Sheep Methane Emission on Semiarid Native Pasture—Potential Impacts of Either Zinc Sulfate or Propylene Glycol as Mitigation Strategies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Use
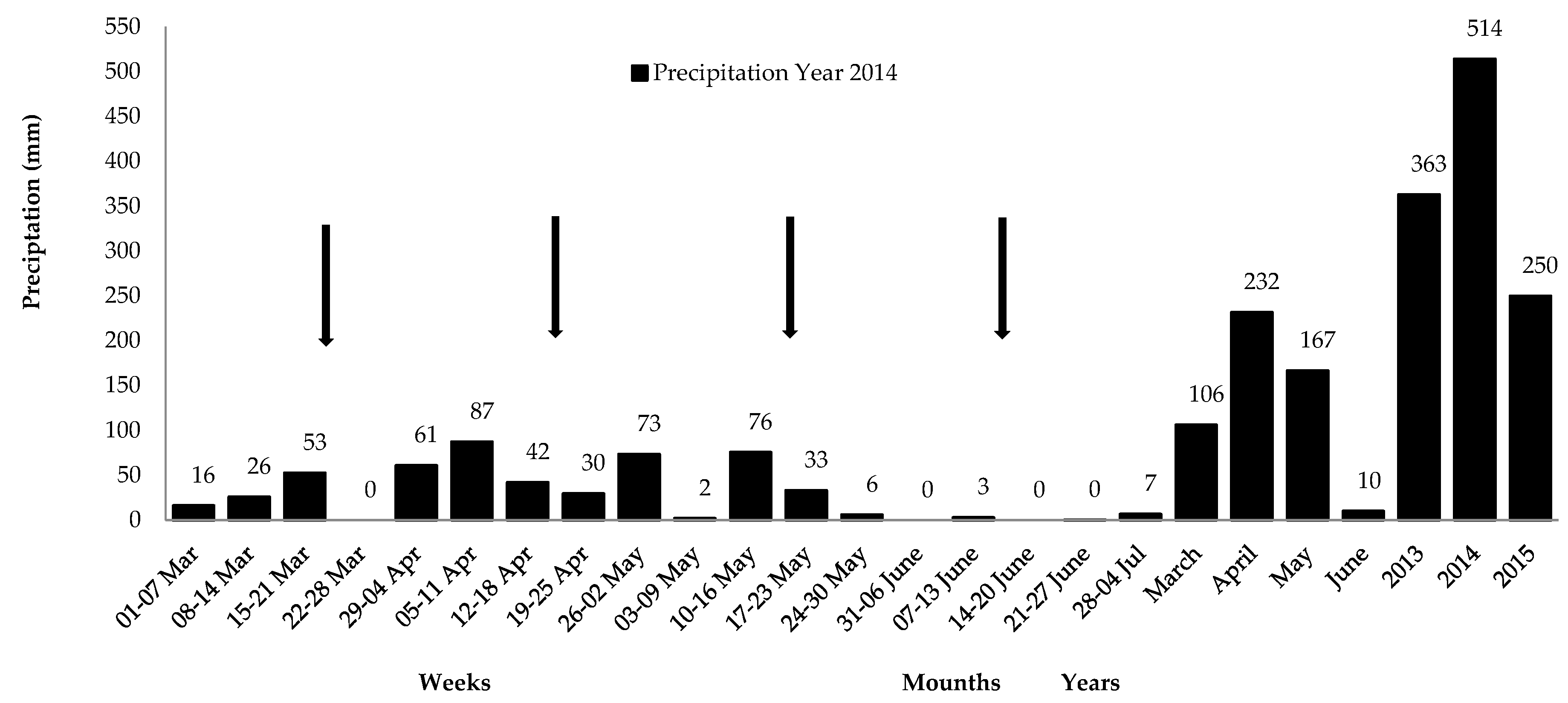
2.2. Characterization of the Experimental Area
2.3. Animals and Experimental Treatments
2.4. Forage Availability and Botanical Composition
2.5. Determination of Nutrient Intake and Pasture Sampling
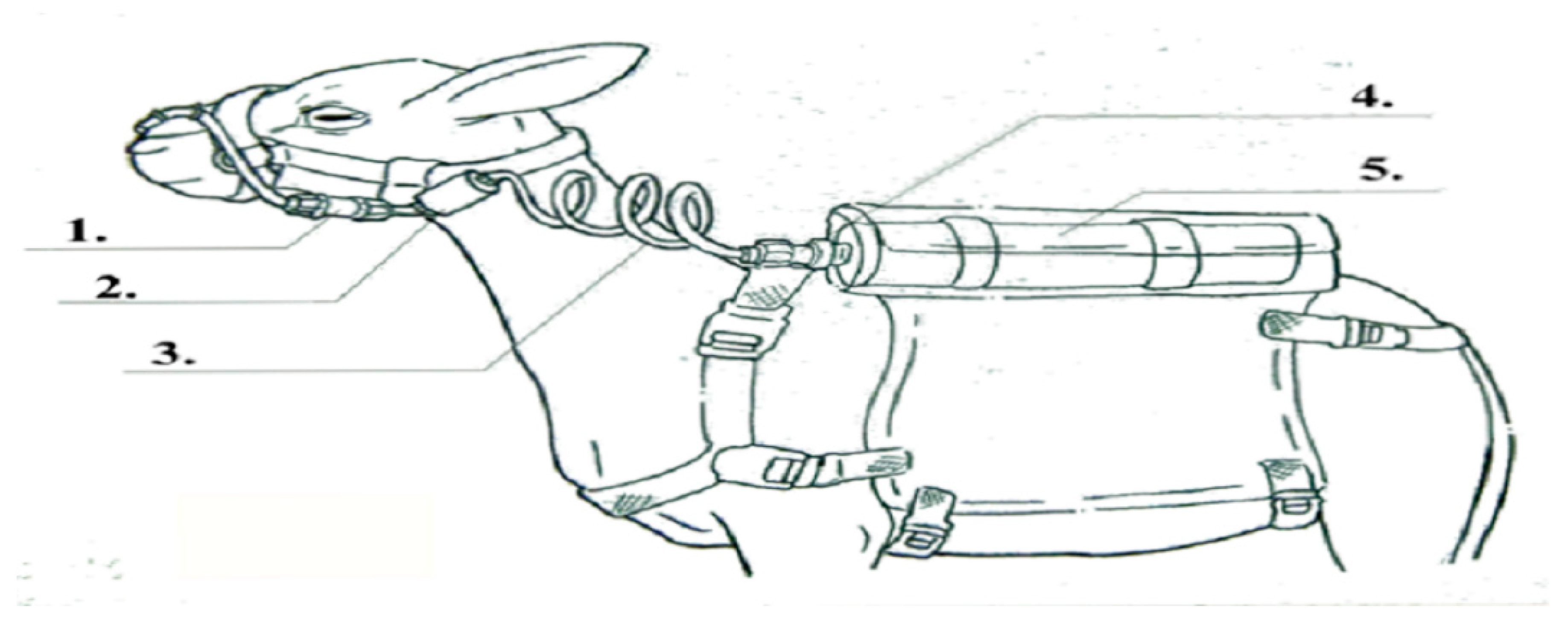
2.6. Determination of Enteric CH4 Emission
2.7. Chemical Analyses
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Dingenen, R.; Crippa, M.J.; Anssens-Maenhout, G.; Guizzardi, D.; Dentener, F. Global Trends of Methane Emissions and Their Impacts on Ozone Concentrations; Publications Office of the European Union: Brussels, Belgium, 2018; EUR29394EN; ISBN 9789279965500. [Google Scholar]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Blaxter, K.L.; Clapperton, J.L. Prediction of the amount of methane produced by ruminants. Br. J. Nutr. 1965, 19, 511–522. [Google Scholar] [CrossRef]
- Hook, S.E.; Wright, A.D.G.; McBride, B.W. Methanogens: Methane producers of the rumen and mitigation strategies. Archaea 2010, 2010, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Pedreira, M.d.S.; Primavesi, O. Aspectos ambientais na bovinocultura. In Nutrição de Ruminantes; Berchielli, T.T., Pires, A.V., Oliveira, S.G., Eds.; FUNEP: Jaboticabal, Brazil, 2011; pp. 521–535. [Google Scholar]
