Finding an Effective Freezing Protocol for Turkey Semen: Benefits of Ficoll as Non-Permeant Cryoprotectant and 1:4 as Dilution Rate
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Chemicals
2.3. Animals
2.4. Extender Preparation
2.5. Semen Processing
2.6. Assessment of Sperm Quality
2.7. Statistical Analysis
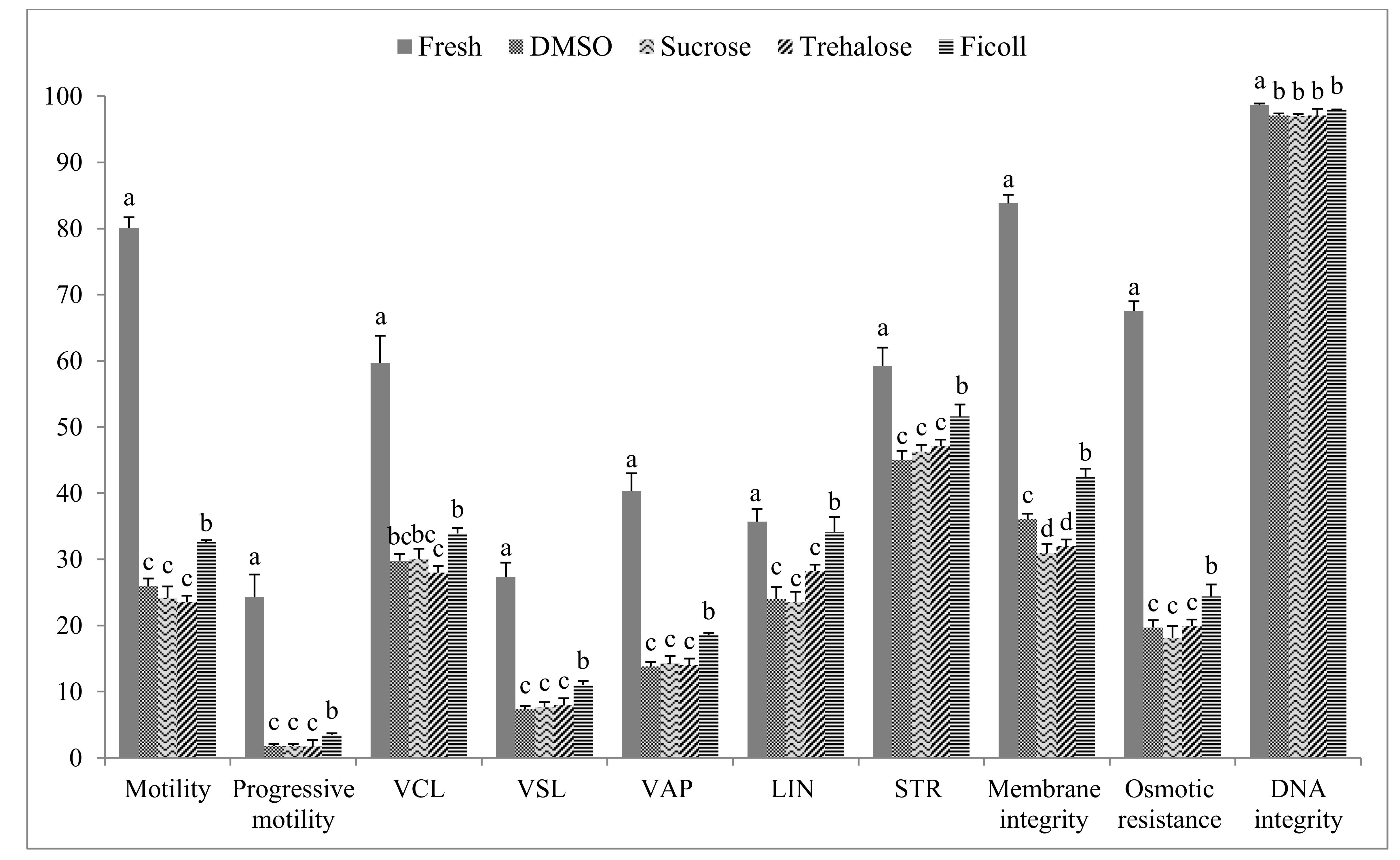
3. Results
4. Discussion
5. Conclusions
Author Contributions
Ethics Statement
Funding
Acknowledgments
Conflicts of Interest
References
- Mara, L.; Casu, S.; Carta, A. Cryobanking of farm animal gametes and embryos as a means of conserving livestock genetics. Anim. Reprod. Sci. 2013, 138, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Joost, S.; Bruford, M.W. Editorial: Advances in farm animal genomic resources. Front. Genet. 2015, 6, 333. [Google Scholar] [CrossRef] [PubMed]
- Thélie, A.; Bailliard, A.; Seigneurin, F.; Zerjal, T.; Tixier-Boichard, M.; Blesbois, E. Chicken semen cryopreservation and use for the restoration of rare genetic resources. Poult. Sci. 2018, 98, 447–455. [Google Scholar] [CrossRef]
- Blesbois, E.; Seigneurin, F.; Grasseau, I.; Limouzin, C.; Besnard, J.; Gourichon, D.; Coquerelle, G.; Rault, P.; Tixier-Boichard, M. Semen cryopreservation for ex situ management of genetic diversity in chicken: Creation of the French avian cryobank. Poult. Sci. 2007, 86, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E.; Grasseau, I.; Seigneurin, F.; Mignon-Grasteau, S.; Saint Jalme, M.; Mialon-Richard, M.M. Predictors of success of semen cryopreservation in chickens. Theriogenology 2008, 69, 252–261. [Google Scholar] [CrossRef]
- Váradi, E.; Vegi, B.; Liptoi, K.; Barna, J. Methods of cryopreservation of guinea fowl sperm. PLoS ONE 2013, 8, e62759. [Google Scholar] [CrossRef]
- Madeddu, M.; Mosca, F.; Abdel Sayed, A.; Zaniboni, L.; Mangiagalli, M.G.; Colombo, E.; Cerolini, S. Effect of cooling rate on the survival of cryopreserved rooster sperm: Comparison of different distances in the vapor above the surface of the liquid nitrogen. Anim. Reprod. Sci. 2016, 171, 58–64. [Google Scholar] [CrossRef]
- Thananurak, P.; Chuaychu-noo, N.; Thélie, A.; Phasuk, Y.; Vongpralub, T.; Blesbois, E. Sucrose increases the quality and fertilizing ability of cryopreserved chicken sperms in contrast to raffinose. Poult. Sci. 2019, 98, 4161–4171. [Google Scholar] [CrossRef]
- Long, J.A. Avian semen cryopreservation: What are the biological challenges? Poult. Sci. 2006, 85, 232–236. [Google Scholar] [CrossRef]
- Blesbois, E. Freezing avian semen. Avian Biol. Res. 2011, 4, 52–58. [Google Scholar] [CrossRef]
- Ehling, C.; Taylor, U.; Baulain, U.; Weigend, S.; Henning, M.; Rath, D. Cryopreservation of semen from genetic resource chicken lines. Agric. Forest. Res. 2012, 62, 151–158. [Google Scholar]
