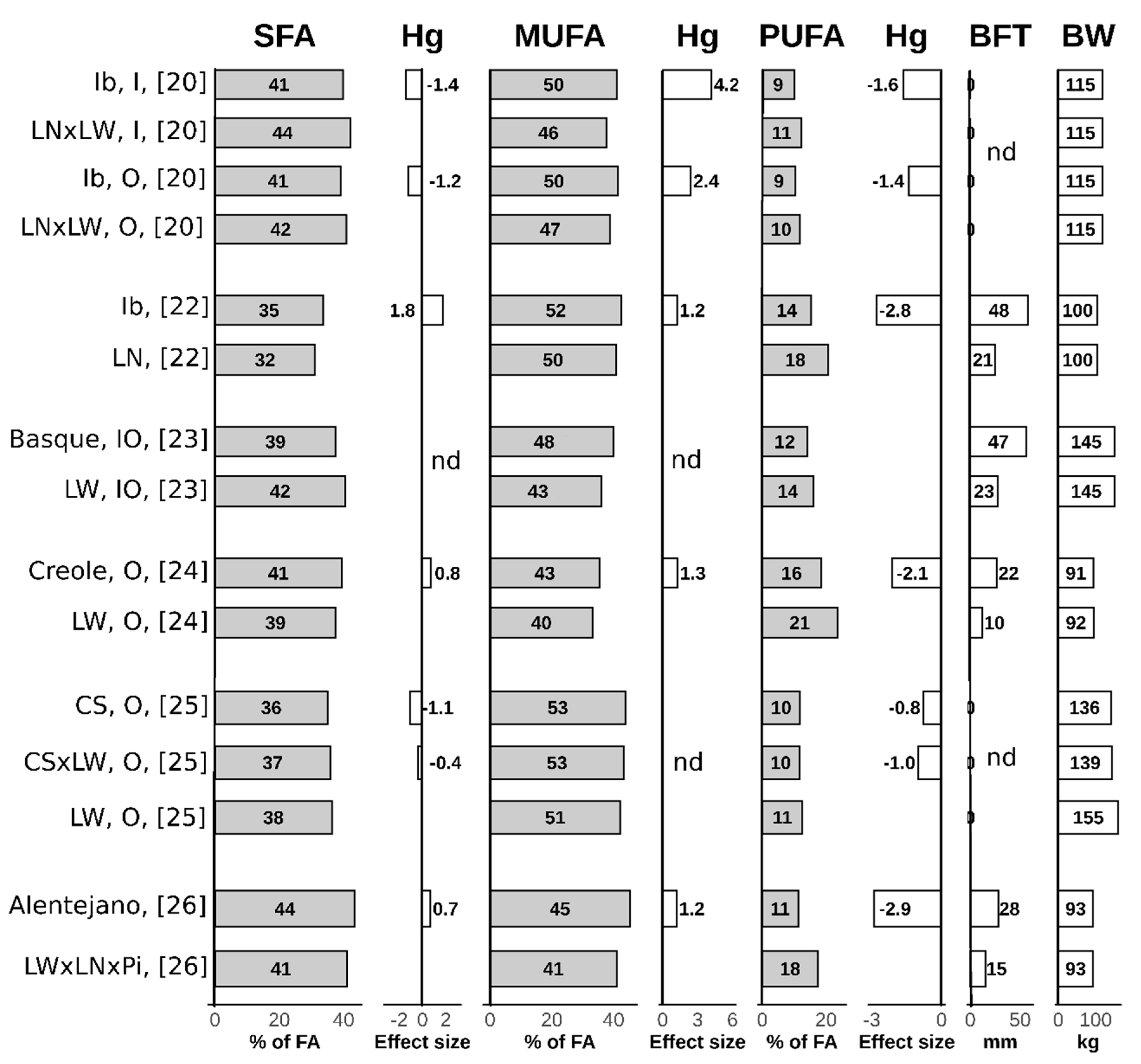

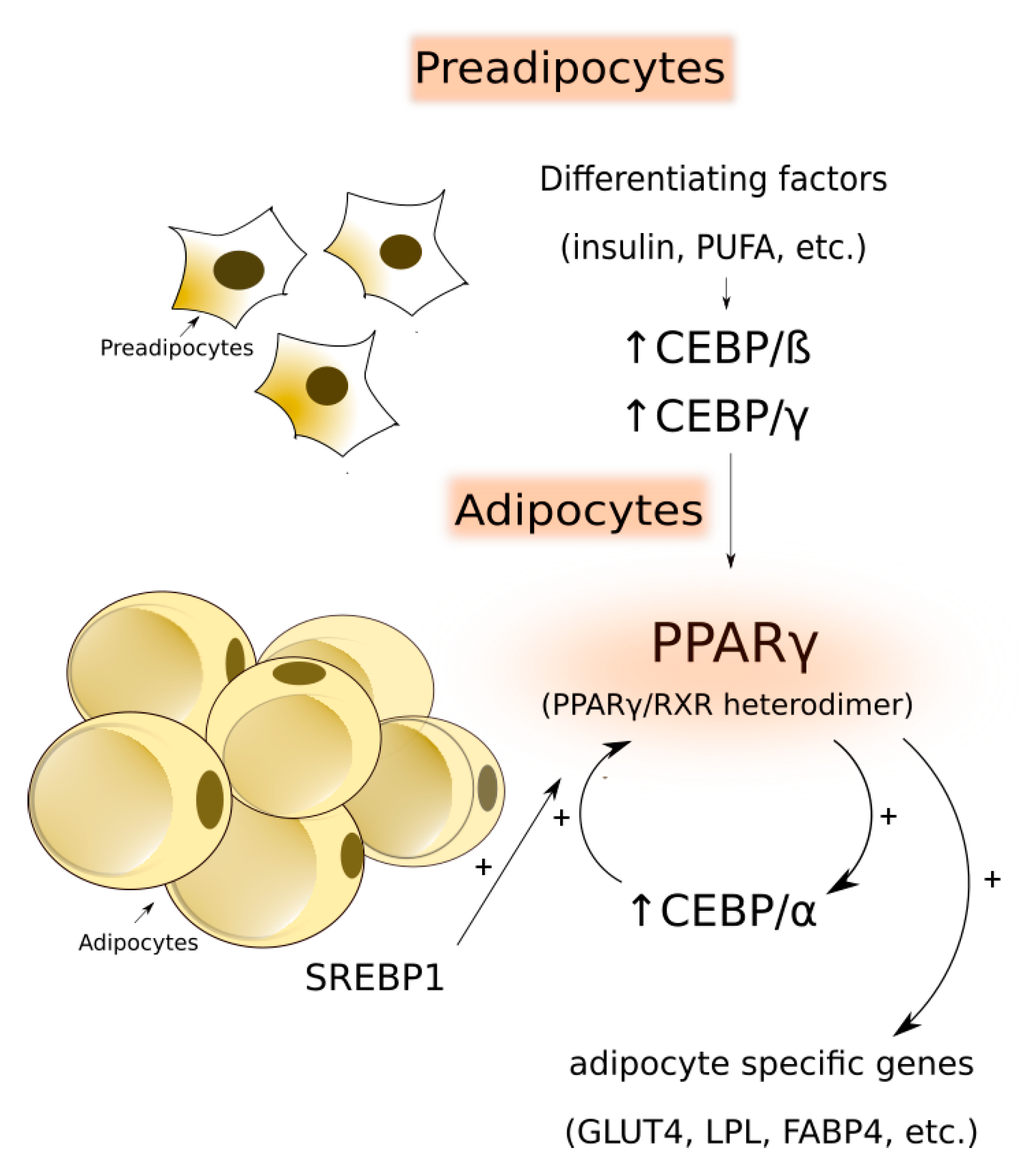
| Lipogenic Enzyme | Function |
|---|---|
| Acetyl-CoA carboxylase (ACACA) | Irreversible formation of malonyl-CoA from acetyl-CoA. |
| Fatty acid synthase (FAS) | Synthesis of palmitate from acetyl-CoA and malonyl-CoA. |
| Glucose-6-phosphate DH (G6PDH) | Providing NADPH for reductive biosynthesis of fatty acids. |
| Malic enzyme (ME) | Providing NADPH for reductive biosynthesis of fatty acids. |
| Stearoyl-CoA desaturase (SCD) | Transformation of MUFA from SFA. |
| Hormone-sensitive lipase (HSL) | Hydrolyses triglycerides to free fatty acids. |
| Lipoprotein lipase (LPL) | Catalyses the hydrolysis of triglycerides from circulating chylomicrons and very low-density lipoproteins. |
| Ref. | Breed | Enzyme activities | ||||
|---|---|---|---|---|---|---|
| ACACA | FAS | G6PDH | ME | SCD | ||
| [23] | Basque vs. LW | / | ↓ * | / | ↓ * | / |
| [34] | Alentejano vs. LW | ↑2.7–3.7 FC | / | ↑3.1–5.8 FC | ↑6.2–6.9 FC | / |
| [35] | Ib vs. LNxLW | / | ↑1.1–1.9 FC | ↑1.2 FC | ↑1.4–1.5 FC | / |
| [36] | Meishan vs. LW | / | / | / | / | ↓1.4–1.8 FC |
| Ref. | Breed | Enzyme Activities | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | ACA | FAS | G6PDH | ME | SCD | LPL | HSL | ||
| [35] | Ib vs. LNxLW | LD | / | ↑2.4 FC | ns | ns | / | / | / |
| [35] | Ib vs. LNxLW | BF | / | ns | ns | ↑1.2–2.1 FC | / | / | / |
| [55] | Wujin vs. LN | LD | / | ↑1.9 FC | / | / | ↑2.1 FC | / | ↓3.5 FC |
| [57] | Bas vs. LW | SM | ↑ 1.4 FC | / | ↑2.9 FC | ↑1.8 FC | / | / | / |
| [58] | Ms vs. LW | SM | ↑ * | / | ↑ * | ↑ * | / | / | / |