- Grainger, C.; Williams, R.; Eckard, R.J.; Hannah, M.C. A high dose of monensin does not reduce methane emissions of dairy cows offered pasture supplemented with grain. J. Dairy Sci. 2010, 93, 5300–5308. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. SPECIAL TOPICS — Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options1. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.A.; Malechek, J.C. The Voluntary Forage Intake and Nutrition of Goats and Sheep in the Semi-Arid Tropics of Northeastern Brazil. J. Anim. Sci. 1986, 63, 1078–1086. [Google Scholar] [CrossRef]
- Andrade, E.M.; Meireles, A.C.M.; Palácio, H.A.Q. O semiárido cearense e suas águas. In O semiárido e o manejo dos recursos naturais; Andrade, E.M., Pereira Omar, P.J., Dantas, F.E.R., Eds.; Imprensa Universitária: Fortaleza, Brazil, 2010; pp. 71–94. [Google Scholar]
- Beauchemin, K. Dietary mitigation of enteric methane from cattle. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2009, 4. [Google Scholar] [CrossRef]
- O’hara, P.; Freney, J.; Ulyatt, M. Abatement of Agricultural Non-Carbon Dioxide Greenhouse Gas Emissions A Study of Research Requirements Report prepared for the Ministry of Agriculture and Forestry on behalf of the Convenor, Ministerial Group on Climate Change, the Minister of Agriculture; New Zealand Ministry of Agriculture and Forestry: Wellington, New Zealand, 2003; ISBN 0-478-07754-8.
- Hungate, R.E. Quantities of Carbohydrate Fermentation Products. In The Rumen and its Microbes; Hungate, R.E., Ed.; Elsevier: New York, NY, USA, 1966; pp. 245–280. [Google Scholar]
- Eryavuz, A.; Dehority, B.A. Effects of supplemental zinc concentration on cellulose digestion and cellulolytic and total bacterial numbers in vitro. Anim. Feed Sci. Technol. 2009, 151, 175–183. [Google Scholar] [CrossRef]
- Arelovich, H.M.; Amela, M.I.; Martínez, M.F.; Bravo, R.D.; Torrea, M.B. Influence of different sources of zinc and protein supplementation on digestion and rumen fermentation parameters in sheep consuming low-quality hay. Small Rumin. Res. 2014, 121, 175–182. [Google Scholar] [CrossRef]