- Liptoi, K.; Horvath, G.; Gal, J.; Varadi, E.; Barna, J. Preliminary results of the application of gonadal tissue transfer in various chicken breeds in the poultry gene conservation. Anim. Reprod. Sci. 2013, 141, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y. Poultry genetic resource conservation using primordial germ cells. J. Reprod. Develop. 2016, 62, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sztan, N.; Lazar, B.; Bodzsar, N.; Vegi, B.; Liptoi, K.; Pain, B.; Varkonyi, E.P. Successful chimera production in the Hungarian goose (Anser anser domestica) by intracardiac injection of blastodermal cells in 3-day-old embryos. Reprod. Fert. Develop. 2017, 29, 2206–2216. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Castaño, C.; Toledano-Díaz, A.; Coloma, M.A.; López-Sebastián, A.; Prieto, M.T.; Campo, J.L. Cryoprotective and contraceptive properties of egg yolk as an additive in rooster sperm diluents. Cryobiology 2012, 65, 230–234. [Google Scholar] [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Blesbois, E. Cryoprotective effect of glycerol concentrations on Indian Red Jungle Fowl (Gallus gallus murgha) spermatozoa. Avian Biol. Res. 11, 80–88. [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Naseer, A.; Hussain, I.; Blesbois, E.; Santiago-Moreno, J. Use of dimethylsulfoxide for semen cryopreservation in Indian red jungle fowl (Gallus gallus Murghi). Theriogenology 2018, 122, 61–67. [Google Scholar] [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Zafar, Z.; Naseer, A.; Hussain, I.; Santiago-Moreno, J.; Blesbois, E. Cryoprotectant effects of egg yolk on Indian red jungle fowl (Gallus gallus murghi) sperm. Theriogenology 2018, 119, 150–155. [Google Scholar] [CrossRef]
- Chuaychu-noo, N.; Thananurak, P.; Chankitisakul, V.; Vongpralub, T. Supplementing rooster sperm with Cholesterol-Loaded-Cyclodextrin improves fertility after cryopreservation. Cryobiology 2017, 74, 8–12. [Google Scholar] [CrossRef]
- Blackburn, H.D. The National Animal Germplasm Program: Challenges and opportunities for poultry genetic resources. Poult. Sci. 2006, 85, 210–215. [Google Scholar] [CrossRef]
- Woelders, H.; Zuidberg, C.A.; Hiemstra, S.J. Animal genetic resources conservation in the Netherlands and Europe: Poultry perspective. Poult. Sci. 2006, 85, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E. Current status in avian semen cryopreservation. World Poult. Sci. J. 2007, 63, 213–222. [Google Scholar] [CrossRef]
- Blanco, J.M.; Wildt, D.E.; Hofle, U.; Voelker, W.; Donoghue, A.M. Implementing artificial insemination as an effective tool for ex situ conservation of endangered avian species. Theriogenology 2009, 71, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Łukaszewicz, E.; Rzońca, Z. Successful preservation of capercaillie (Tetrao urogallus L.) semen in liquid and frozen states. Theriogenology 2012, 77, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, N.; Romagnoli, L.; Manchisi, A.; Rosato, M.P. Cryopreservation of turkey semen by the pellet method: Effects of variables