| [59] | Mas vs. LW | LD | ns | ↑ * | / | / | / | ns | ↓ * |
| Metabolic Pathway | Breed | Tissue | Platform | Ref. |
|---|---|---|---|---|
| Adipocyte growth and lipid deposition | ||||
| - ↑ lipogenesis, desaturation (ME1, ELOVL6, SCD) | Ib vs. Du | SCAT | qPCR | [38] |
| - ↑ lipogenesis, desaturation (FASN, SREBP-1, SCD) | Wujin vs. LN | LD-IMF | qPCR | [55] |
| - ↑ lipogenesis, desaturation (FASN, SCD) | DSP and Tibetan vs. LN and YY | LD-IMF | RNA-seq | [91] |
| - ↑ lipogenesis (ACACB) | Basque vs. LW | LD-IMF | microarray | [82] |
| - ↑ lipogenesis, desaturation (ELOVL6, ME1, PTGES3, AGPAT5, GNPAT, SCD) | Ib vs. Ib x Du | LD-IMF | microarray, qPCR | [83] |
| - ↑ lipogenesis (PCK1, FASN), desaturation (↑ SCD expression pigs at day 30, ↓ SCD expression pigs at day 150) | Jinhua vs. LN | LD-IMF | microarray | [92] |
| - ↑ lipogenesis (ME1, PCK1) | Ib vs. Du | SCAT | RNA-seq | [86] |
| - ↑ lipogenic and adipogenic gene expression after insulin and glucose exposure | Bamei vs. LW | SCAT, LD-IMF | qPCR | [84] |
| - ↑ adipogenesis (C/EBPγ, C/EBPα, PPARγ), lipogenesis (FASN) | Mashen vs. LN | LD-IMF | qPCR | [59] |
| - ↑ lipogenesis (e.g., PCK1, ACACB) | Songliao vs. LN | SCAT | RNA-seq | [88] |
| - ↑ adipogenic genes expression in preadipocyte cell culture in early stage of differentiation (PPARγ, CEBPα), ↑ lipogenic gene expression in late stage of differentiation (SREBP1, FASN) | Wujin vs. LN | LD-IMF | qPCR | [93] |
| Lipid mobilization and expenditure | ||||
| - ↓ lipolysis (HSL, ATGL) | Wujin vs. LN | LD-IMF | qPCR | [55] |
| - ↑ lipolysis (PON, PLA1A) | Ib vs. Ib x Du | LD-IMF | microarray | [83] |