- Garg, A.K.; Mudgal, V.; Dass, R.S. Effect of organic zinc supplementation on growth, nutrient utilization and mineral profile in lambs. Anim. Feed Sci. Technol. 2008, 144, 82–96. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Danfær, A.; Røjen, B.A.; Raun, B.-M.L.; Weisbjerg, M.R.; Hvelplund, T. Metabolism of propionate and 1,2-propanediol absorbed from the washed reticulorumen of lactating cows. J. Anim. Sci. 2002, 80, 2168. [Google Scholar] [CrossRef]
- Kim, Y.K.; Choi, H.; Myung, K.H. Effects of propylene glycol on carcass traits and its related gene expression in Korean native steers. J. Anim. Sci. 2005, 83, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Czerkawski, J.W.; Breckenridge, G. Dissimilation of 1,2-propanediol by rumen micro-organisms. Br. J. Nutr. 1973, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Ingvartsen, K. Propylene glycol for dairy cows. Anim. Feed Sci. Technol. 2004, 115, 191–213. [Google Scholar] [CrossRef]
- Lane, S.F.; Hogue, D.E. Effects of butylene and propylene glycols on body composition and fatty acid synthetase in lambs. J. Anim. Sci. 1981, 53, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Araújo Filho, J.A. de Manejo Pastoril Sustentável da Caatinga | Instituto Interamericano de Cooperação para a Agricultura, 1st ed.; Projeto Dom Helder Camara: Recife, Brazil, 2013; ISBN 978-85-64154-04-9. [Google Scholar]
- BDMEP. BDMEP—Banco de Dados Meteorológicos para Ensino e Pesquisa. Available online: http://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep (accessed on 16 August 2019).