such as the extender, cryoprotectant concentration, cooling time and warming temperature on sperm quality determined through principal components analysis. Theriogenology 2011, 76, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, N.; Di Iorio, M.; Miranda, M.; Zaniboni, L.; Manchisi, A.; Cerolini, S. Cryopreserving turkey semen in straws and nitrogen vapour using DMSO or DMA: Effects of cryoprotectant concentration, freezing rate and thawing rate on post-thaw semen quality. Brit. Poult. Sci. 2016, 57, 264–270. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Cerolini, S.; Manchisi, A. Overview of turkey semen storage: Focus on cryopreservation—A review. Ann. Anim. Sci. 16, 961–974. [CrossRef]
- Long, J.A.; Purdy, P.H.; Zuidberg, K.; Sipke-Joost, H.; Velleman, S.G.; Woelders, H. Cryopreservation of turkey semen: Effect of breeding line and freezing method on post-thaw sperm quality, fertilization, and hatching. Cryobiology 2014, 68, 371–378. [Google Scholar] [CrossRef]
- Blanco, J.M.; Gee, G.; Wildt, D.E.; Donoghue, L. Species variation in osmotic cryoprotectant, and cooling rate tolerance in poultry, eagle and peregrine falcon spermatozoa. Biol. Reprod. 2000, 63, 1164–1171. [Google Scholar] [CrossRef]
- Massip, A.; Leibo, S.P.; Blesbois, E. Cryobiology of gametes and the breeding of domestic animals. In Life in the Frozen State; Benson, E., Fuller, B., Lane, N., Eds.; Taylor & Francis Group: London, UK, 2004; pp. 371–393. [Google Scholar]
- Blanco, J.M.; Long, J.A.; Gee, G.; Donoghue, A.M.; Wildt, D.E. Osmotic tolerance of avian spermatozoa: Influence of time, temperature, cryoprotectant and membrane ion pump function on sperm viability. Cryobiology 2008, 56, 8–14. [Google Scholar] [CrossRef]
- Blanco, J.M.; Long, J.A.; Gee, G.; Wildt, D.E.; Donoghue, A.M. Comparative cryopreservation of avian spermatozoa: Benefits of non-permeating osmoprotectants and ATP on turkey and crane sperm cryosurvival. Anim. Reprod. Sci. 2011, 123, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.M.; Long, J.A.; Gee, G.; Wildt, D.E.; Donoghue, A.M. Comparative cryopreservation of avian spermatozoa: Effects of freezing and thawing rates on turkey and sandhill crane sperm cryosurvival. Anim. Reprod. Sci. 2012, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Madeddu, M.; Sayed, A.; Zaniboni, L.; Iaffaldano, N.; Cerolini, S. Combined effect of permeant and non-permeant cryoprotectants on the quality of frozen/thawed chicken sperm. Cryobiology 2016, 73, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Kulíková, B.; Vašíček, J.; Olexiková, L.; Iaffaldano, N.; Chrenek, P. Effect of cryoprotectants and thawing temperatures on chicken sperm quality. Reprod. Domest. Anim. 2018, 53, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Rakha, B.A.; Ansari, M.S.; Hussain, I.; Akhter, S.; Santiago-Moreno, S.; Blesbois, E. Cryopreservation of Indian red jungle fowl (Gallus gallus murghi) semen with polyvinylpyrrolidone. Cryobiology 2017, 78, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Toscani, T.; Robbe, D.; Parrillo, S.; De Amicis, I.; Contri, A. Cryopreservation of turkey spermatozoa without permeant cryoprotectants. Anim. Reprod. Sci. 2019, 2011, 106218. [Google Scholar] [CrossRef]