| - ↑ lipolysis, fatty acid transport (LPL, LIPE, FABP3) | Jinhua vs. LN | LD-IMF | microarray | [92] |
| - ↑ lipolysis, fatty acid transport, oxidation (PPAP2A, LIPE, FABP3, SLC25A20, PPARδ) | Basque vs. LW | LD-IMF | microarray | [82] |
| - ↑ fatty acid transport, oxidation (FABP3, FABP4, CPT-1B) | Wujin vs. LN | LD-IMF | qPCR | [55] |
| - ↓ oxidoreductase activity, fatty acid degradation, mitochondrial function (e.g., ACAD, HADHA, ACAA2, HSD17B4) | Min vs. LN | SCAT | RNA-seq | [90] |
| - ↑ oxidoreductase activity | Chinese breeds * vs. YY | SCAT, LD-IMF | RNA-seq | [89] |
| - ↓ mitochondrial energy metabolism (e.g., SIRT3) | Basque vs. LW | SCAT | microarray | [72] |
| Regulation | ||||
| - ↑ response to steroid hormone stimulus | DSP and Tibetan vs. LN and YY | LD-IMF | RNA-seq | [91] |
| - ↑ LEP | Ib vs. Du | SCAT | qPCR | [38] |
| - ↑ LEP | Ib vs. Du | SCAT | RNA-seq | [86] |
| - ↑ insulin signaling pathway, insulin resistance | Songliao vs. LN | SCAT | RNA-seq | [88] |
| Other | ||||
| - ↑ immune response (e.g., CSF1R) | Basque vs. LW | SCAT | microarray | [72] |
| - ↑ immune response | Chinese breeds * vs. YY | SCAT, LD-IMF | RNA-seq | [89] |
| - ↑ immune response, ↓ extracellular matrix formation, ↓ growth, ↑ carbohydrate metabolism | Ib vs. Du | SCAT | RNA-seq | [86] |
| - ↑ glycolysis, ↑ gluconeogenesis | Songliao vs. LN | SCAT | RNA-seq | [88] |
| Metabolic pathway | Breed | Tissue | Ref. |
|---|---|---|---|
| Lipid deposition | |||
| ↑ lipogenesis (ME, G6PDH) | Basque vs. LW | SCAT | [105] |
| Lipid mobilization and expenditure | |||