- Arelovich, H.M.; Owens, F.N.; Horn, G.W.; Vizcarra, J.A. Effects of supplemental zinc and manganese on ruminal fermentation, forage intake, and digestion by cattle fed prairie hay and urea. J. Anim. Sci. 2000, 78, 2972. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Small Ruminants, 1st ed.; National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-10213-1. [Google Scholar]
- Faria, E.P.; Rodriguez, N.M.; Moreira, G.R.; Barbosa, I.; Sampaio, M.; Luiza, A.; Cruz, C.; Saliba, E.d.O.S.; Faria, E.P.; Rodriguez, N.M.; et al. Use of Infrared Spectroscopy to Estimate Fecal Output with Marker Lipe®. Int. J. Food Sci. Nutr. Diet. (IJFS) 2015, 1–10. [Google Scholar]
- Olson, K.C. Diet Sample Collection by Esophageal Fistula and Rumen Evacuation Techniques. J. Range Manag. 1991, 44, 515. [Google Scholar] [CrossRef][Green Version]
- Tilley, J.M.A.; Terry, R.A. A two-satge technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Saliba, E.O.S.; Pilo-Veloso, D.; Rodriguez, N.M.; Capanema, E.A.; Saliba, J.S.; Borges, A.L.C.C.; Gonçalves, L.C.; Borges, I.; Jayme, D.G.; Silva, R.R. Characterization of Lignin before and after Exposure to the Gastrointestinal Tract of Ruminants. Am. J. Anal. Chem. 2016, 7, 748–753. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis of AOAC International. Assoc. Off. Anal. Chem. Int. 1990, 15, CD-ROM.
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses; U.S. Department Government Printing Office: Washington, DC, USA, 1975; pp. 387–598.
- Senger, C.C.D.; Kozloski, G.V.; Bonnecarrère Sanchez, L.M.; Mesquita, F.R.; Alves, T.P.; Castagnino, D.S. Evaluation of autoclave procedures for fibre analysis in forage and concentrate feedstuffs. Anim. Feed Sci. Technol. 2008, 146, 169–174. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Jung, H.-J.G.; Ralph, J.; Buxton, D.R.; Weimer, P.J. A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J. Sci. Food Agric. 1994, 65, 51–58. [Google Scholar] [CrossRef]
- Makkar, H.P. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Pinares-Patiño, C.S.; McEwan, J.C.; Dodds, K.G.; Cárdenas, E.A.; Hegarty, R.S.; Koolaard, J.P.; Clark, H. Repeatability of methane emissions from sheep. Anim. Feed Sci. Technol. 2011, 166, 210–218. [Google Scholar] [CrossRef]
- Pfister, J.A.; Malechek, J.C.; Balph, D.F. Foraging Behaviour of Goats and Sheep in the Caatinga of Brazil. J. Appl. Ecol. 1988, 25, 379. [Google Scholar] [CrossRef]
- Holter, J.B.; Young, A.J. Methane Prediction in Dry and Lactating Holstein Cows. J. Dairy Sci. 1992, 75, 2165–2175. [Google Scholar] [CrossRef]
- Kurihara, M.; Shibata, M.; Nishida, T.; Purnomoad, A.; Terada, F. Methane Production and Its Dietary Manipulation in Ruminants. In Rumen microbes and digestive physiology in ruminants; Onodera, R., Itabashi, H., Ushida, K., Yano, H., Sasaki, Y., Eds.; Japan Scientific Societies: Tokyo, Japan, 1997; pp. 199–208. ISBN 4762208647. [Google Scholar]