- Tselutin, K.; Narubina, L.; Mavrodina, T.; Tur, B. Cryopreservation of poultry semen. Brit. Poult. Sci. 1995, 36, 805–811. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Manchisi, A.; Rosato, M.P. The preservability of turkey semen quality during liquid storage in relation to strain and age of males. Anim. Reprod. Sci. 2008, 109, 266–273. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Mannina, M.; Paventi, G.; Rosato, M.P.; Cerolini, S.; Sobolev, A.P. Age-dependent changes in metabolic profile of turkey spermatozoa as assessed by NMR analysis. PLoS ONE 2018, 13, e019421. [Google Scholar] [CrossRef]
- Gandini, L.; Lombardo, F.; Lenzi, A.; Spano, M.; Dondero, F. Cryopreservation and sperm DNA integrity. Cell Tissue Bank 2006, 7, 91–98. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Rosato, M.P.; Manchisi, A. Cryopreservation of rabbit semen using non-permeable cryoprotectants: Effectiveness of different concentrations of low-density lipoproteins (LDL) from egg yolk versus egg yolk or sucrose. Anim. Reprod. Sci. 2014, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Colonna, M.A.; Miranda, M.; Principe, P.; Schiavitto, M.; Manchisi, A.; Iaffaldano, N. Initial cooling time before freezing affects post-thaw quality and reproductive performance of rabbit semen. Anim. Sci. J. 2018, 89, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Łukaszewicz, E. Simple and Effective Methods of Freezing Capercaillie (Tetrao urogallus L.) Semen. PLoS ONE 2015, 10, e0116797. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, N.; Meluzzi, A. Effect of dialysis on quality characteristics of turkey semen during liquid storage. Theriogenology 2003, 60, 421–427. [Google Scholar] [CrossRef]
- Douard, V.; Hermier, D.; Magistrini, M.; Blesbois, E. Role of seminal plasma in damage to turkey spermatozoa during in vitro storage. Theriogenology 2005, 63, 126–137. [Google Scholar] [CrossRef]
- Swain, J.E.; Smith, G.D. Cryoprotectants. In Fertility Cryopreservation; Chian, R.C., Quinn, P., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 24–38. [Google Scholar]
- El-Sheshtawy, R.I.; Sisy, G.A.; El-Nattat, W.S. Effects of different concentrations of sucrose or trehalose on the post-thawing quality of cattle bull semen. Asian Pac. J. Reprod. 2015, 4, 26–31. [Google Scholar] [CrossRef]
- Aisen, E.; Medina, V.; Venturino, A. Cryopreservation and post thawed fertility of ram semen frozen in different trehalose concentration. Theriogenology 2002, 57, 1801–1808. [Google Scholar] [CrossRef]