| ↑ fatty acid transport capacity (albumin, fatty acid binding protein) | Korean vs. LN | LD-IMF | [50] |
| ↑ fatty acid transport capacity (albumin), ↑ lipolysis (CES) | Basque vs. LW | SCAT | [105] |
| ↑ fatty acid transport capacity (albumin) | Lantang vs. LN | LD-IMF | [106] |
| Other | |||
| ↑ acute phase response (ITIH) and low-grade inflammation (serpins), ↑ selenium binding protein | Basque vs. LW | SCAT | [105] |
| ↑ carbohydrate metabolism (pyruvate dehydrogenase), ↑ oxidative metabolism (COX5A, ATP5), ↓ glycolytic metabolism (β-enolase) | Lantang vs. LN | LD-IMF | [106] |
| ↑ glycolysis and glycolysis-related pathways (β-enolase, TPI, PGM1, LDH, CK and GPDH) | Casertana vs. LW | LD-IMF | [107] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poklukar, K.; Čandek-Potokar, M.; Batorek Lukač, N.; Tomažin, U.; Škrlep, M. Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review. Animals 2020, 10, 424. https://doi.org/10.3390/ani10030424
Poklukar K, Čandek-Potokar M, Batorek Lukač N, Tomažin U, Škrlep M. Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review. Animals. 2020; 10(3):424. https://doi.org/10.3390/ani10030424
Chicago/Turabian StylePoklukar, Klavdija, Marjeta Čandek-Potokar, Nina Batorek Lukač, Urška Tomažin, and Martin Škrlep. 2020. "Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review" Animals 10, no. 3: 424. https://doi.org/10.3390/ani10030424
APA StylePoklukar, K., Čandek-Potokar, M., Batorek Lukač, N., Tomažin, U., & Škrlep, M. (2020). Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review. Animals, 10(3), 424. https://doi.org/10.3390/ani10030424