- Makkar, H.P.S.; Makkar, H.P.S. Treatment of Plant Material, Extraction of Tannins, and an Overview of Tannin Assays Presented in the Manual. Quantif. Tann. Tree Shrub Foliage 2013, 43–48. [Google Scholar]
- Archimède, H.; Rira, M.; Eugène, M.; Fleury, J.; Lastel, M.L.; Periacarpin, F.; Silou-Etienne, T.; Morgavi, D.P.; Doreau, M. Intake, total-tract digestibility and methane emissions of Texel and Blackbelly sheep fed C4 and C3 grasses tested simultaneously in a temperate and a tropical area. J. Clean. Prod. 2018, 185, 455–463. [Google Scholar] [CrossRef]
- Mallaki, M.; Norouzian, M.A.; Khadem, A.A. Effect of organic zinc supplementation on growth, nutrient utilization, and plasma zinc status in lambs. Turkish J. Vet. Anim. Sci. 2015, 39, 75–80. [Google Scholar] [CrossRef]
- Cozzi, G.; Berzaghi, P.; Gottardo, F.; Gabai, G.; Andrighetto, I. Effects of feeding propylene glycol to mid-lactating dairy cows. Anim. Feed Sci. Technol. 1996, 64, 43–51. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional ecology of the ruminant, 2nd ed.Comstock Pub; Cornel University: Ithaca, NY, USA, 1994; ISBN 0-8014-2772-X. [Google Scholar]
- Leuning, R.; Baker, S.K.; Jamie, I.M.; Hsu, C.H.; Klein, L.; Denmead, O.T.; Griffith, D.W.T. Methane emission from free-ranging sheep: A comparison of two measurement methods. Atmos. Environ. 1999, 33, 1357–1365. [Google Scholar] [CrossRef]
- Moreira, G.D.; Lima, P.d.M.T.; Borges, B.O.; Primavesi, O.; Longo, C.; McManus, C.; Abdalla, A.; Louvandini, H. Tropical tanniniferous legumes used as an option to mitigate sheep enteric methane emission. Trop. Anim. Health Prod. 2013, 45, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.M. Fitossociologia e fluxo de emissão de metano entérico em áreas de Caatinga; Universidade Estadual Vale do Acaraú: Ceará, Brazil, 2014. [Google Scholar]
- Clapperton, J.L.; Czerkawski, J.W. Metabolism of propane-1:2-diol infused into the rumen of sheep. Br. J. Nutr. 1972, 27, 553–560. [Google Scholar] [CrossRef] [PubMed]
| DM Availability, kg/ha | Floristic Composition, g/kg | |||
|---|---|---|---|---|
| Legumes | Grasses | Total | Legumes | Grasses |
| 1364 | 533 | 1897 | 719 | 281 |
| Variables | Periods | Concentrate β | |||
|---|---|---|---|---|---|
| March | April | May | June | ||
| Dry Matter ¥, g/kg | 118 | 128 | 142 | 158 | 877 |
| g/kg DM | |||||
| OM | 819 | 810 | 798 | 819 | 913 |
| Crude Protein | 192 | 187 | 176 | 131 | 254 |
| Neutral Detergent Insoluble Nitrogen (NDIN) | 2.99 | 2.87 | 3.03 | 3.01 | 3.04 |
| NDIN, % of total nitrogen | 98.3 | 96.3 | 108 | 145 | 74.6 |
| Ether Extract | 76.0 | 76.5 | 86.8 | 111 | 64.0 |
| Neutral Detergent Fiber | 524 | 590 | 610 | 564 | 159 |
| Ash-free values of Neutral Detergent Fiber (aNDFom-NDF) ‡ | 437 | 496 | 504 | 478 | 113 |
| Acid Detergent Fiber | 430 | 476 | 487 | 473 | 103 |
| Hemicellulose | 94.1 | 114 | 123 | 91.5 | 56.1 |
| Cellulose | 208 | 250 | 261 | 243 | 45.6 |
| Acid Detergent Lignin | 35.4 | 45.4 | 52.5 | 37.8 | 11.3 |