- Woelders, H.; Matthijs, A.; Engel, B. Effects of trehalose and sucrose, osmolarity of the frozen medium, and cooling rate on viability and intactness of bull sperm after freezing and thawing. Cryobiology 1997, 35, 93–105. [Google Scholar] [CrossRef]
- Aboagla, E.M.; Terada, T. Trehalose-enhanced fluidity of the goat sperm membrane and its protection during freezing. Biol. Reprod. 2003, 69, 1245–1250. [Google Scholar] [CrossRef]
- Yamashiro, H.; Narita, K.; Sugimura, S.; Han, Y.J.; Sugawara, A.; Morohaku, K.; Nakazato, F.; Konno, T.; Yoshida, M.; Sato, E. Trehalose enhanced the freezability of Poodle dog sperm collected by an artificial vagina (AV). Anim. Reprod. Sci. 2007, 102, 165–171. [Google Scholar] [CrossRef]
- Gutiérrez-Pérez, O.; Juàrez-Mosqueda, M.L.; Carvajal, S.U.; Ortega, M.E. Boar spermatozoa cryopreservation in low glycerol/trehalose enriched freezing media improves cellular integrity. Cryobiology 2009, 58, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Singhc, R.P.; Pukazhenthia, B.; Keeferb, L.C.; Songsasen, L. Cryopreservation effects on sperm function and fertility in two threatened crane species. Cryobiology 2018, 82, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kulíková, B.; Di Iorio, M.; Kubovicova, E.; Kuželová, L.; Iaffaldano, N.; Chrenek, P. The cryoprotective effect of Ficoll on the rabbit spermatozoa quality. Zygote 2015, 23, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Han, X.; Zhang, Y. Cryoprotective mechanism of using Ficoll for cell cryopreservation at non-cryogenic temperatures: A molecular dynamics study. Int. J. Heat Mass Tran. 2018, 127, 319–325. [Google Scholar] [CrossRef]
- Dumoulin, J.C.; Bergers-Janssen, J.M.; Pieters, M.H.; Enginsu, M.E.; Geraedts, J.P.; Evers, J.L. The protective effects of polymers in the cryopreservation of human and mouse zonae pellucidae and embryos. Fertil. Steril. 1994, 62, 793–798. [Google Scholar] [CrossRef]
- Shaw, J.M.; Kuleshowa, L.L.; MacFarlane, D.R.; Trounson, A.O. Vitrification properties of solutions of ethylene glycol in saline containing PVP, Ficoll, or dextran. Cryobiology 1997, 35, 219–229. [Google Scholar] [CrossRef]
- Kuleshova, L.L.; Shaw, J.M.; Trounson, A.O. Studies on replacing most of the penetrating cryoprotectant by polymers for embryo cryopreservation. Cryobiology 2001, 43, 21–31. [Google Scholar] [CrossRef]
- Kasai, M.; Hamaguchi, Y.; Zhu, S.E.; Miyake, T.; Sakurai, T.; Machida, T. High survival of rabbit morulae after vitrification in an ethylene glycol-based solution by a simple method. Biol. Reprod. 1992, 46, 1042–1046. [Google Scholar] [CrossRef]
- Papis, K.; Sypecka, J.; Korwin-Kossakowski, M.; Wenta-Muchalska, E.; Bilska, B. Banking of embryos of mutated, paralytic tremor rabbit by means of vitrification. Lab. Anim. 2005, 39, 284–289. [Google Scholar] [CrossRef]
- Makarevich, A.V.; Chrenek, P.; Olexikova, L.; Popelkova, M.; Turanova, Z.; Ostro, A.; Pivko, J. Post-thaw survival, cell death and actin cytoskeleton in gene-microinjected rabbit embryos after vitrification. Theriogenology 2008, 70, 675–681. [Google Scholar] [CrossRef]
- Lagares, M.A.; Castanheira, P.N.; Amaral, D.C.G.; Vasconcelos, A.B.; Veado, J.C.C.; Arantes, R.M.E.; Stahlberg, R. Addition of ficoll and disaccharides to vitrification solutions improve in vitro viability of vitrified equine embryos. CryoLetters 2009, 30, 408–413. [Google Scholar] [CrossRef] [PubMed]

| Components | mM |
|---|---|
| Glucose | 44.4 |
| Sodium glutamate | 128.0 |
| Di-Potassium hydrogen phosphate | 20.0 |
| Magnesium acetate | 7.0 |
| Glycine | 13.3 |
| Glutamic acid | 7.68 |
| Inositol | 11.1 |
| MOT.% | MOT. PROG. % | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | Membrane Integrity (%) | Osmotic Resistance (%) | DNA Integrity (%) | Sperm Concentration × 109 |
|---|---|---|---|---|---|---|---|---|---|---|
| 80.1 ± 1.6 | 24.3 ± 3.4 | 59.7 ± 4.1 | 27.3 ± 2.2 | 40.3 ± 2.7 | 35.7 ± 1.9 | 59.2 ± 2.8 | 83.8 ± 1.3 | 67.5 ± 1.5 | 98.7 ± 0.2 | 8.9 ± 0.4 |
| Semen Treatment | Sperm Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dilution rate | MOT. % | MOT. PROG. % | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | Membrane integrity (%) | Osmotic resistance (%) | DNA integrity (%) | |
| DMSO | 1:2 | 25.4 ± 1.0 | 1.8 ± 0.2 | 31.4 ± 0.8 | 7.1 ± 0.5 | 14.1 ± 0.5 | 21.1 ± 1.1 | 41.8 ± 0.8 | 34.8 ± 0.8 | 19.1 ± 1.4 | 96.8 ± 0.3 |
| DMSO | 1:4 | 26.0 ± 1.1 | 1.9 ± 0.3 | 29.8 ± 1.0 | 7.3 ± 0.5 | 13.8 ± 0.7 | 24.0 ± 1.8 | 45.0 ± 1.4 | 36.1 ± 0.8 | 19.7 ± 1.1 | 97.1 ± 0.3 |
| Semen Treatment | Sperm Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NP-CPA Concentration | Dilution rate | MOT. % | MOT. PROG. % | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | Membrane Integrity (%) | Osmotic Resistance (%) | DNA Integrity (%) |
| 50 mM | 1:2 | 24.2 ± 1.7a | 1.8 ± 0.3a | 30.1 ± 1.5a | 7.7 ± 0.7a | 14.3 ± 1.1a | 23.5 ± 1.6abc | 46.3 ± 1.0b-e | 31.0 ± 1.3ab | 18.1 ± 1.8abc | 97.0 ± 0.3ab |
| 100 mM | 1:2 | 19.6 ± 1.9b | 1.3 ± 0.4ab | 28.3 ± 2.8a | 5.8 ± 0.7ab | 12.0 ± 1.0ab | 18.6 ± 1.5c | 41.9 ± 1.5e | 31.2 ± 1.4ab | 17.5 ± 1.7abc | 96.5 ± 0.2bc |
| 200 mM | 1:2 | 22.1 ± 1.7ab | 1.2 ± 0.3ab | 28.3 ± 2.1a | 6.8 ± 0.9a | 12.4 ± 1.1ab | 24.0 ± 2.1abc | 46.1± 1.3c-e | 29.2 ± 2.0b | 17.0 ± 1.3abc | 96.0 ± 0.3cd |
| 400 mM | 1:2 | 13.0 ± 0.4c | 0.7 ± 0.2c | 21.2 ± 1.3b | 4.5 ± 0.4b | 8.2 ± 0.4c | 20.6 ± 2.0bc | 47.4 ± 1.4a | 22.3 ± 1.4d | 14.7 ± 0.9c | 95.5 ± 0.2d |
| 50 mM | 1:4 | 20.8 ± 1.0ab | 1.3 ± 0.2ab | 27.6 ± 1.3a | 6.6 ± 0.4ab | 13.2 ± 0.8a | 24.7 ± 2.0abc | 44.2 ± 1.0c-e | 28.5 ± 2.1bc | 19.2 ± 1.3ab | 96.9 ± 0.3ab |
| 100 mM | 1:4 | 22.9 ± 1.1ab | 1.6 ± 0.3a | 28.5 ± 1.6a | 7.3 ± 0.8a | 13.6 ± 1.1a | 27.0 ± 2.9ab | 46.7 ± 2.2ab | 34.7 ± 1.2a | 20.7 ± 1.8a | 97.3 ± 0.2a |
| 200 mM | 1:4 | 20.5 ± 1.2ab | 1.4 ± 0.1ab | 27.4 ± 1.5a | 7.6 ± 0.5a | 13.3 ± 0.8a | 29.9 ± 1.5a | 48.5 ± 1.1a | 31.3 ± 0.8ab | 19.8 ± 0.8ab | 96.5 ± 0.2bc |