| Klason Lignin | 40.7 | 50.4 | 65.4 | 52.8 | 17.8 |
| Total tannins | 0.64 | 8.14 | 8.33 | 14.8 | - |
| In Vitro Dry Matter Digestibility † | 537 | 408 | 424 | 441 | 954 |
| In Vitro Organic Matter Digestibility | 468 | 333 | 353 | 359 | 939 |
| Variable £ | Treatment ‡ | Period β | SEM ¥ | P-value † | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | Zn | PG | Mar | Apr | May | Jun | T | P | T × P | ||
| Intake, g/day | |||||||||||
| OM | 527 | 542 | 551 | 607 a | 539 b | 509 b | 505 b | 9.39 | 0.56 | <0.01 | 0.57 |
| NDF | 231 | 236 | 246 | 279 a | 233 b | 235 b | 204 c | 5.47 | 0.33 | <0.01 | 0.52 |
| CH4 emission | |||||||||||
| g/day | 15.8 b | 15.6 b | 19.2 a | 16.5 ab | 17.2 ab | 18.8 a | 15.0 b | 0.56 | 0.01 | 0.04 | 0.11 |
| mg/OM | 30.0 | 29.0 | 35.0 | 27.9b | 32.2 b | 37.3 a | 29.7 b | 1.24 | 0.09 | 0.04 | 0.11 |
| g/OMkgLW0.75 | 0.29 | 0.30 | 0.35 | 0.27b | 0.31 b | 0.37 a | 0.31 b | 0.01 | 0.09 | 0.04 | 0.12 |
| mg/NDF | 69.9 | 68.9 | 79.4 | 61.1 | 75.6 | 81.0 | 73.3 | 2.89 | 0.17 | 0.07 | 0.18 |
| g/NDFkgLW0.75 | 0.68 | 0.71 | 0.79 | 0.60 | 0.73 | 0.80 | 0.77 | 0.03 | 0.25 | 0.06 | 0.17 |
| Variable | Treatment ‡ | SEM ¥ | P-value | ||
|---|---|---|---|---|---|
| CT | Zn | PG | |||
| Production parameter | |||||
| Cold carcass, in kg | 7.45 | 8.16 | 8.33 | 0.270 | 0.37 |
| Body weight gain in the period, in kg | 3.33 | 4.08 | 4.31 | 0.365 | 0.29 |
| CH4 relation with production parameters | |||||
| β Total CH4, kg | 1.81 b | 1.71 b | 2.20 a | 0.057 | 0.01 |
| kg CH4/kg of total body weight gain | 0.688 | 0.411 | 0.700 | 0.072 | 0.09 |
| kg CH4/kg of cold carcass weight | 0.248 | 0.209 | 0.271 | 0.009 | 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, H.; Saliba, E.; Bomfim, M.; Lana, Â.M.; Borges, A.L.; Landim, A.; Mota, C.; Tonucci, R.; Faciola, A.P. Sheep Methane Emission on Semiarid Native Pasture—Potential Impacts of Either Zinc Sulfate or Propylene Glycol as Mitigation Strategies. Animals 2020, 10, 395. https://doi.org/10.3390/ani10030395
Costa H, Saliba E, Bomfim M, Lana ÂM, Borges AL, Landim A, Mota C, Tonucci R, Faciola AP. Sheep Methane Emission on Semiarid Native Pasture—Potential Impacts of Either Zinc Sulfate or Propylene Glycol as Mitigation Strategies. Animals. 2020; 10(3):395. https://doi.org/10.3390/ani10030395
Chicago/Turabian StyleCosta, Hélio, Eloisa Saliba, Marco Bomfim, Ângela Maria Lana, Ana Luiza Borges, Aline Landim, Carlos Mota, Rafael Tonucci, and Antonio P. Faciola. 2020. "Sheep Methane Emission on Semiarid Native Pasture—Potential Impacts of Either Zinc Sulfate or Propylene Glycol as Mitigation Strategies" Animals 10, no. 3: 395. https://doi.org/10.3390/ani10030395
APA StyleCosta, H., Saliba, E., Bomfim, M., Lana, Â. M., Borges, A. L., Landim, A., Mota, C., Tonucci, R., & Faciola, A. P. (2020). Sheep Methane Emission on Semiarid Native Pasture—Potential Impacts of Either Zinc Sulfate or Propylene Glycol as Mitigation Strategies. Animals, 10(3), 395. https://doi.org/10.3390/ani10030395