| 400 mM | 1:4 | 15.8 ± 1.0c | 0.6 ± 0.2c | 21.5 ± 1.0b | 6.2 ± 1.0ab | 10.2 ± 1.1bc | 24.3 ± 4.2abc | 47.6 ± 2.5a | 24.8 ± 1.3cd | 16.2 ± 0.5bc | 96.5 ± 0.1bc |
| Concentration effect | p = 0.000 | p = 0.002 | p = 0.000 | p = 0.035 | p = 0.000 | p = 0.258 | p = 0.146 | p = 0.000 | p = 0.034 | p = 0.000 | |
| Dilution rate effect | p = 0.755 | p = 0.941 | p = 0.498 | p = 0.215 | p = 0.156 | p = 0.258 | p = 0.009 | p = 0.187 | p = 0.027 | p = 0.080 | |
| Concentration × dilution | p = 0.129 | p = 0.463 | p = 0.787 | p = 0.363 | p = 0.212 | p = 0.182 | p = 0.524 | p = 0.201 | p = 0.848 | p = 0.053 | |
| Semen Treatment | Sperm Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NP-CPA Concentration | Dilution rate | MOT. % | MOT. PROG. % | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | Membrane Integrity (%) | Osmotic Resistance (%) | DNA Integrity (%) |
| 50 mM | 1:2 | 21.1 ± 2.2ab | 1.6 ± 0.5ab | 29.4 ± 2.5a | 6.8 ± 0.9abc | 13.2 ± 1.6ab | 20.9 ± 2.0ab | 44.3 ± 1.1ab | 29.2 ± 1.7ab | 16.8 ± 1.4a | 96.8 ± 0.2ab |
| 100 mM | 1:2 | 18.7 ± 1.7bc | 1.5 ± 0.3ab | 27.9 ± 2.4a | 5.4 ± 0.9ab | 10.9 ± 1.5abc | 18.1 ± 1.6b | 42.2 ± 0.9ab | 30.6 ± 1.9a | 18.5 ± 1.4a | 96.9 ± 0.1ab |
| 200 mM | 1:2 | 16.7 ± 1.6cd | 0.9 ± 0.1bc | 23.7 ± 1.2a | 4.9 ± 0.6a | 9.8 ± 0.9bc | 17.7 ± 2.0b | 40.9 ± 1.7b | 27.5 ± 1.8ab | 16.9 ± 1.9a | 95.9 ± 0.3c |
| 400 mM | 1:2 | 13.3 ± 0.9d | 0.5 ± 0.1c | 20.0 ± 1.5b | 4.3 ± 0.7b | 8.1 ± 1.1c | 18.1 ± 2.4b | 45.6 ± 1.7ab | 21.9 ± 1.8c | 17.1 ± 1.3a | 95.1 ± 0.5d |
| 50 mM | 1:4 | 21.6 ± 1.2ab | 1.5 ± 0.2ab | 29.2 ± 1.8a | 7.6 ± 0.7ab | 13.7 ± 1.1a | 26.0 ± 2.9a | 46.3 ± 2.1ab | 31.9 ± 1.1a | 19.1 ± 1.3a | 96.9 ± 0.2ab |
| 100 mM | 1:4 | 23.5 ± 1.1a | 1.7 ± 0.3a | 28.0 ± 1.9a | 8.0 ± 1.0a | 14.0 ± 1.4a | 28.2 ± 1.5a | 47.1 ± 0.8a | 32.0 ± 1.4a | 19.9 ± 0.6a | 97.1 ± 0.2a |
| 200 mM | 1:4 | 18.6 ± 0.9bc | 1.0 ± 0.2bc | 25.4 ± 1.6a | 6.7 ± 0.7abc | 11.8 ± 0.8abc | 27.0 ± 3.0a | 47.1 ± 2.4a | 30.9 ± 1.5a | 19.1 ± 0.9a | 96.6 ± 0.2abc |
| 400 mM | 1:4 | 14.2 ± 0.9d | 0.5 ± 0.1c | 24.9 ± 1.2b | 5.6 ± 0.6abc | 11.0 ± 1.1abc | 22.9 ± 2.7ab | 45.9 ± 1.9ab | 25.2 ± 1.3bc | 17.6 ± 1.4a | 96.1 ± 0.2bc |
| Concentration effect | p = 0.000 | p = 0.000 | p = 0.002 | p = 0.039 | p = 0.013 | p = 0.417 | p = 0.613 | p = 0.000 | p = 0.573 | p = 0.000 | |
| Dilution rate effect | p = 0.037 | p = 0.999 | p = 0.212 | p = 0.006 | p = 0.017 | p = 0.000 | p = 0.008 | p = 0.017 | p = 0.090 | p = 0.009 | |
| Concentration × dilution | p = 0.366 | p = 0.796 | p = 0.473 | p = 0.720 | p = 0.711 | p = 0.824 | p = 0.375 | p = 0.899 | p = 0.894 | p = 0.336 | |
| Semen Treatment | Sperm Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NP-CPA Concentration | Dilution rate | MOT. % | MOT. PROG. % | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | Membrane Integrity (%) | Osmotic Resistance (%) | DNA Integrity (%) |
| 0.5 mM | 1:2 | 26.9 ± 1.1bcd | 2.8 ± 0.3abc | 33.3 ± 1.3ab | 8.5 ± 0.5b | 15.8 ± 0.7abc | 24.0 ± 1.1b | 45.3 ± 0.9c | 34.1 ± 1.5c | 19.1 ± 2.0a | 97.4 ± 0.3bc |
| 0.75 mM | 1:2 | 30.7 ± 2.2ab | 3.0 ± 0.4ab | 34.0 ± 1.3a | 8.5 ± 0.4b | 16.3 ± 0.8abc | 24.2 ± 0.9b | 44.7 ± 0.7c | 37.1 ± 1.8bc | 22.4 ± 2.4a | 97.8 ± 0.2ab |
| 1 mM | 1:2 | 29.0 ± 1.6bc | 2.7 ± 0.3abc | 33.0 ± 1.3ab | 8.9 ± 0.6b | 16.2 ± 0.9abc | 24.3 ± 1.6b | 45.4 ± 0.9c | 36.0 ± 1.7bc | 21.4 ± 1.9a | 97.5 ± 0.2abc |
| 1.5 mM | 1:2 | 23.5 ± 1.7de | 1.9 ± 0.3cd | 30.0 ± 1.9ab | 8.0 ± 0.9b | 14.4 ± 1.3bc | 24.2 ± 1.9b | 46.5 ± 1.3bc | 36.0 ± 1.0bc | 19.8 ± 1.5a | 96.7 ± 0.1d |
| 0.5 mM | 1:4 | 25.1 ± 0.8cde | 1.8 ± 0.3d | 29.3 ± 1.4b | 7.7 ± 0.2b | 13.7 ± 0.4c | 28.5 ± 1.3b | 49.5 ± 0.9ab | 37.1 ± 1.2bc | 23.1 ± 1.7a | 97.1 ± 0.1cd |
| 0.75 mM | 1:4 | 30.8 ± 0.6ab | 2.4 ± 0.2bcd | 32.8 ± 0.6ab | 8.8 ± 0.1b | 17.5 ± 1.6ab | 26.9 ± 0.8b | 48.0 ± 0.9abc | 40.5 ± 0.9ab | 23.9 ± 1.9a | 97.8 ± 0.2ab |
| 1 mM | 1:4 | 32.6 ± 0.3a | 3.5 ± 0.2a | 33.9 ± 0.8a | 11.0 ± 0.6a | 18.5 ± 0.4a | 34.1 ± 2.3a | 51.6 ± 1.8a | 42.5 ± 1.2a | 24.4 ± 1.8a | 97.9 ± 0.1a |
| 1.5 mM | 1:4 | 22.1 ± 1.4e | 1.6 ± 0.1d | 31.2 ± 1.2ab | 7.8 ± 0.9b | 15.5 ± 1.8abc | 27.0 ± 2.6b | 48.3 ± 1.8abc | 35.7 ± 1.9c | 22.3 ± 1.5a | 96.6 ± 0.1d |
| Concentration effect | p = 0.000 | p = 0.000 | p = 0.059 | p = 0.005 | p = 0.052 | p = 0.123 | p = 0.431 | p = 0.024 | p = 0.534 | p = 0.000 | |
| Dilution rate effect | p = 0.510 | p = 0.198 | p = 0.378 | p = 0.435 | p = 0.453 | p = 0.000 | p = 0.000 | p = 0.004 | p = 0.073 | p = 0.735 | |
| Concentration × dilution | p = 0.034 | p = 0.013 | p = 0.162 | p = 0.276 | p = 0.099 | p = 0.152 | p = 0.391 | p = 0.158 | p = 0.931 | p = 0.112 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Iorio, M.; Rusco, G.; Iampietro, R.; Colonna, M.A.; Zaniboni, L.; Cerolini, S.; Iaffaldano, N. Finding an Effective Freezing Protocol for Turkey Semen: Benefits of Ficoll as Non-Permeant Cryoprotectant and 1:4 as Dilution Rate. Animals 2020, 10, 421. https://doi.org/10.3390/ani10030421
Di Iorio M, Rusco G, Iampietro R, Colonna MA, Zaniboni L, Cerolini S, Iaffaldano N. Finding an Effective Freezing Protocol for Turkey Semen: Benefits of Ficoll as Non-Permeant Cryoprotectant and 1:4 as Dilution Rate. Animals. 2020; 10(3):421. https://doi.org/10.3390/ani10030421
Chicago/Turabian StyleDi Iorio, Michele, Giusy Rusco, Roberta Iampietro, Maria Antonietta Colonna, Luisa Zaniboni, Silvia Cerolini, and Nicolaia Iaffaldano. 2020. "Finding an Effective Freezing Protocol for Turkey Semen: Benefits of Ficoll as Non-Permeant Cryoprotectant and 1:4 as Dilution Rate" Animals 10, no. 3: 421. https://doi.org/10.3390/ani10030421
APA StyleDi Iorio, M., Rusco, G., Iampietro, R., Colonna, M. A., Zaniboni, L., Cerolini, S., & Iaffaldano, N. (2020). Finding an Effective Freezing Protocol for Turkey Semen: Benefits of Ficoll as Non-Permeant Cryoprotectant and 1:4 as Dilution Rate. Animals, 10(3), 421. https://doi.org/10.3390/ani10030421







